Conditioned Medium from Mesenchymal Stem Cells Alleviates Endothelial Dysfunction of Vascular Grafts Submitted to Ischemia/Reperfusion Injury in 15-Month-Old Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Bone Marrow-Derived MSCs-CM
2.3. Antibody Array
2.4. Rat Model of Endothelial Dysfunction Induced by Cold Ischemic Storage and Reperfusion
2.4.1. Preparation and Conservation of Aortic Rings
2.4.2. Experimental Groups
2.4.3. Ex Vivo Organ Bath Experiments
2.5. Aortic Histomorphometry
2.6. Acid Fuchsin Orange (AFOG) Staining
2.7. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (PCR) Analysis
2.8. Caspase-12 Immunolabeling
2.9. Tibial Lengths
2.10. Statistical Analysis
3. Results
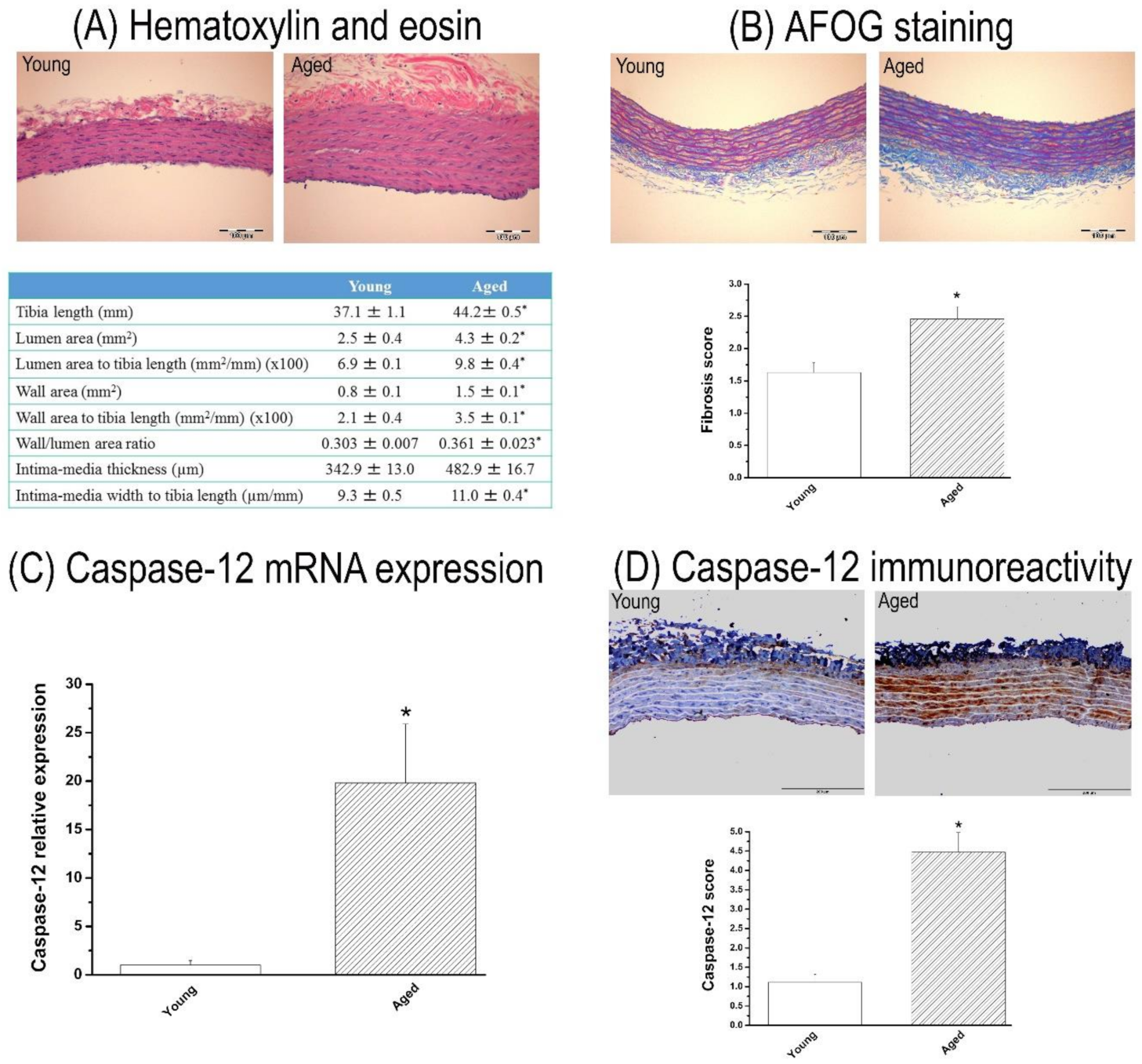
3.1. Characterization of 15-Month-Old Rat Model
3.1.1. Body Weight and Aortic Morphometry
3.1.2. Fibrosis in the Aorta
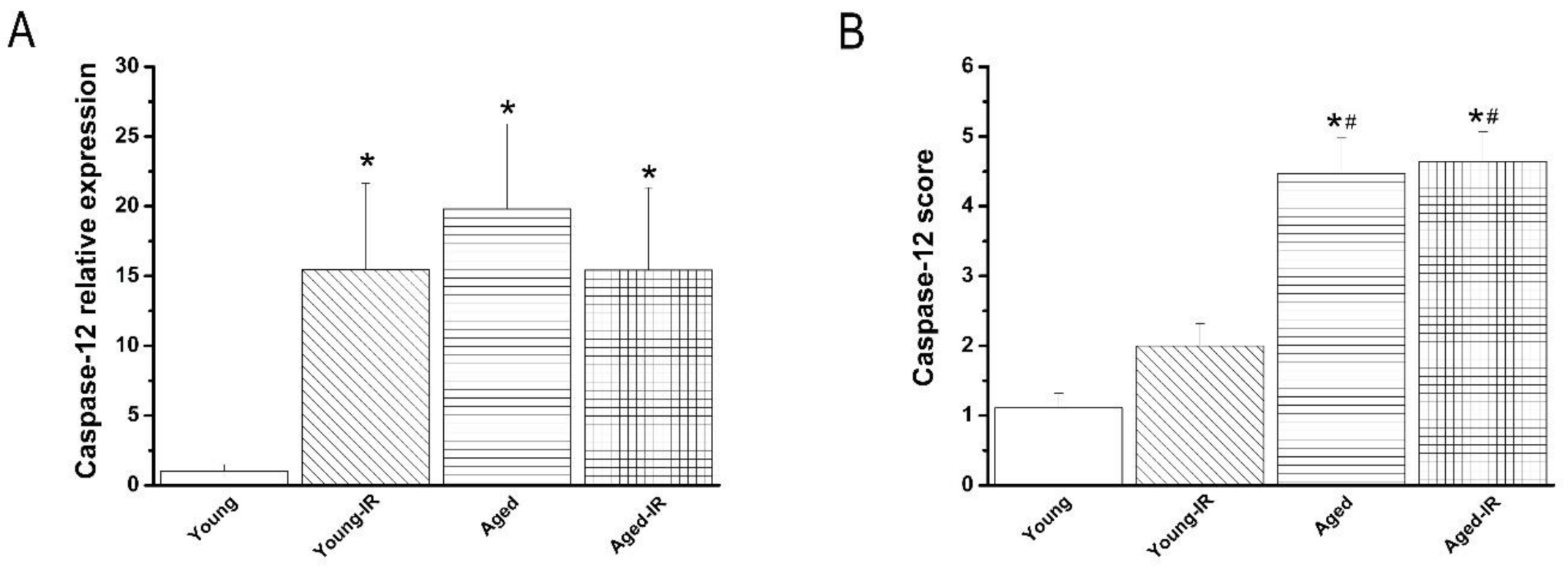
3.1.3. Caspase-12 Expression in the Aorta
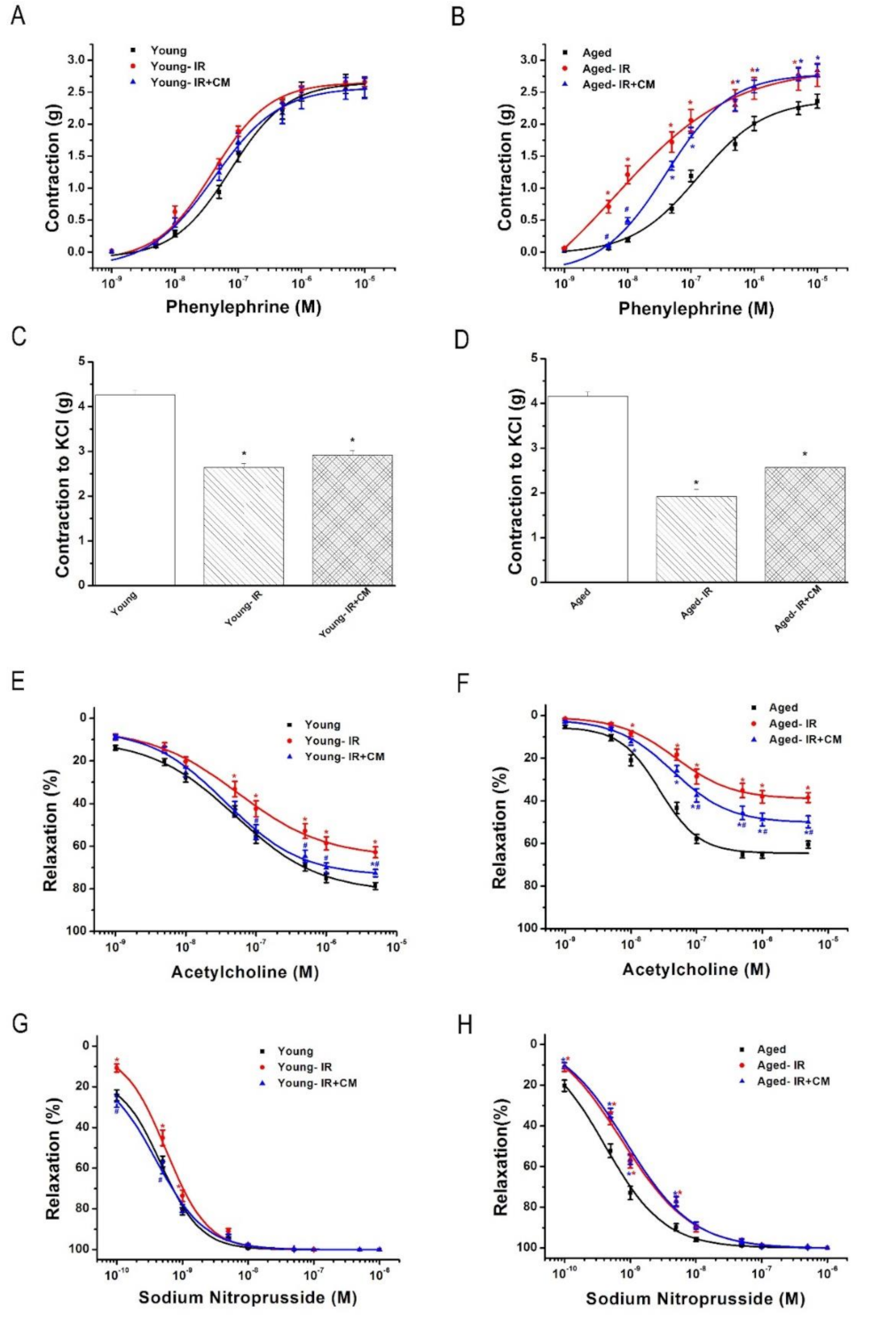
3.1.4. Contractile and Relaxant Responses in the Aortic Rings
3.2. Effect of IRI in Young and Aged Rats’ Aorta
3.2.1. Effect of IRI on Contractile Responses of Aortic Rings
3.2.2. Effect of IRI on Endothelium-Dependent Vasorelaxation of Aortic Rings
3.2.3. Effect of IRI on Endothelium-Independent Vasorelaxation of Aortic Rings
3.2.4. Effect of IRI on Caspase-12 mRNA Expression and Immunoreactivity in the Aorta
3.3. Effect of CM Against IRI in Young and Aged Rats’ Aorta
3.3.1. Characterization of the CM
3.3.2. Effects of CM on Contractile Responses After IRI
3.3.3. Effects of CM on Endothelium-Dependent Vasorelaxation After IRI
3.3.4. Effects of CM on Endothelium-Independent Vasorelaxation After IRI
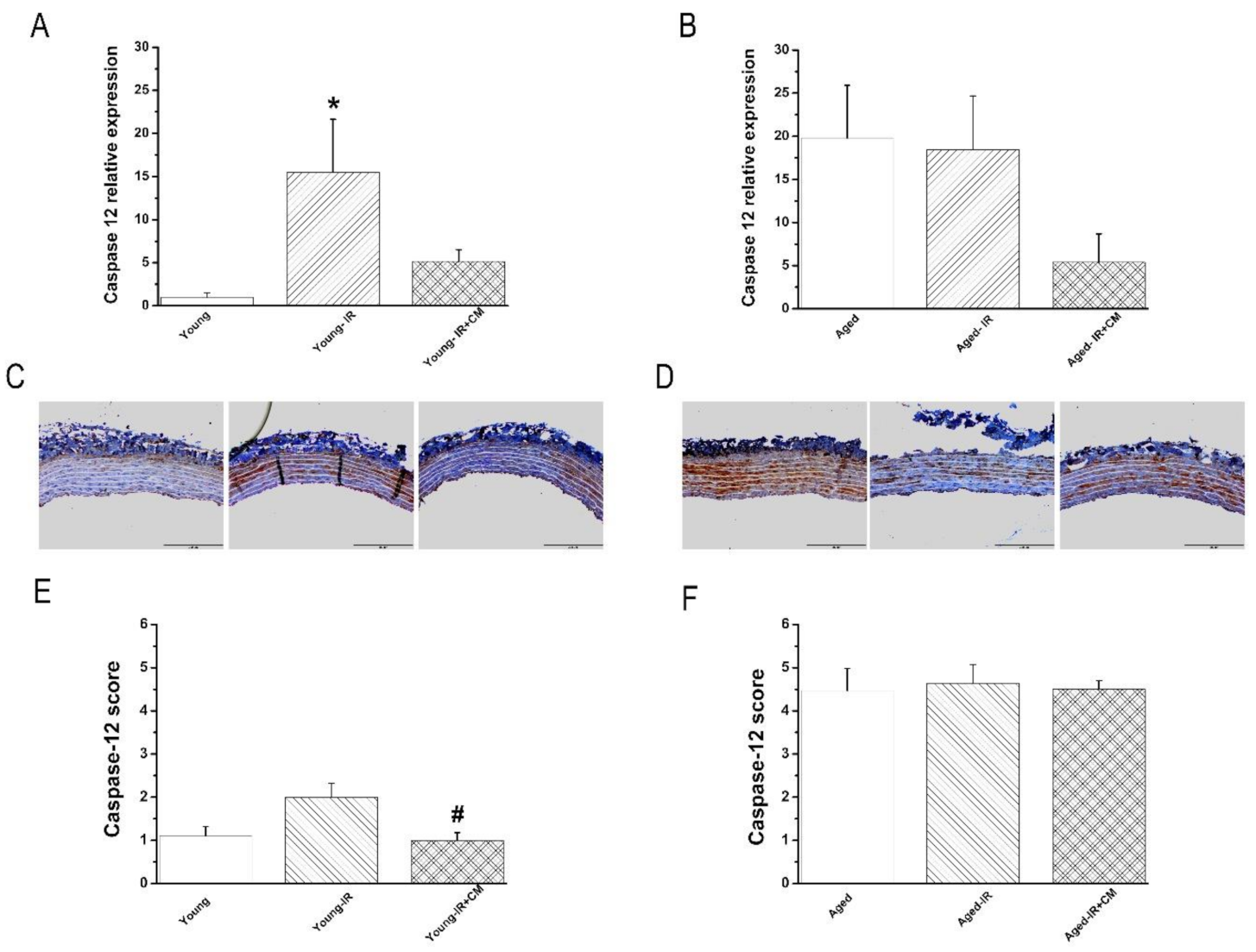
3.3.5. Effects of CM on Caspase-12 Expression After IRI
4. Discussion
4.1. Mechanisms Underlying the Protective Effects of CM after IRI in Vascular
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birnbaum, Y.; Leor, J.; Kloner, R.A. Pathobiology and Clinical Impact of Reperfusion Injury. J. Thromb. Thrombolysis 1995, 2, 177–186. [Google Scholar]
- Hearse, D.J.; Bolli, R. Reperfusion induced injury: Manifestations, mechanisms, and clinical relevance. Cardiovasc. Res. 1992, 26, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Anversa, P.; Cheng, W.; Liu, Y.; Leri, A.; Redaelli, G.; Kajstura, J. Apoptosis and myocardial infarction. Basic Res. Cardiol. 1998, 93, s8–s12. [Google Scholar] [CrossRef]
- Marin, J. Age-related changes in vascular responses: A review. Mech. Ageing Dev. 1995, 79, 71–114. [Google Scholar] [CrossRef]
- Radovits, T.; Seres, L.; Gero, D.; Berger, I.; Szabo, C.; Karck, M.; Szabo, G. Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction. Exp. Gerontol. 2007, 42, 676–685. [Google Scholar] [CrossRef][Green Version]
- Radovits, T.; Seres, L.; Gero, D.; Lin, L.N.; Beller, C.J.; Chen, S.H.; Zotkina, J.; Berger, I.; Groves, J.T.; Szabo, C.; et al. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech. Ageing Dev. 2007, 128, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, U.; Tanner, B.; Strohschneider, T.; Stadtmuller, A.; Hannekum, A. Damage to arterial and venous endothelial cells in bypass grafts induced by several solutions used in bypass surgery. Thorac. Cardiovasc. Surg. 1997, 45, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Chou, C.C.; Frantzen, M.; Miyamoto, T.; Kar, S.; Lee, S.; Shah, P.K.; Martin, B.J.; Lill, M.; Forrester, J.S.; et al. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int. J. Cardiol. 2006, 111, 231–239. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007, 1, 129–137. [Google Scholar] [CrossRef]
- Korkmaz-Icoz, S.; Li, S.; Huttner, R.; Ruppert, M.; Radovits, T.; Loganathan, S.; Sayour, A.A.; Brlecic, P.; Lasitschka, F.; Karck, M.; et al. Hypothermic perfusion of donor heart with a preservation solution supplemented by mesenchymal stem cells. J. Heart Lung Transplant. 2019, 38, 315–326. [Google Scholar] [CrossRef]
- Korkmaz-Icoz, S.; Zhou, P.; Guo, Y.; Loganathan, S.; Brlecic, P.; Radovits, T.; Sayour, A.A.; Ruppert, M.; Veres, G.; Karck, M.; et al. Mesenchymal stem cell-derived conditioned medium protects vascular grafts of brain-dead rats against in vitro ischemia/reperfusion injury. Stem Cell Res. Ther. 2021, 12, 144. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Barnucz, E.; Veres, G.; Hegedus, P.; Klein, S.; Zoller, R.; Radovits, T.; Korkmaz, S.; Horkay, F.; Merkely, B.; Karck, M.; et al. Prolyl-hydroxylase inhibition preserves endothelial cell function in a rat model of vascular ischemia reperfusion injury. J. Pharmacol. Exp. Ther. 2013, 345, 25–31. [Google Scholar] [CrossRef]
- Radovits, T.; Lin, L.N.; Zotkina, J.; Koch, A.; Rauen, U.; Kohler, G.; Karck, M.; Szabo, G. Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: Effects of iron chelators and N-alpha-acetyl-L-histidine. J. Heart Lung Transplant. 2008, 27, 208–216. [Google Scholar] [CrossRef]
- Veres, G.; Hegedus, P.; Barnucz, E.; Zoller, R.; Radovits, T.; Korkmaz, S.; Kolonics, F.; Weymann, A.; Karck, M.; Szabo, G. Addition of vardenafil into storage solution protects the endothelium in a hypoxia-reoxygenation model. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 242–248. [Google Scholar] [CrossRef][Green Version]
- Korkmaz-Icoz, S.; Vater, A.; Li, S.; Lehner, A.; Radovits, T.; Hegedus, P.; Ruppert, M.; Brlecic, P.; Zorn, M.; Karck, M.; et al. Mild type 2 diabetes mellitus improves remote endothelial dysfunction after acute myocardial infarction. J. Diabetes Complicat. 2015, 29, 1253–1260. [Google Scholar] [CrossRef]
- Korkmaz-Icoz, S.; Brlecic, P.; Ruppert, M.; Radovits, T.; Karck, M.; Szabo, G. Mechanical pressure unloading therapy reverses thoracic aortic structural and functional changes in a hypertensive rat model. J. Hypertens 2018, 26, 2350–2361. [Google Scholar] [CrossRef]
- Eckl, S.; Heim, C.; Abele-Ohl, S.; Hoffmann, J.; Ramsperger-Gleixner, M.; Weyand, M.; Ensminger, S.M. Combination of clopidogrel and everolimus dramatically reduced the development of transplant arteriosclerosis in murine aortic allografts. Transpl. Int. 2010, 23, 959–966. [Google Scholar] [CrossRef]
- Chirco, R.; Liu, X.W.; Jung, K.K.; Kim, H.R. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006, 25, 99–113. [Google Scholar] [CrossRef] [PubMed]
- De Gennaro Colonna, V.; Rossoni, G.; Bonacci, D.; Ciceri, S.; Cattaneo, L.; Muller, E.; Berti, F. Worsening of ischemic damage in hearts from rats with selective growth hormone deficiency. Eur. J. Pharmacol. 1996, 314, 333–338. [Google Scholar] [CrossRef]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995, 1, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L.E.; Golijanin, D.; Itin, A.; Pode, D.; Keshet, E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Investig. 1999, 103, 159–165. [Google Scholar] [CrossRef]
- Harrison, C.A.; Gray, P.C.; Vale, W.W.; Robertson, D.M. Antagonists of activin signaling: Mechanisms and potential biological applications. Trends Endocrinol. Metab. 2005, 16, 73–78. [Google Scholar] [CrossRef]
- Hung, S.C.; Pochampally, R.R.; Chen, S.C.; Hsu, S.C.; Prockop, D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- De Gracia, D.J.; Montie, H.L. Cerebral ischemia and the unfolded protein response. J. Neurochem. 2004, 91, 1–8. [Google Scholar] [CrossRef]
- Lenna, S.; Han, R.; Trojanowska, M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 2014, 66, 530–537. [Google Scholar] [CrossRef]
- Liu, W.; Ding, Y.; Zhang, X.; Wang, L. Bone marrow stromal cells inhibit caspase-12 expression in rats with spinal cord injury. Exp. Ther. Med. 2013, 6, 671–674. [Google Scholar] [CrossRef]
- Xue, L.X.; Liu, H.Y.; Cui, Y.; Dong, Y.; Wang, J.Q.; Ji, Q.Y.; He, J.T.; Yao, M.; Wang, Y.Y.; Shao, Y.K.; et al. Neuroprotective effects of Activin A on endoplasmic reticulum stress-mediated apoptotic and autophagic PC12 cell death. Neural. Regen. Res. 2017, 12, 779–786. [Google Scholar]

| Young | Young-IR | Aged | Aged-IR | |
|---|---|---|---|---|
| PE (g) | 2.63 ± 0.15 | 2.66 ± 0.07 | 2.36 ± 0.11 | 2.77 ± 0.18 |
| pD2 to PE | 7.07 ± 0.04 | 7.45 ± 0.06 * | 6.57 ± 0.22 | 8.35 ± 0.21 $ |
| KCl (g) | 4.26 ± 0.11 | 2.64 ± 0.09 * | 4.16 ± 0.10 | 1.92 ± 0.16 $ |
| Rmax to ACh (%) | 79.81 ± 1.41 | 65.01 ± 1.93 * | 65.45 ± 1.40 * | 38.49 ± 2.32 #,$ |
| pD2 to ACh | 7.25 ± 0.08 | 7.05 ± 0.11 | 6.95 ± 0.27 | 6.81 ± 0.18 |
| Rmax to SNP (%) | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| pD2 to SNP | 9.30 ± 0.02 | 9.26 ± 0.05 | 9.31 ± 0.06 | 9.14 ± 0.09 $ |
| Young | Young-IR | Young-IR + CM | |
|---|---|---|---|
| PE (g) | 2.63 ± 0.15 | 2.66 ± 0.07 | 2.57 ± 0.17 |
| pD2 to PE | 7.07 ± 0.04 | 7.45 ± 0.06 * | 7.30 ± 0.05 * |
| KCl (g) | 4.26 ± 0.11 | 2.64 ± 0.09 * | 2.92 ± 0.10 * |
| Rmax to ACh (%) | 79.81 ± 1.41 | 65.01 ± 1.93 * | 72.63 ± 1.78 *,# |
| pD2 to ACh | 7.25 ± 0.08 | 7.05 ± 0.11 | 7.23 ± 0.12 |
| Rmax to SNP (at 5 × 10−7 M) (%) | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| pD2 to SNP | 9.30 ± 0.02 | 9.26 ± 0.05 | 9.55 ± 0.10 # |
| Aged | Aged-IR | Aged-IR + CM | |
| PE (g) | 2.36 ± 0.11 | 2.77 ± 0.18 * | 2.84 ± 0.10 * |
| pD2 to PE | 6.57 ± 0.22 | 8.35 ± 0.21 * | 7.34 ± 0.04 * |
| KCl (g) | 4.16 ± 0.10 | 1.92 ± 0.16 * | 2.57 ± 0.10 * |
| Rmax to ACh (%) | 65.45 ± 1.40 | 38.49 ± 2.32 * | 53.89 ± 2.46 *,# |
| pD2 to ACh | 6.95 ± 0.27 | 6.81 ± 0.18 * | 7.03 ± 0.19 * |
| Rmax to SNP (at 5 × 10−7 M) (%) | 99.7 ± 0.2 | 99.7 ± 0.2 | 99.7 ± 0.2 |
| pD2 to SNP | 9.31 ± 0.06 | 9.14 ± 0.09 * | 9.09 ± 0.07 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz-Icöz, S.; Sun, X.; Li, S.; Brlecic, P.; Loganathan, S.; Ruppert, M.; Sayour, A.A.; Radovits, T.; Karck, M.; Szabó, G. Conditioned Medium from Mesenchymal Stem Cells Alleviates Endothelial Dysfunction of Vascular Grafts Submitted to Ischemia/Reperfusion Injury in 15-Month-Old Rats. Cells 2021, 10, 1231. https://doi.org/10.3390/cells10051231
Korkmaz-Icöz S, Sun X, Li S, Brlecic P, Loganathan S, Ruppert M, Sayour AA, Radovits T, Karck M, Szabó G. Conditioned Medium from Mesenchymal Stem Cells Alleviates Endothelial Dysfunction of Vascular Grafts Submitted to Ischemia/Reperfusion Injury in 15-Month-Old Rats. Cells. 2021; 10(5):1231. https://doi.org/10.3390/cells10051231
Chicago/Turabian StyleKorkmaz-Icöz, Sevil, Xiaoxin Sun, Shiliang Li, Paige Brlecic, Sivakkanan Loganathan, Mihály Ruppert, Alex Ali Sayour, Tamás Radovits, Matthias Karck, and Gábor Szabó. 2021. "Conditioned Medium from Mesenchymal Stem Cells Alleviates Endothelial Dysfunction of Vascular Grafts Submitted to Ischemia/Reperfusion Injury in 15-Month-Old Rats" Cells 10, no. 5: 1231. https://doi.org/10.3390/cells10051231
APA StyleKorkmaz-Icöz, S., Sun, X., Li, S., Brlecic, P., Loganathan, S., Ruppert, M., Sayour, A. A., Radovits, T., Karck, M., & Szabó, G. (2021). Conditioned Medium from Mesenchymal Stem Cells Alleviates Endothelial Dysfunction of Vascular Grafts Submitted to Ischemia/Reperfusion Injury in 15-Month-Old Rats. Cells, 10(5), 1231. https://doi.org/10.3390/cells10051231





