MAGI1, a Scaffold Protein with Tumor Suppressive and Vascular Functions
Abstract
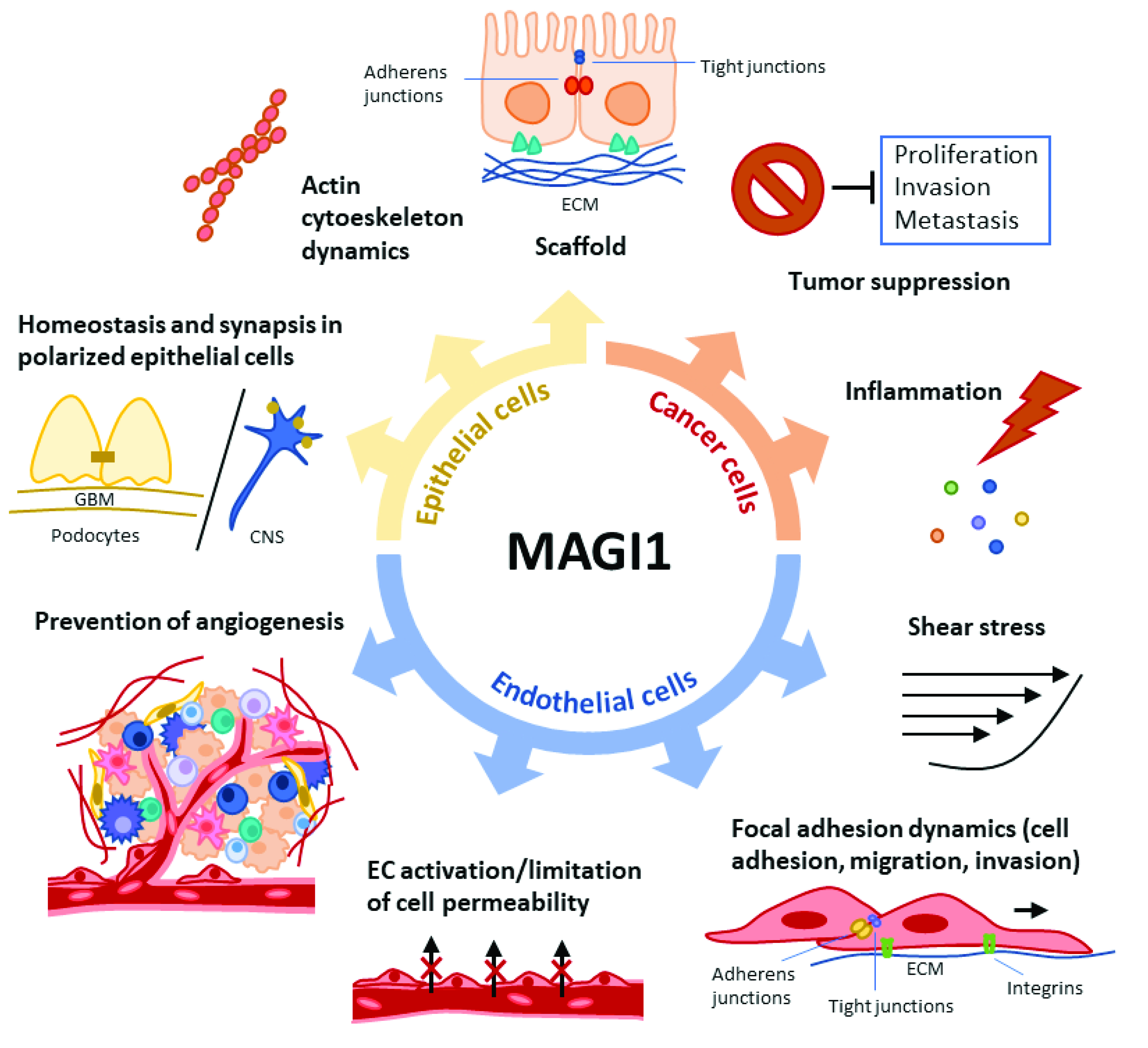
1. Introduction
1.1. MAGI1 Structure and Expression
1.2. MAGI1 Role as a Scaffold Molecule
1.3. MAGI1 Role as Tumor Suppressor
1.4. MAGI1 Vascular Functions
1.5. MAGI1 Function in Podocytes and the Nervous System
1.6. Regulation of MAGI1 Expression
2. Conclusions and Open Questions
2.1. MAGI1 as Tumor Suppressor
2.2. MAGI1 Link with Inflammation
2.3. Regulation of Endothelial Function
2.4. Clinical Significance
2.5. Therapeutic Implications
Authors contribution
Funding
Acknowledgments
Conflicts of Interest
References
- Shiratsuchi, T.; Futamura, M.; Oda, K.; Nishimori, H.; Nakamura, Y.; Tokino, T. Cloning and characterization of BAI-associated protein 1: A PDZ domain-containing protein that interacts with BAI1. Biochem. Biophys. Res. Commun. 1998, 247, 597–604. [Google Scholar] [CrossRef]
- Ide, N.; Hata, Y.; Nishioka, H.; Hirao, K.; Yao, I.; Deguchi, M.; Mizoguchi, A.; Nishimori, H.; Tokino, T.; Nakamura, Y.; et al. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene 1999, 18, 7810–7815. [Google Scholar] [CrossRef]
- Dobrosotskaya, I.; Guy, R.; James, G.L. MAGI-1, a Membrane-associated Guanylate Kinase with a Unique Arrangement of Protein-Protein Interaction Domains. J. Biol. Chem. 1997, 272, 31589–31597. [Google Scholar] [CrossRef]
- Feng, X.; Jia, S.; A Martin, T.; Jiang, W. Regulation and involvement in cancer and pathological conditions of MAGI1, a tight junction protein. Anticancer. Res. 2014, 34, 3251-6. [Google Scholar]
- De Mendoza, A.; Suga, H.; Ruiz-Trillo, I. Evolution of the MAGUK protein gene family in premetazoan lineages. BMC Evol. Biol. 2010, 10, 93. [Google Scholar] [CrossRef]
- Velthuis, A.J.T.; Admiraal, J.F.; Bagowski, C.P. Molecular evolution of the MAGUK family in metazoan genomes. BMC Evol. Biol. 2007, 7, 129-10. [Google Scholar] [CrossRef] [PubMed]
- Saras, J.; Heldin, C.H. PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem. Sci. 1996, 21, 455–458. [Google Scholar] [CrossRef]
- Ponting, C.P. Evidence for PDZ domains in bacteria, yeast, and plants. Protein Sci. 1997, 6, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shang, Y.; Chen, J.; Zhang, M. Structure and function of the guanylate kinase-like domain of the MAGUK family scaffold proteins. Front. Biol. 2012, 7, 379–396. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Ávila-Flores, A. MAGUK proteins: Structure and role in the tight junction. Semin. Cell Dev. Biol. 2000, 11, 315–324. [Google Scholar] [CrossRef]
- Olsen, O.; Bredt, D.S. Functional Analysis of the Nucleotide Binding Domain of Membrane-associated Guanylate Kinases. J. Biol. Chem. 2003, 278, 6873–6878. [Google Scholar] [CrossRef]
- Gomperts, S.N. Clustering Membrane Proteins: It’s All Coming Together with the PSD-95/SAP90 Protein Family. Cell 1996, 84, 659–662. [Google Scholar] [CrossRef]
- Fanning, A.S.; Anderson, J.M. Protein–protein interactions: PDZ domain networks. Curr. Biol. 1996, 6, 1385–1388. [Google Scholar] [CrossRef]
- Hirao, K.; Hata, Y.; Ide, N.; Takeuchi, M.; Irie, M.; Yao, I.; Deguchi, M.; Toyoda, A.; Sudhof, T.C.; Takai, Y. A Novel Multiple PDZ Domain-containing Molecule Interacting withN-Methyl-d-aspartateReceptors and Neuronal Cell Adhesion Proteins. J. Biol. Chem. 1998, 273, 21105–21110. [Google Scholar] [CrossRef]
- Wu, Y.; Dowbenko, D.; Spencer, S.; Laura, R.; Lee, J.; Gu, Q.; Lasky, L.A. Interaction of the Tumor Suppressor PTEN/MMAC with a PDZ Domain of MAGI3, a Novel Membrane-associated Guanylate Kinase. J. Biol. Chem. 2000, 275, 21477–21485. [Google Scholar] [CrossRef]
- Adamsky, K.; Arnold, K.; Sabanay, H.; Peles, E. Junctional protein MAGI-3 interacts with receptor tyrosine phosphatase beta (RPTP beta) and tyrosine-phosphorylated proteins. J. Cell Sci. 2003, 116, 1279–1289. [Google Scholar] [CrossRef]
- Laura, R.P.; Ross, S.; Koeppen, H.; Lasky, L.A. MAGI-1: A Widely Expressed, Alternatively Spliced Tight Junction Protein. Exp. Cell Res. 2002, 275, 155–170. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Guillemot, L.; Paschoud, S.; Pulimeno, P.; Foglia, A.; Citi, S. The cytoplasmic plaque of tight junctions: A scaffolding and signalling center. Biochim. Biophys. Acta Biomembr. 2008, 1778, 601–613. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Tajima, M.; Yao, I.; Nishimura, W.; Mori, H.; Hata, Y. JAM4, a Junctional Cell Adhesion Molecule Interacting with a Tight Junction Protein, MAGI-1. Mol. Cell. Biol. 2003, 23, 4267–4282. [Google Scholar] [CrossRef]
- Wegmann, F.; Ebnet, K.; Du Pasquier, L.; Vestweber, D.; Butz, S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp. Cell Res. 2004, 300, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Dobrosotskaya, I.Y. Identification of mNET1 as a Candidate Ligand for the First PDZ Domain of MAGI-1. Biochem. Biophys. Res. Commun. 2001, 283, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Mino, A.; Ohtsuka, T.; Inoue, E.; Takai, Y. Membrane-associated guanylate kinase with inverted orientation (MAGI)-1/brain angiogenesis inhibitor 1-associated protein (BAP1) as a scaffolding molecule for Rap small G protein GDP/GTP exchange protein at tight junctions. Genes Cells 2000, 5, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Dobrosotskaya, Y.I.; James, G.L. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem. Biophys Res. Commun. 2000, 270, 903–909. [Google Scholar] [CrossRef]
- Kotelevets, L.; Van Hengel, J.; Bruyneel, E.; Mareel, M.; Van Roy, F.; Chastre, E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 2004, 19, 115–117. [Google Scholar] [CrossRef]
- Nishimura, W.; Iizuka, T.; Hirabayashi, S.; Tanaka, N.; Hata, Y. Localization of BAI-associated protein1/membrane-associated guanylate kinase-1 at adherens junctions in normal rat kidney cells: Polarized targeting mediated by the carboxyl-terminal PDZ domains. J. Cell Physiol. 2000, 185, 358–365. [Google Scholar] [CrossRef]
- Chastre, E.; Abdessamad, M.; Kruglov, A.; Bruyneel, E.; Bracke, M.; Di Gioia, Y.; Beckerle, M.C.; Van Roy, F.; Kotelevets, L. TRIP6, a novel molecular partner of the MAGI-1 scaffolding molecule, promotes invasiveness. FASEB J. 2008, 23, 916–928. [Google Scholar] [CrossRef]
- Zmajkovicova, K.; Jesenberger, V.; Catalanotti, F.; Baumgartner, C.; Reyes, G.; Baccarini, M. MEK1 Is Required for PTEN Membrane Recruitment, AKT Regulation, and the Maintenance of Peripheral Tolerance. Mol. Cell 2013, 50, 43–55. [Google Scholar] [CrossRef]
- Glaunsinger, B.A.; Lee, S.S.; Thomas, M.; Banks, L.; Javier, R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 2000, 19, 5270–5280. [Google Scholar] [CrossRef]
- Makokha, G.N.; Takahashi, M.; Higuchi, M.; Saito, S.; Tanaka, Y.; Fujii, M. Human T-cell leukemia virus type 1 Tax protein interacts with and mislocalizes the PDZ domain protein MAGI-1. Cancer Sci. 2013, 104, 313–320. [Google Scholar] [CrossRef]
- Patrie, K.M.; Drescher, A.J.; Welihinda, A.; Mundel, P.; Margolis, B. Interaction of Two Actin-binding Proteins, Synaptopodin and α-Actinin-4, with the Tight Junction Protein MAGI-1. J. Biol. Chem. 2002, 277, 30183–30190. [Google Scholar] [CrossRef]
- Patrie, K.M.; Drescher, A.J.; Goyal, M.; Wiggins, R.C.; Margolis, B. The membrane-associated guanylate kinase protein MAGI-1 binds megalin and is present in glomerular podocytes. J. Am. Soc. Nephrol. 2001, 12, 667–677. [Google Scholar] [CrossRef]
- Patrie, K.M. Identification and characterization of a novel tight junction-associated family of proteins that interacts with a WW domain of MAGI-1. Biochim. Biophys. Acta Bioenergy 2005, 1745, 131–144. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Mori, H.; Kansaku, A.; Kurihara, H.; Sakai, T.; Shimizu, F.; Kawachi, H.; Hata, Y. MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab. Investig. 2005, 85, 1528–1543. [Google Scholar] [CrossRef]
- Wood, J.D.; Yuan, J.; Margolis, R.L.; Colomer, V.; Duan, K.; Kushi, J.; Kaminsky, Z.; Kleiderlein, J.J., Jr.; Sharp, A.H.; Ross, C.A. Atrophin-1, the DRPLA Gene Product, Interacts with Two Families of WW Domain-Containing Proteins. Mol. Cell. Neurosci. 1998, 11, 149–160. [Google Scholar] [CrossRef]
- Hruska-Hageman, A.M.; Benson, C.; Leonard, A.S.; Price, M.P.; Welsh, M.J. PSD-95 and Lin-7b Interact with Acid-sensing Ion Channel-3 and Have Opposite Effects on H+-gated Current. J. Biol. Chem. 2004, 279, 46962–46968. [Google Scholar] [CrossRef]
- Yao, R.; Natsume, Y.; Noda, T. MAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and Ltap. Oncogene 2004, 23, 6023–6030. [Google Scholar] [CrossRef]
- Shoji, H.; Tsuchida, K.; Kishi, H.; Yamakawa, N.; Matsuzaki, T.; Liu, Z.; Nakamura, T.; Sugino, H. Identification and Characterization of a PDZ Protein That Interacts with Activin Type II Receptors. J. Biol. Chem. 2000, 275, 5485–5492. [Google Scholar] [CrossRef]
- Wu, X.; Hepner, K.; Castelino-Prabhu, S.; Do, D.; Kaye, M.B.; Yuan, X.-J.; Wood, J.; Ross, C.; Sawyers, C.L.; Whang, Y.E. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl. Acad. Sci. USA 2000, 97, 4233–4238. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, L.; Miranda, J.; Ortega-Olvera, J.M.; Gallego-Gutierrez, H.; Raya-Sandino, A.; Vargas-Sierra, O. Involvement of Tight Junction Plaque Proteins in Cancer. Curr. Pathobiol. Rep. 2016, 4, 117–133. [Google Scholar] [CrossRef]
- Nagashima, S.; Kodaka, M.; Iwasa, H.; Hata, Y. MAGI2/S-SCAM outside brain. J. Biochem. 2015, 157, 177–184. [Google Scholar] [CrossRef]
- Hultin, S.; Zheng, Y.; Mojallal, M.; Vertuani, S.; Gentili, C.; Balland, M.; Milloud, R.; Belting, H.-G.; Affolter, M.; Helker, C.; et al. AmotL2 links VE-cadherin to contractile actin fibres necessary for aortic lumen expansion. Nat. Commun. 2014, 5, 3743. [Google Scholar] [CrossRef]
- Sakurai, A.; Fukuhara, S.; Yamagishi, A.; Sako, K.; Kamioka, Y.; Masuda, M.; Nakaoka, Y.; Mochizuki, N. MAGI-1 Is Required for Rap1 Activation upon Cell-Cell Contact and for Enhancement of Vascular Endothelial Cadherin-mediated Cell Adhesion. Mol. Biol. Cell 2006, 17, 966–976. [Google Scholar] [CrossRef]
- Kimura, R.; Ishida, T.; Kuriyama, M.; Hirata, K.-I.; Hayashi, Y. Interaction of endothelial cell-selective adhesion molecule and MAGI-1 promotes mature cell-cell adhesion via activation of RhoA. Genes Cells 2010, 15, 385–396. [Google Scholar] [CrossRef]
- Alberts, A.S.; Treisman, R. Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J. 1998, 17, 4075–4085. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Kotelevets, L.; Van Hengel, J.; Bruyneel, E.; Mareel, M.; Van Roy, F.; Chastre, E. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J. Cell Biol. 2001, 155, 1129–1136. [Google Scholar] [CrossRef]
- Chalhoub, N.; Baker, S.J. PTEN and the PI3-Kinase Pathway in Cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 127–150. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z. MAGI1 inhibits cancer cell migration and invasion of hepatocellular carcinoma via regulating PTEN. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011, 36, 381–385. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, T.; Wang, Z. Downregulation of MAGI1 Associates with Poor Prognosis of Hepatocellular Carcinoma. J. Investig. Surg. 2011, 25, 93–99. [Google Scholar] [CrossRef]
- Kozakai, T.; Takahashi, M.; Higuchi, M.; Hara, T.; Saito, K.; Tanaka, Y.; Masuko, M.; Takizawa, J.; Sone, H.; Fujii, M. MAGI-1 expression is decreased in several types of human T-cell leukemia cell lines, including adult T-cell leukemia. Int. J. Hematol. 2017, 107, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Z.; Guo, L.; Wang, L.; Zhang, L.; Cai, X.; Zhao, H.; Zha, X. MAGI-2 Inhibits cell migration and proliferation via PTEN in human hepatocarcinoma cells. Arch. Biochem. Biophys. 2007, 467, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Gu, J.; Danen, E.; Takino, T.; Miyamoto, S.; Yamada, K.M. PTEN Interactions with Focal Adhesion Kinase and Suppression of the Extracellular Matrix-dependent Phosphatidylinositol 3-Kinase/Akt Cell Survival Pathway. J. Biol. Chem. 1999, 274, 20693–20703. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, Y.; Meng, R.; Xie, K.M.; Xiong, Y.; Lin, S.; He, Z.L.K.; Tao, T.; Yang, Y.; Zhao, J.Z.; et al. MAGI3 Suppresses Glioma Cell Proliferation via Upregulation of PTEN Expression. Biomed. Environ. Sci. 2015, 28, 502–509. [Google Scholar] [CrossRef]
- Kitamura, K.; Seike, M.; Okano, T.; Matsuda, K.; Miyanaga, A.; Mizutani, H.; Noro, R.; Minegishi, Y.; Kubota, K.; Gemma, A. MiR-134/487b/655 cluster regulates TGF-beta-induced epithelial-mesenchymal transition and drug resistance to gefitinib by targeting MAGI2 in lung adenocarcinoma cells. Mol. Cancer Ther. 2014, 13, 444–453. [Google Scholar] [CrossRef]
- Brenner, J.C.; Chinnaiyan, A.M. Disruptive Events in the Life of Prostate Cancer. Cancer Cell 2011, 19, 301–303. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, W.; Zhang, L.; Li, J. Silencing Of MAGI1 Promotes The Proliferation And Inhibits Apoptosis Of Glioma Cells Via The Wnt/beta-Catenin And PTEN/AKT Signaling Pathways. Onco. Targets Ther. 2019, 12, 9639–9650. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, X.-H.; Tian, G.-W.; Zhang, D.-Y.; Gao, H.; Wang, Z.-Y. MAGI1 Inhibits the Proliferation, Migration and Invasion of Glioma Cells. OncoTargets Ther. 2019, ume 12, 11281–11290. [Google Scholar] [CrossRef]
- Qian, M.; Yang, Y.; Feng, D.; Zheng, S.; Meng, R.; Fa, P.; Zhao, C.; Liu, H.; Song, R.; Tao, T.; et al. MAGI3 negatively regulates Wnt/beta-catenin signaling and suppresses malignant phenotypes of glioma cells. Oncotarget 2015, 6, 35851–35865. [Google Scholar]
- Zaric, J.; Joseph, J.-M.; Tercier, S.; Sengstag, T.; Ponsonnet, L.; Delorenzi, M.; Rüegg, C.; Zaric, J.; Joseph, J.-M.; Tercier, S.; et al. Identification of MAGI1 as a tumor-suppressor protein induced by cyclooxygenase-2 inhibitors in colorectal cancer cells. Oncogene 2011, 31, 48–59. [Google Scholar] [CrossRef]
- Alday-Parejo, B.; Richard, F.; Wörthmüller, J.; Rau, T.; Galván, J.A.; Desmedt, C.; Santamaria-Martinez, A.; Rüegg, C. MAGI1, a New Potential Tumor Suppressor Gene in Estrogen Receptor Positive Breast Cancer. Cancers 2020, 12, 223. [Google Scholar] [CrossRef]
- Kantar, D.; Mur, E.B.; Mancini, M.; Slaninova, V.; Ben Salah, Y.; Costa, L.; Forest, E.; Lassus, P.; Géminard, C.; Boissière-Michot, F.; et al. MAGI1 inhibits the AMOTL2/p38 stress pathway and prevents luminal breast tumorigenesis. Sci. Rep. 2021, 11, 1–20. [Google Scholar] [CrossRef]
- Jia, S.; Lu, J.; Qu, T.; Feng, Y.; Wang, X.; Liu, C.; Ji, J. MAGI1 inhibits migration and invasion via blocking MAPK/ERK signaling pathway in gastric cancer. Chin. J. Cancer Res. 2017, 29, 25–35. [Google Scholar] [CrossRef]
- Latorre, I.J.; Roh, M.H.; Frese, K.K.; Weiss, R.S.; Margolis, B.; Javier, R.T. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J. Cell Sci. 2005, 118, 4283–4293. [Google Scholar] [CrossRef]
- Coradini, D.; Casarsa, C.; Oriana, S. Epithelial cell polarity and tumorigenesis: New perspectives for cancer detection and treatment. Acta Pharmacol. Sin. 2011, 32, 552–564. [Google Scholar] [CrossRef]
- Kranjec, C.; Banks, L. A Systematic Analysis of Human Papillomavirus (HPV) E6 PDZ Substrates Identifies MAGI-1 as a Major Target of HPV Type 16 (HPV-16) and HPV-18 Whose Loss Accompanies Disruption of Tight Junctions. J. Virol. 2010, 85, 1757–1764. [Google Scholar] [CrossRef]
- Kranjec, C.; Massimi, P.; Banks, L. Restoration of MAGI-1 Expression in Human Papillomavirus-Positive Tumor Cells Induces Cell Growth Arrest and Apoptosis. J. Virol. 2014, 88, 7155–7169. [Google Scholar] [CrossRef]
- Vestweber, D.; Winderlich, M.; Cagna, G.; Nottebaum, A.F. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009, 19, 8–15. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Wu, M.H. Endothelial focal adhesions and barrier function. J. Physiol. 2005, 569, 359–366. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Kaur, B.; Brat, D.J.; Calkins, C.C.; Van Meir, E.G. Brain Angiogenesis Inhibitor 1 Is Differentially Expressed in Normal Brain and Glioblastoma Independently of p53 Expression. Am. J. Pathol. 2003, 162, 19–27. [Google Scholar] [CrossRef]
- Fukushima, Y.; Oshika, Y.; Tsuchida, T.; Tokunaga, T.; Hatanaka, H.; Kijima, H.; Yamazaki, H.; Ueyama, Y.; Tamaoki, N.; Nakamura, M. Brain-specific angiogenesis inhibitor 1 expression is inversely correlated with vascularity and distant metastasis of colorectal cancer. Int. J. Oncol. 1998, 13, 967–1037. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, H.; Oshika, Y.; Abe, Y.; Yoshida, Y.; Hashimoto, T.; Handa, A.; Kijima, H.; Yamazaki, H.; Inoue, H.; Ueyama, Y.; et al. Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1). Int. J. Mol. Med. 2000, 5, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Iomini, C.; Hyink, D.; Wilson, P.D. PRKX critically regulates endothelial cell proliferation, migration, and vascular-like structure formation. Dev. Biol. 2011, 356, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Alday-Parejo, B.; Ghimire, K.; Coquoz, O.; Albisetti, G.W.; Tamò, L.; Zaric, J.; Stalin, J.; Rüegg, C. MAGI1 localizes to mature focal adhesion and modulates endothelial cell adhesion, migration and angiogenesis. Cell Adhes. Migr. 2021, 15, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Zaric, J.; Alday-Parejo, B.; Seebach, J.; Bousquenaud, M.; Stalin, J.; Bieler, G.; Schnittler, H.-J.; Rüegg, C. MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress. Cells 2019, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid Shear Stress Sensing by the Endothelial Layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Stuehr, D.J. Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 339–359. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.-C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Abe, J.-I.; Ko, K.A.; Kotla, S.; Wang, Y.; Paez-Mayorga, J.; Shin, I.J.; Imanishi, M.; Vu, H.T.; Tao, Y.; Leiva-Juarez, M.M.; et al. MAGI1 as a link between endothelial activation and ER stress drives atherosclerosis. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Couzens, A.L.; Knight, J.D.R.; Kean, M.J.; Teo, G.; Weiss, A.; Dunham, W.H.; Lin, Z.-Y.; Bagshaw, R.D.; Sicheri, F.; Pawson, T.; et al. Protein Interaction Network of the Mammalian Hippo Pathway Reveals Mechanisms of Kinase-Phosphatase Interactions. Sci. Signal. 2013, 6, rs15. [Google Scholar] [CrossRef]
- Abe, R.J.; Savage, H.; Imanishi, M.; Banerjee, P.; Kotla, S.; Paez-Mayorga, J.; Taunton, J.; Fujiwara, K.; Won, J.H.; Yusuf, S.W.; et al. p90RSK-MAGI1 Module Controls Endothelial Permeability by Post-translational Modifications of MAGI1 and Hippo Pathway. Front. Cardiovasc. Med. 2020, 7, 542485. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.A.; van Buul, J.D. Endothelial junction regulation: A prerequisite for leukocytes crossing the vessel wall. J. Innate. Immun. 2013, 5, 324–335. [Google Scholar] [CrossRef]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef]
- Wessel, F.; Winderlich, M.; Holm, M.; Frye, M.; Rivera-Galdos, R.; Vockel, M.; Linnepe, R.; Ipe, U.; Stadtmann, A.; Zarbock, A.; et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 2014, 15, 223–230. [Google Scholar] [CrossRef]
- Esser, S.; Lampugnani, M.G.; Corada, M.; Dejana, E.; Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 1998, 111, 1853–1865. [Google Scholar] [CrossRef]
- Bradfield, P.F.; Nourshargh, S.; Aurrand-Lions, M.; Imhof, B.A. JAM Family and Related Proteins in Leukocyte Migration (Vestweber Series). Arter. Thromb. Vasc. Biol. 2007, 27, 2104–2112. [Google Scholar] [CrossRef]
- Wegmann, F.; Petri, B.; Khandoga, A.G.; Moser, C.; Khandoga, A.; Volkery, S.; Oliver, B.; Nasdala, I.; Brandau, O.; Fassler, R.; et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J. Exp. Med. 2006, 203, 1671–1677. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Muller, W.A. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003, 24, 326–333. [Google Scholar] [CrossRef]
- Kobayashi, N.; Gao, S.-Y.; Chen, J.; Saito, K.; Miyawaki, K.; Li, C.-Y.; Pan, L.; Saito, S.; Terashita, T.; Matsuda, S. Process formation of the renal glomerular podocyte: Is there common molecular machinery for processes of podocytes and neurons? Anat. Sci. Int. 2004, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Armelloni, S.; Corbelli, A.; Giardino, L.; Li, M.; Ikehata, M.; Mattinzoli, D.; Messa, P.; Pignatari, C.; Watanabe, S.; Rastaldi, M.P. Podocytes: Recent biomolecular developments. Biomol. Concepts 2014, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.; Anderson, J.M.; Farquhar, M.G. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J. Cell Biol. 1990, 111, 1255–1263. [Google Scholar] [CrossRef]
- Kaufman, L.; Potla, U.; Coleman, S.; Dikiy, S.; Hata, Y.; Kurihara, H.; He, J.C.; D’Agati, V.D.; Klotman, P.E. Up-regulation of the homophilic adhesion molecule sidekick-1 in podocytes contributes to glomerulosclerosis. J. Biol. Chem. 2010, 285, 25677–25685. [Google Scholar] [CrossRef]
- Yamagata, M.; Sanes, J.R. Synaptic Localization and Function of Sidekick Recognition Molecules Require MAGI Scaffolding Proteins. J. Neurosci. 2010, 30, 3579–3588. [Google Scholar] [CrossRef]
- Tanemoto, M.; Toyohara, T.; Abe, T.; Ito, S. MAGI-1a Functions as a Scaffolding Protein for the Distal Renal Tubular Basolateral K+ Channels. J. Biol. Chem. 2008, 283, 12241–12247. [Google Scholar] [CrossRef]
- Ridgway, L.D.; Kim, E.Y.; Dryer, S.E. MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface. Am. J. Physiol. Physiol. 2009, 297, C55–C65. [Google Scholar] [CrossRef]
- Ni, J.; Bao, S.; Johnson, R.I.; Zhu, B.; Li, J.; Vadaparampil, J.; Smith, C.M.; Campbell, K.N.; Grahammer, F.; Huber, T.B.; et al. MAGI-1 Interacts with Nephrin to Maintain Slit Diaphragm Structure through Enhanced Rap1 Activation in Podocytes. J. Biol. Chem. 2016, 291, 24406–24417. [Google Scholar] [CrossRef]
- Balbas, M.D.; Burgess, M.R.; Murali, R.; Wongvipat, J.; Skaggs, B.J.; Mundel, P.; Weins, A.; Sawyers, C.L. MAGI-2 scaffold protein is critical for kidney barrier function. Proc. Natl. Acad. Sci. USA 2014, 111, 14876–14881. [Google Scholar] [CrossRef]
- Ihara, K.-I.; Asanuma, K.; Fukuda, T.; Ohwada, S.; Yoshida, M.; Nishimori, K. MAGI-2 Is Critical for the Formation and Maintenance of the Glomerular Filtration Barrier in Mouse Kidney. Am. J. Pathol. 2014, 184, 2699–2708. [Google Scholar] [CrossRef]
- Yamada, H.; Shirata, N.; Makino, S.; Miyake, T.; Trejo, J.A.O.; Yamamoto-Nonaka, K.; Kikyo, M.; Empitu, M.A.; Kadariswantiningsih, I.N.; Kimura, M.; et al. MAGI-2 orchestrates the localization of backbone proteins in the slit diaphragm of podocytes. Kidney Int. 2021, 99, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Shirata, N.; Ihara, K.-I.; Yamamoto-Nonaka, K.; Seki, T.; Makino, S.-I.; Trejo, J.A.O.; Miyake, T.; Yamada, H.; Campbell, K.N.; Nakagawa, T.; et al. Glomerulosclerosis Induced by Deficiency of Membrane-Associated Guanylate Kinase Inverted 2 in Kidney Podocytes. J. Am. Soc. Nephrol. 2017, 28, 2654–2669. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.; Clarkson, M.; Massa, F.; Bradford, S.T.; Charlet, A.; Buske, F.; Lacas-Gervais, S.; Schulz, H.; Gimpel, C.; Hata, Y.; et al. Alternatively spliced isoforms of WT1 control podocyte-specific gene expression. Kidney Int. 2015, 88, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Bierzynska, A.; Soderquest, K.; Dean, P.; Colby, E.; Rollason, R.; Jones, C.; Inward, C.D.; McCarthy, H.J.; Simpson, M.A.; Lord, G.M.; et al. MAGI2 Mutations Cause Congenital Nephrotic Syndrome. J. Am. Soc. Nephrol. 2016, 28, 1614–1621. [Google Scholar] [CrossRef]
- Ashraf, S.; Kudo, H.; Rao, J.; Kikuchi, A.; Widmeier, E.; Lawson, J.A.; Tan, W.; Hermle, T.; Warejko, J.K.; Shril, S.; et al. Mutations in six nephrosis genes delineate a pathogenic pathway amenable to treatment. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Empitu, M.A.; Kadariswantiningsih, I.N.; Aizawa, M.; Asanuma, K. MAGI-2 and scaffold proteins in glomerulopathy. Am. J. Physiol. Physiol. 2018, 315, F1336–F1344. [Google Scholar] [CrossRef]
- Sumita, K.; Sato, Y.; Iida, J.; Kawata, A.; Hamano, M.; Hirabayashi, S.; Ohno, K.; Peles, E.; Hata, Y. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J. Neurochem. 2007, 100, 154–166. [Google Scholar] [CrossRef]
- Iida, J.; Ishizaki, H.; Okamoto-Tanaka, M.; Kawata, A.; Sumita, K.; Ohgake, S.; Sato, Y.; Yorifuji, H.; Nukina, N.; Ohashi, K.; et al. Synaptic Scaffolding Molecule α Is a Scaffold To Mediate N-Methyl-d-Aspartate Receptor-Dependent RhoA Activation in Dendrites. Mol. Cell. Biol. 2007, 27, 4388–4405. [Google Scholar] [CrossRef]
- Zou, S.; Pita-Almenar, J.D.; Eskin, A. Regulation of glutamate transporter GLT-1 by MAGI-1. J. Neurochem. 2011, 117, 833–840. [Google Scholar] [CrossRef]
- Hammad, M.; Dunn, H.A.; Ferguson, S.S.G. MAGI Proteins Regulate the Trafficking and Signaling of Corticotropin-Releasing Factor Receptor 1 via a Compensatory Mechanism. J. Mol. Signal. 2016, 11, 5. [Google Scholar] [CrossRef]
- Karlsson, R.; Graae, L.; Lekman, M.; Wang, D.; Favis, R.; Axelsson, T.; Galter, D.; Belin, A.C.; Paddock, S. MAGI1 Copy Number Variation in Bipolar Affective Disorder and Schizophrenia. Biol. Psychiatry 2012, 71, 922–930. [Google Scholar] [CrossRef]
- Ferentinos, P.; Rivera, M.; Ising, M.; Spain, S.; Cohen-Woods, S.; Butler, A.W.; Craddock, N.; Owen, M.J.; Korszun, A.; Jones, L.; et al. Investigating the genetic variation underlying episodicity in major depressive disorder: Suggestive evidence for a bipolar contribution. J. Affect. Disord. 2014, 155, 81–89. [Google Scholar] [CrossRef]
- De Moor, M.H.M.; Berg, S.V.D.; Verweij, K.J.H.; Krueger, R.F.; Luciano, M.; Vasquez, A.A.; Matteson, L.K.; Derringer, J.; Esko, T.; Amin, N.; et al. Meta-analysis of Genome-wide Association Studies for Neuroticism, and the Polygenic Association With Major Depressive Disorder. JAMA Psychiatry 2015, 72, 642–650. [Google Scholar] [CrossRef]
- Berger, F.M.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220. [Google Scholar] [CrossRef]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.-L.; Ordóñez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nat. Cell Biol. 2009, 463, 191–196. [Google Scholar] [CrossRef]
- Berger, M.F.; Hodis, E.; Heffernan, T.P.; Deribe, Y.L.; Lawrence, M.S.; Protopopov, A.; Ivanova, E.; Watson, I.; Nickerson, E.; Ghosh, P.; et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nat. Cell Biol. 2012, 485, 502–506. [Google Scholar] [CrossRef]
- Cabrita, R.; Mitra, S.; Sanna, A.; Ekedahl, H.; Lövgren, K.; Olsson, H.; Ingvar, C.; Isaksson, K.; Lauss, M.; Carneiro, A.; et al. The Role of PTEN Loss in Immune Escape, Melanoma Prognosis and Therapy Response. Cancers 2020, 12, 742. [Google Scholar] [CrossRef]
- Sachdeva, M.; Wu, H.; Ru, P.; Hwang, L.; Trieu, V.; Mo, Y.-Y. MicroRNA-101-mediated Akt activation and estrogen-independent growth. Oncogene 2010, 30, 822–831. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Pencheva, N.; Tavazoie, S.F. Control of metastatic progression by microRNA regulatory networks. Nat. Cell Biol. 2013, 15, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Mahdian, R.; Nodouzi, V.; Asgari, M.; Rezaie, M.; Alizadeh, J.; Yousefi, B.; Shahrokh, H.; Abolhasani, M.; Nowroozi, M. Expression profile of MAGI2 gene as a novel biomarker in combination with major deregulated genes in prostate cancer. Mol. Biol. Rep. 2014, 41, 6125–6131. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Huang, R.L.; Huang, Y.K.; Liao, Y.P.; Su, P.H.; Wang, H.C.; Chang, C.C.; Lin, Y.W.; Yu, M.H.; Chu, T.Y.; et al. Methylomics analysis identifies epigenetically silenced genes and implies an activation of beta-catenin signaling in cervical cancer. Int. J. Cancer 2014, 135, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Cibulskis, K.; Rangel-Escareno, C.; Brown, K.K.; Carter, S.L.; Frederick, A.M.; Lawrence, M.S.; Sivachenko, A.Y.; Sougnez, C.; Zou, L.; et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nat. Cell Biol. 2012, 486, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Chen, X.; Shao, S.; Hu, S.; Zhang, T. MAGI1 mediates tumor metastasis through c-Myb/miR-520h/MAGI1 signaling pathway in renal cell carcinoma. Apoptosis 2019, 24, 837–848. [Google Scholar] [CrossRef]
- Sakuta, K.; Sasaki, Y.; Abe, Y.; Sato, H.; Shoji, M.; Yaoita, T.; Yagi, M.; Mizumoto, N.; Onozato, Y.; Kon, T.; et al. Somatic alterations and mutational burden are potential predictive factors for metachronous development of early gastric cancer. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ravi, N.; Yang, M.; Mylona, N.; Wennerberg, J.; Paulsson, K. Global RNA Expression and DNA Methylation Patterns in Primary Anaplastic Thyroid Cancer. Cancers 2020, 12, 680. [Google Scholar] [CrossRef]
- Lakshminarasimhan, R.; Liang, G. The Role of DNA Methylation in Cancer. Adv. Exp. Med. Biol. 2016, 945, 151–172. [Google Scholar] [CrossRef]
- Kuang, S.-Q.; Tong, W.-G.; Yang, H.; Lin, W.; Lee, M.K.; Fang, Z.H.; Wei, Y.; Jelinek, J.; Issa, J.-P.; Garcia-Manero, G. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia. Leuk. 2008, 22, 1529–1538. [Google Scholar] [CrossRef]
- Taher, M.M.; Hassan, A.A.; Saeed, M.; Jastania, R.A.; Nageeti, T.H.; Alkhalidi, H.; Dairi, G.; Abduljaleel, Z.; Athar, M.; Bouazzaoui, A.; et al. Next generation DNA sequencing of atypical choroid plexus papilloma of brain: Identification of novel mutations in a female patient by Ion Proton. Oncol. Lett. 2019, 18, 5063–5076. [Google Scholar] [CrossRef]
- Cui, M.; Hu, Y.; Bi, Y.; Wang, W.; Wang, M.; Zhang, X.; Zhang, R.; Wang, P.; Su, Z.; Gao, X.; et al. Preliminary exploration of potential molecular therapeutic targets in recurrent and metastatic parathyroid carcinomas. Int. J. Cancer 2019, 144, 525–532. [Google Scholar] [CrossRef]
- Pfarr, N.; Allgäuer, M.; Steiger, K.; Weichert, W.; Schirmacher, P.; Noske, A.; Stenzinger, A. Several genotypes, one phenotype: PIK3CA/AKT1 mutation-negative hidradenoma papilliferum show genetic lesions in other components of the signalling network. Pathology 2019, 51, 362–368. [Google Scholar] [CrossRef]
- De Lara, C.L.; Jaitovich-Groisman, I.; Cruceanu, C.; Mamdani, F.; Lebel, V.; Yerko, V.; Beck, A.; Young, L.T.; Rouleau, G.; Grof, P.; et al. Implication of synapse-related genes in bipolar disorder by linkage and gene expression analyses. Int. J. Neuropsychopharmacol. 2010, 13, 1397–1410. [Google Scholar] [CrossRef]
- Gregorc, U.; Ivanova, S.; Thomas, M.; Guccione, E.; Glaunsinger, B.; Javier, R.; Turk, V.; Banks, L.; Turk, B. Cleavage of MAGI-1, a tight junction PDZ protein, by caspases is an important step for cell-cell detachment in apoptosis. Apoptosis 2007, 12, 343–354. [Google Scholar] [CrossRef]
- Ivanova, S.; Repnik, U.; Banks, L.; Turk, V.; Turk, B. Cellular localization of MAGI-1 caspase cleavage products and their role in apoptosis. Biol. Chem. 2007, 388, 1195–1198. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Pang, L.Y.; Hurst, E.A.; Argyle, D.J. Cyclooxygenase-2: A Role in Cancer Stem Cell Survival and Repopulation of Cancer Cells during Therapy. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Gupta, R.A.; DuBois, R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer 2001, 1, 11–21. [Google Scholar] [CrossRef]
- Fischer, S.M.; Hawk, E.T.; Lubet, R.A. Coxibs and Other Nonsteroidal Anti-Inflammatory Drugs in Animal Models of Cancer Chemoprevention. Cancer Prev. Res. 2011, 4, 1728–1735. [Google Scholar] [CrossRef]
- Shono, T.; Tofilon, P.J.; Bruner, J.M.; Owolabi, O.; Lang, F.F. Cyclooxygenase-2 expression in human gliomas: Prognostic significance and molecular correlations. Cancer Res. 2001, 61, 4375–4381. [Google Scholar]
- Sareddy, R.G.; Geeviman, K.; Ramulu, C.; Babu, P.P. The nonsteroidal anti-inflammatory drug celecoxib suppresses the growth and induces apoptosis of human glioblastoma cells via the NF-kappaB pathway. J. Neurooncol. 2012, 106, 99–109. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Baumgarten, S.C.; Frasor, J. Minireview: Inflammation: An Instigator of More Aggressive Estrogen Receptor (ER) Positive Breast Cancers. Mol. Endocrinol. 2012, 26, 360–371. [Google Scholar] [CrossRef]
- Hayes, E.L.; Lewis-Wambi, J.S. Mechanisms of endocrine resistance in breast cancer: An overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015, 17, 42. [Google Scholar] [CrossRef]
- Moris, D.; Kontos, M.; Spartalis, E.; Fentiman, I.S. The Role of NSAIDs in Breast Cancer Prevention and Relapse: Current Evidence and Future Perspectives. Breast Care 2016, 11, 339–344. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Forget, P.; Bentin, C.; Machiels, J.-P.; Berliere, M.; Coulie, P.G.; De Kock, M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br. J. Anaesth. 2014, 113, i82–i87. [Google Scholar] [CrossRef]
- Desmedt, C.; Demicheli, R.; Fornili, M.; Bachir, I.; Duca, M.; Viglietti, G.; Berlière, M.; Piccart, M.; Sotiriou, C.; Sosnowski, M.; et al. Potential Benefit of Intra-operative Administration of Ketorolac on Breast Cancer Recurrence According to the Patient’s Body Mass Index. J. Natl. Cancer Inst. 2018, 110, 1115–1122. [Google Scholar] [CrossRef]
- Forget, P.; Bouche, G.; Duhoux, F.P.; Coulie, P.G.; Decloedt, J.; Dekleermaker, A.; Guillaume, J.-E.; Ledent, M.; Machiels, J.-P.; Mustin, V.; et al. Intraoperative ketorolac in high-risk breast cancer patients. A prospective, randomized, placebo-controlled clinical trial. PLoS ONE 2019, 14, e0225748. [Google Scholar] [CrossRef]
- Frisk, G.; Ekberg, S.; Lidbrink, E.; Eloranta, S.; Sund, M.; Fredriksson, I.; Lambe, M.; Smedby, K.E. No association between low-dose aspirin use and breast cancer outcomes overall: A Swedish population-based study. Breast Cancer Res. 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.I.; Dubois, R.N. NSAIDs and Cancer Prevention: Targets Downstream of COX-2. Annu. Rev. Med. 2007, 58, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Carlson, P.; Valentin, N.; Acosta, A.; O’Neill, J.; Eckert, D.J.; Dyer, R.; Na, J.; Klee, E.W.; Murray, J.A. Pilot study of small bowel mucosal gene expression in patients with irritable bowel syndrome with diarrhea. Am. J. Physiol. Liver Physiol. 2016, 311, G365–G376. [Google Scholar] [CrossRef]
- Julia, A.; Pinto, J.A.; Gratacos, J.; Queiro, R.; Ferrandiz, C.; Fonseca, E.; Montilla, C.; Torre-Alonso, J.C.; Puig, L.; Venegas, J.J.P.; et al. A deletion at ADAMTS9-MAGI1 locus is associated with psoriatic arthritis risk. Ann. Rheum. Dis. 2015, 74, 1875–1881. [Google Scholar] [CrossRef]
- Alonso, A.; Domènech, E.; Julià, A.; Panés, J.; García-Sánchez, V.; Mateu, P.N.; Gutiérrez, A.; Gomollón, F.; Mendoza, J.L.; Garcia-Planella, E.; et al. Identification of Risk Loci for Crohn’s Disease Phenotypes Using a Genome-Wide Association Study. Gastroenterol. 2015, 148, 794–805. [Google Scholar] [CrossRef]
- Jauregi-Miguel, A.; Fernandez-Jimenez, N.; Irastorza, I.; Plaza-Izurieta, L.; Vitoria, J.C.; Bilbao, J.R. Alteration of Tight Junction Gene Expression in Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 762–767. [Google Scholar] [CrossRef]
- Norén, E.; Mellander, M.-R.; Almer, S.; Söderman, J. Genetic Variation and Gene Expression Levels of Tight Junction Genes Indicates Relationships Between PTEN as well as MAGI1 and Microscopic Colitis. Dig. Dis. Sci. 2017, 63, 105–112. [Google Scholar] [CrossRef]
- Norén, E.; Almer, S.; Söderman, J. Genetic variation and expression levels of tight junction genes identifies association between MAGI3 and inflammatory bowel disease. BMC Gastroenterol. 2017, 17, 1–8. [Google Scholar] [CrossRef][Green Version]
- McGovern, D.P.; Taylor, K.D.; Landers, C.; Derkowski, C.; Dutridge, D.; Dubinsky, M.; Ippoliti, A.; Vasiliauskas, E.; Mei, L.; Mengesha, E.; et al. MAGI2 genetic variation and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 75–83. [Google Scholar] [CrossRef]
- Wapenaar, M.C.; Monsuur, A.J.; Van Bodegraven, A.A.; Weersma, R.K.; Bevova, M.R.; Linskens, R.K.; Howdle, P.; Holmes, G.; Mulder, C.J.; Dijkstra, G.; et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitisAn unusual case of ascites. Gut 2008, 57, 463–467. [Google Scholar] [CrossRef]
- Available online: https://www.proteinatlas.org/ENSG00000151276-MAGI1/pathology (accessed on 29 April 2021).
- Sun, Y.-H.; Cao, Z.; Ji, J.; Wang, F.-B.; Kong, C.; Xu, H.; Xu, Y.-L.; Chen, X.; Yu, Y.-W. MAGI-2 downregulation: A potential predictor of tumor progression and early recurrence in Han Chinese patients with prostate cancer. Asian J. Androl. 2020, 22, 616–622. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Conciatori, F.; Pallocca, M.; Falcone, I.; Fanciulli, M.; Cognetti, F.; Milella, M.; Ciuffreda, L. PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers 2019, 11, 435. [Google Scholar] [CrossRef]
- Ichiki, T.; Izumi, R.; Cataliotti, A.; Larsen, A.M.; Sandberg, S.M.; Burnett, J.C. Endothelial permeability in vitro and in vivo: Protective actions of ANP and omapatrilat in experimental atherosclerosis. Peptides 2013, 48, 21–26. [Google Scholar] [CrossRef]

| Subfamily Member | Function | Mechanism | Model | Reference |
|---|---|---|---|---|
| MAGI1-b | Tumor suppressor role | Ectopic expression potentiates interaction with PTEN, promotes cell–cell aggregation, reverts Src-induced invasiveness, decreases AKT activity | Kidney epithelial MDCKts-src cells | Kotelevets et al., 2001 |
| MAGI1 | Tumor suppressor in hepatocellular carcinoma (HCC) | MAGI1 transfection inhibits cell migration and invasion by upregulating PTEN | HepG2 cells | Zhang et al., 2011 |
| Decreased expression associated with poor prognosis in HCC patients, and its downregulation correlates with vascular invasion of HCC tissues | HCC datasets | Zhang et al., 2012 | ||
| MAGI2 | Tumor suppressor in hepatocellular carcinoma (HCC) | Inhibits cell migration and proliferation through downregulation of p-FAK and p-AKT | HCC cell lines | Hu et al., 2007 |
| MAGI1 | Tumor suppressor in adult T-cell leukemia (ATL) | Inhibits AKT activity through PTEN and MEK1. Knockdown increases AKT and MEK activity. Upregulation reduces cell growth | T-cells | Kozakai et al., 2018 |
| MAGI3/ MAGI1 | Tumor suppressor in glioma | Overexpression of MAGI3 upregulates PTEN, inhibits phosphorylation of AKT, and suppresses cell proliferation | Glioma cell lines | Ma et al., 2015 |
| MAGI3 regulates negatively Wnt/β-catenin signaling. Knockdown enhances cell proliferation in vitro; overexpression suppresses tumor growth in vivo; associated negatively with tumor grade and poor prognosis | Glioma cell lines; in vivo studies; glioma datasets | Ma et al., 2015 | ||
| MAGI1 enhances proliferation and inhibits apoptosis, increases the Wnt/β-catenin signaling pathway, p-AKT and reduces E-cadherin and PTEN expression. Overexpression of MAGI1 inhibits tumor growth in vivo | Glioma cell lines; in vivo studies | Lu et al., 2019 | ||
| MAGI1 overexpression inhibits proliferation, migration and invasion of glioma cells by regulating EMT through AKT, MMP2, MMP9, and the E-cadherin/N-cadherin/vimentin pathway | Glioma cell lines | Li et al., 2019 | ||
| MAGI2 | Tumor suppressor in lung adenocarcinoma | Regulated by TGF-β1. TGF-β1 stimulation induces overexpression of miR-134/487b/655, that silence MAGI2 | Lung adenocarcinoma cell lines | Kitamura et al., 2014 |
| MAGI1 | Tumor suppressor in colorectal cancer (CRC) | Negative regulator of Wnt/β-catenin signaling. Silencing enhances Wnt signaling, migration, and invasion in vitro; overexpression attenuates tumor growth and metastasis in vivo by inhibiting Wnt signaling | CRC cancer cell lines; in vivo studies | Zaric et al., 2012 |
| MAGI1 | Tumor suppressor in ER+/HER2− breast cancer (BC) | High expression associated with better prognosis/low with higher histological grade, aggressive phenotype, and worse prognosis. Downregulation promotes cell proliferation and survival through activation of PI3K and Wnt signaling, and enhances tumor growth and metastasis in vivo | BC cell lines; in vivo studies; BC datasets. | Alday-Parejo et al., 2020 |
| Loss of MAGI1 leads to accumulation of E-cadherin and AMOTL2 in the cells, which increases stiffness, decreases YAP activity, and increases ROCK and p38 stress pathways | BC cell lines; in vivo studies | Kantar et al., 2021 | ||
| MAGI1 | Tumor suppressor in gastric cancer | Downregulation enhances migration and invasion by altering the expression of MMPs and EMT-related molecules via inhibiting the MAPK/ERK signaling pathway | Gastric cancer cell lines | Jia et al., 2017 |
| MAGI1 | Tumor suppressor in cervical cancer | Proteolytic substrate of the HPV-16 and HPV-18 E6 oncoproteins. MAGI1 restoration induces apoptosis and represses cell proliferation | Cervical cancer cell lines | Kranjec et al., 2014 |
| MAGI2 | Tumor suppressor in prostate cancer | Its mutation is theorized to contribute to prostate carcinogenesis by driving AKT phosphorylation and altering PI3K signaling, thus disrupting PTEN signaling | Brenner & Chinnaiyan, 2011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wörthmüller, J.; Rüegg, C. MAGI1, a Scaffold Protein with Tumor Suppressive and Vascular Functions. Cells 2021, 10, 1494. https://doi.org/10.3390/cells10061494
Wörthmüller J, Rüegg C. MAGI1, a Scaffold Protein with Tumor Suppressive and Vascular Functions. Cells. 2021; 10(6):1494. https://doi.org/10.3390/cells10061494
Chicago/Turabian StyleWörthmüller, Janine, and Curzio Rüegg. 2021. "MAGI1, a Scaffold Protein with Tumor Suppressive and Vascular Functions" Cells 10, no. 6: 1494. https://doi.org/10.3390/cells10061494
APA StyleWörthmüller, J., & Rüegg, C. (2021). MAGI1, a Scaffold Protein with Tumor Suppressive and Vascular Functions. Cells, 10(6), 1494. https://doi.org/10.3390/cells10061494







