Pathological Role of Pin1 in the Development of DSS-Induced Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Antibodies
2.3. Flow Cytometry
2.4. Real Time PCR
2.5. Histology
2.6. Immunohistochemical Analysis
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Pin1 Expression Is Increased in DSS-Induced Colitis
3.2. Global Pin1 KO Mice Were Resistant to DSS-Induced Colitis Development
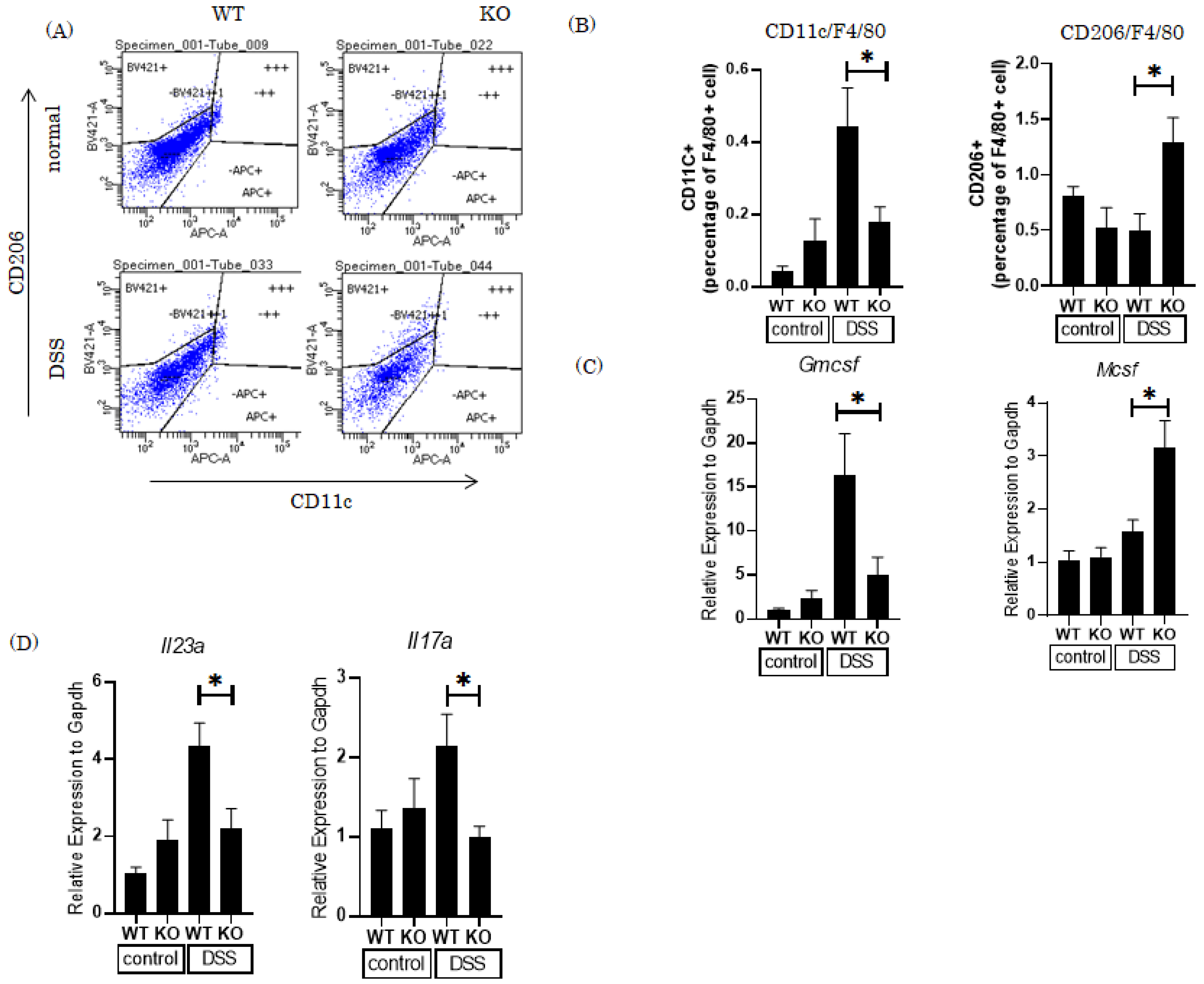
3.3. Knockout of the Pin1 Gene Increases the M2 Macrophage Population and Reduces Expression of Th17 Cell-Related Cytokines in the Colons of DSS-Treated Mice
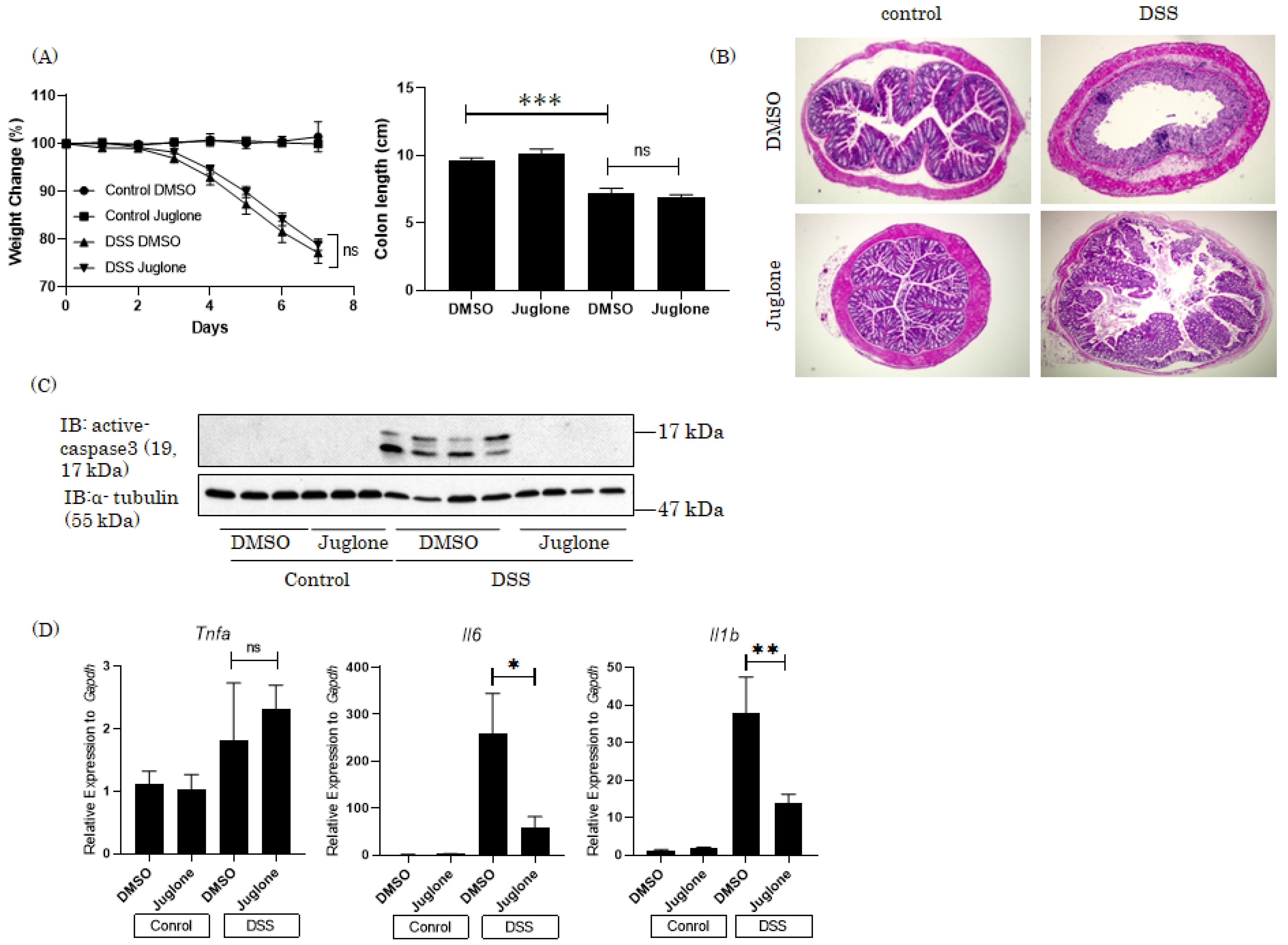
4. Therapeutic Effects of the Pin1 Inhibitor Juglone on DSS-Induced Colitis in Mice
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 2019, 577, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Nowarski, R.; Jackson, R.; Gagliani, N.; Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Nakatsu, Y.; Matsunaga, Y.; Yamamotoya, T.; Ueda, K.; Inoue, Y.; Mori, K.; Sakoda, H.; Fujishiro, M.; Ono, H.; Kushiyama, A.; et al. Physiological and Pathogenic Roles of Prolyl Isomerase Pin1 in Metabolic Regulations via Multiple Signal Transduction Pathway Modulations. Int. J. Mol. Sci. 2016, 17, 1495. [Google Scholar] [CrossRef]
- Lu, K.P.; Zhou, X.Z. The prolyl isomerase PIN 1: A pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.C.; Zhou, X.Z.; Lu, K.P. Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem. Sci. 2011, 36, 501–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryo, A.; Suizu, F.; Yoshida, Y.; Perrem, K.; Liou, Y.C.; Wulf, G.; Rottapel, R.; Yamaoka, S.; Lu, K.P. Regulation of NF-kappa B signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 2003, 12, 1413–1426. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Xia, Y.; Ji, H.; Chen, X.; Guo, F.; Lyssiotis, C.A.; Aldape, K.; Cantley, L.C.; Lu, Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012, 14, 1295–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.J.; Wulf, G.; Zhou, X.Z.; Lu, K.P. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999, 399, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Esnault, S.; Malter, J.S. The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat. Immunol. 2005, 6, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, Y.; Iwashita, M.; Sakoda, H.; Ono, H.; Nagata, K.; Matsunaga, Y.; Fukushima, T.; Fujishiro, M.; Kushiyama, A.; Kamata, H.; et al. Prolyl isomerase Pin1 negatively regulates AMP-activated protein kinase (AMPK) by associating with the CBS domain in the γ subunit. J. Biol. Chem. 2015, 290, 24255–24266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsu, Y.; Otani, Y.; Sakoda, H.; Zhang, J.; Guo, Y.; Okubo, H.; Kushiyama, A.; Fujishiro, M.; Kikuch, T.; Fukushima, T.; et al. Role of Pin1 protein in the pathogenesis of nonalcoholic steatohepatitis in a rodent model. J. Biol. Chem. 2012, 287, 44526–44535. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- El-Behi, M.; Ciric, B.; Dai, H.; Yan, Y.; Cullimore, M.; Safavi, F.; Zhang, G.X.; Dittel, B.N.; Rostami, A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011, 12, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Shen, Z.J.; Hu, J.; Shiizaki, K.; Kuro-o, M.; Malter, J.S. Phosphate-Induced Renal Fibrosis Requires the Prolyl Isomerase Pin1. PLoS ONE 2016, 11, e0150093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussetta, T.; Gougerot-Pocidalo, M.A.; Hayem, G.; Ciappelloni, S.; Raad, H.; Derkawi, R.A.; Bournier, O.; Kroviarski, Y.; Zhou, X.Z.; Malter, J.S.; et al. The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils. Blood 2010, 116, 5795–5802. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.J.; Hu, J.; Kashi, V.; Bochkov, Y.A.; Gern, J.E.; Malter, J.S. TLR-7 Stress Signaling in Differentiating and Mature Eosinophils Is Mediated by the Prolyl Isomerase Pin1. J. Immunol. 2018, 201, 3503–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, C.; Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011, 140, 1729–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, K.L.; Zheng, L.B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Gagliani, N.; Vesely, M.C.A.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; de Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Codarri, L.; Gyülvészi, G.; Tosevski, V.; Hesske, L.; Fontana, A.; Magnenat, L.; Suter, T.; Becher, B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011, 12, 560–567. [Google Scholar] [CrossRef]
- Esnault, S.; Shen, Z.J.; Whitesel, E.; Malter, J.S. The peptidyl-prolyl isomerase Pin1 regulates granulocyte-macrophage colony-stimulating factor mRNA stability in T lymphocytes. J. Immunol. 2006, 177, 6999–7006. [Google Scholar] [CrossRef]


| Forward | Tm | Reverse | Tm | Product Size | |
|---|---|---|---|---|---|
| Gapdh | TGATGGGTGTGAACCACGAG | 63 | GGGCCATCCACAGTCTTCTG | 63 | 178 |
| Tnfa | GAACTGGCAGAAGAGGCACT | 63 | AGGGTCTGGGCCATAGAACT | 63 | 163 |
| Mcp1 | TGGTCCCTGTCATGCTTCTG | 63 | TCTGGACCCATTCCTTCTTG | 63 | 208 |
| Il6 | AGTTGCCTTCTTGGGACTGA | 63 | CAGAATTGCCATTGCACAAC | 63 | 151 |
| Il17a | TCGAGAAGATGCTGGTGGGT | 58.4 | CTCTGTTTAGGCTGCCTGGC | 60.4 | 71 |
| Il23a | GGTGGCTCAGGGAAATGT | 55.8 | GACAGAGCAGGCAGGTACAG | 60.4 | 67 |
| Gmcsf | ATGCCTGTCACGTTGAAT GAAG | 63 | GCGGGTCTGCACAGATGTTA | 63 | 81 |
| Pin1 | CGGCAGGAAAAGATCACCAG | 63 | TCCCCTGTCCGTAGAGCAAA | 63 | 175 |
| Il1b | CGTGGACCTTCCAGGATGAG | 63 | GCTCATATGGGTCCGACAGC | 63 | 146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, Y.; Hasei, S.; Yamamotoya, T.; Honda, H.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Ono, H.; Ito, H.; Okabe, T.; et al. Pathological Role of Pin1 in the Development of DSS-Induced Colitis. Cells 2021, 10, 1230. https://doi.org/10.3390/cells10051230
Matsunaga Y, Hasei S, Yamamotoya T, Honda H, Kushiyama A, Sakoda H, Fujishiro M, Ono H, Ito H, Okabe T, et al. Pathological Role of Pin1 in the Development of DSS-Induced Colitis. Cells. 2021; 10(5):1230. https://doi.org/10.3390/cells10051230
Chicago/Turabian StyleMatsunaga, Yasuka, Shun Hasei, Takeshi Yamamotoya, Hiroaki Honda, Akifumi Kushiyama, Hideyuki Sakoda, Midori Fujishiro, Hiraku Ono, Hisanaka Ito, Takayoshi Okabe, and et al. 2021. "Pathological Role of Pin1 in the Development of DSS-Induced Colitis" Cells 10, no. 5: 1230. https://doi.org/10.3390/cells10051230
APA StyleMatsunaga, Y., Hasei, S., Yamamotoya, T., Honda, H., Kushiyama, A., Sakoda, H., Fujishiro, M., Ono, H., Ito, H., Okabe, T., Asano, T., & Nakatsu, Y. (2021). Pathological Role of Pin1 in the Development of DSS-Induced Colitis. Cells, 10(5), 1230. https://doi.org/10.3390/cells10051230






