Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview
Abstract
:1. Introduction
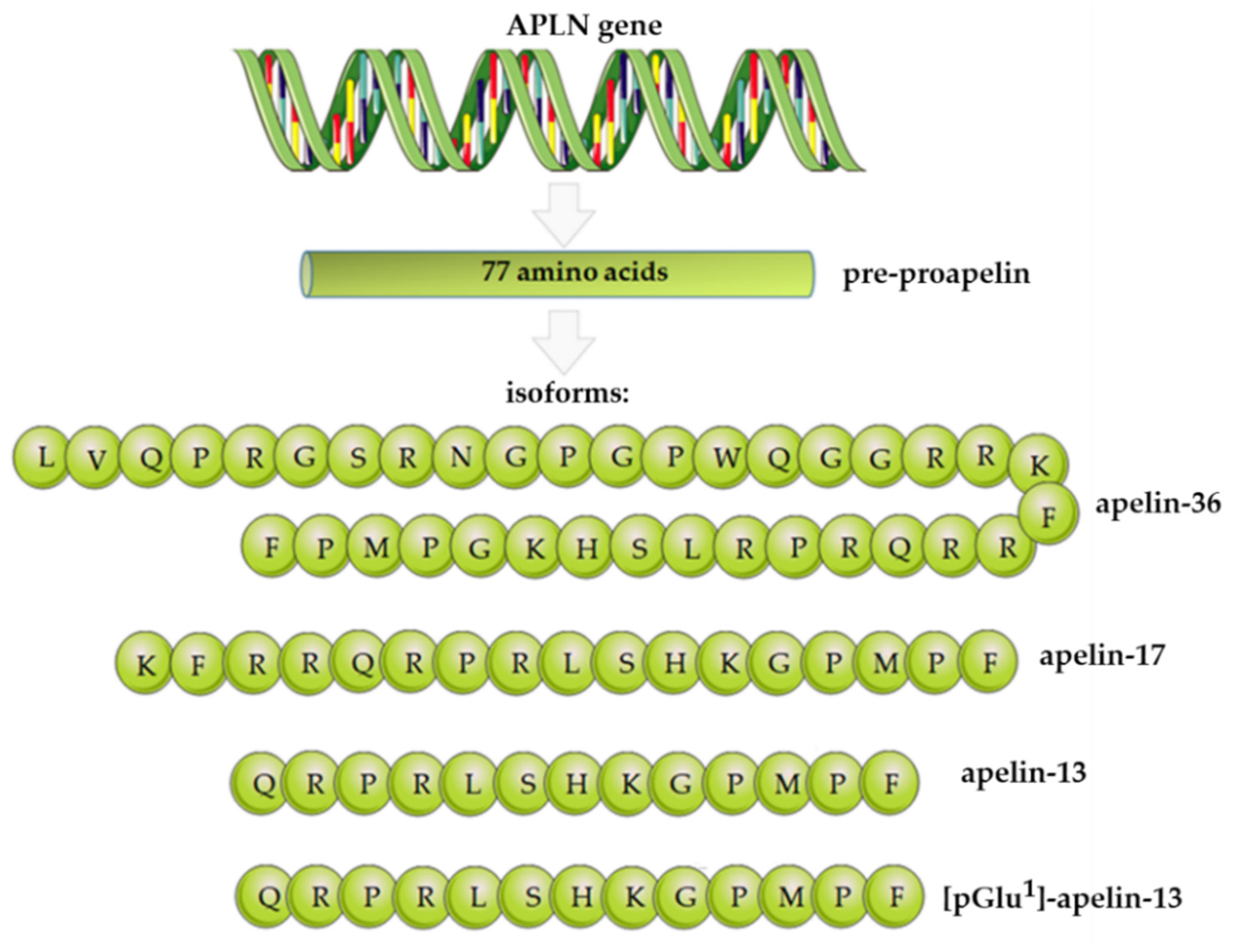
2. Apelin Structure, Expression, and Functions
3. APJ Structure, Expression, and Functions
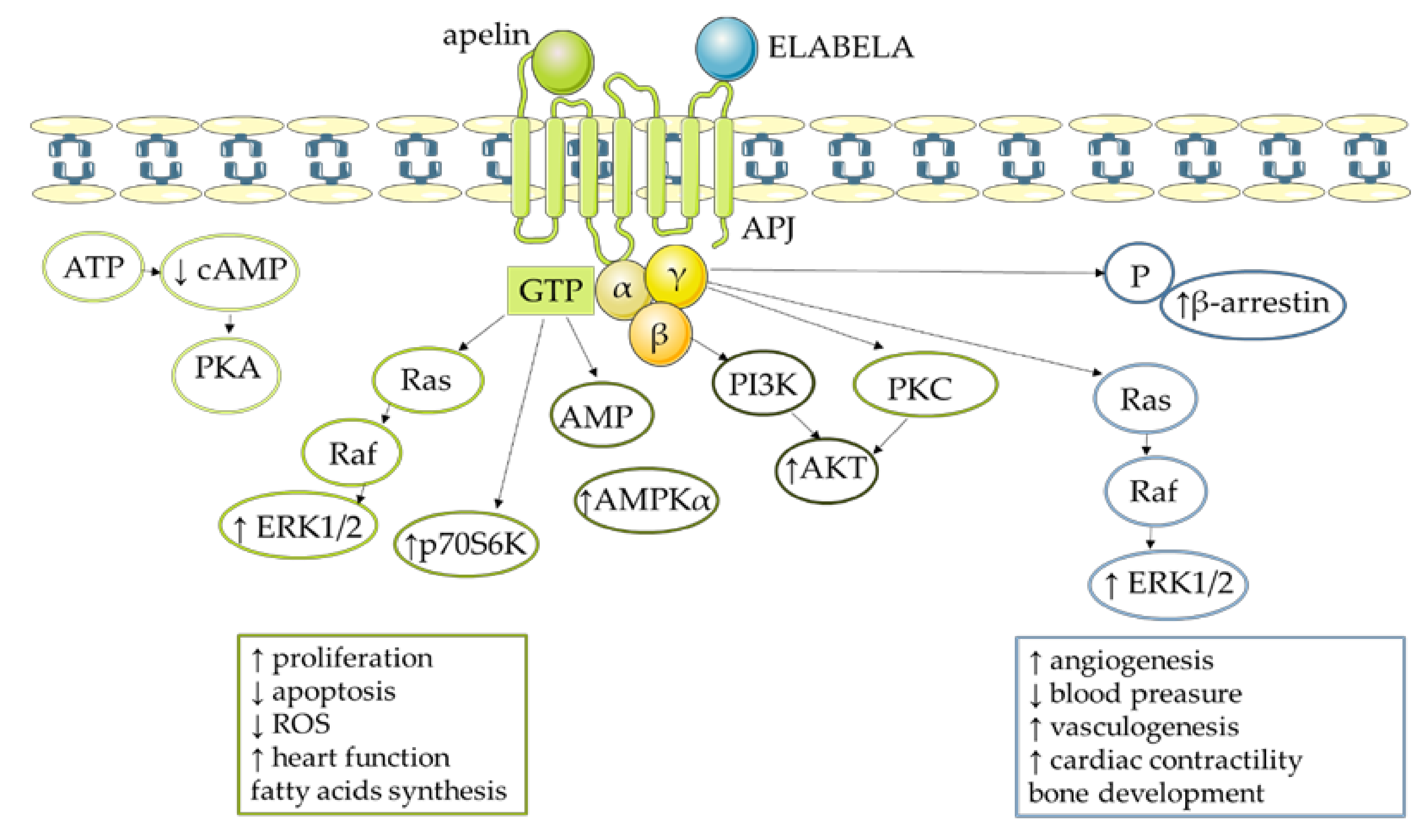
4. Molecular Mechanism of Apelin Action
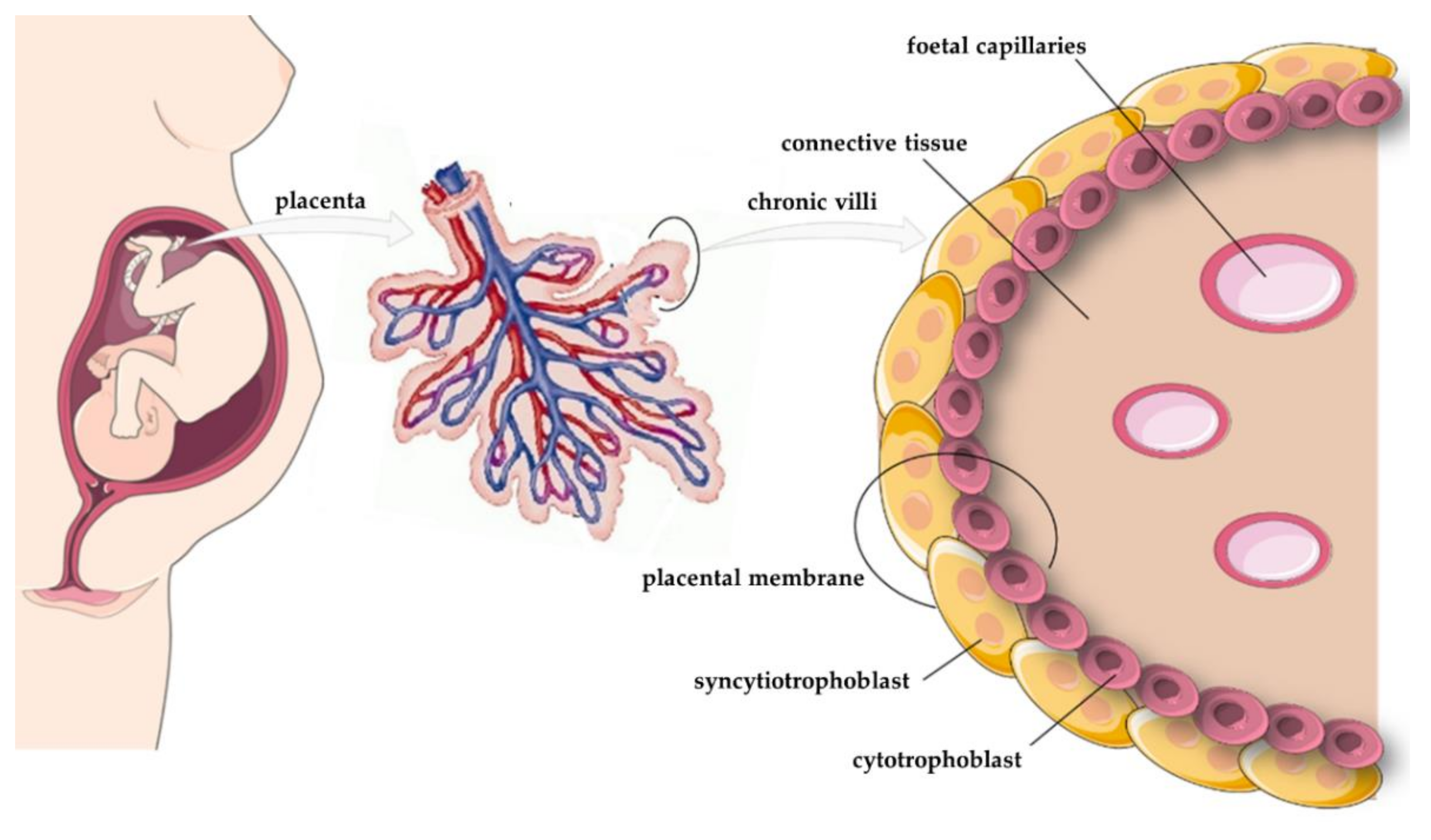
5. Expression of Apelin, APJ, and ELABELA in the Placenta
6. Effects of Apelin, APJ, and ELABELA on Placental Function
6.1. Proliferation
6.2. Apoptosis
6.3. Endocrinology
6.4. Angiogenesis
6.5. Transport and Metabolism
7. Placental Pathology and Pregnancy Pathology
7.1. Preeclampsia
7.2. Intrauterine Growth Restriction
7.3. Gestational Diabetes Mellitus
8. Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erlebacher, A.; Fisher, S.J. Baby’s First Organ. Sci. Am. 2017, 317, 46–53. [Google Scholar] [CrossRef]
- Tsai, K.; Tullis, B.; Jensen, T.; Graff, T.; Reynolds, P.; Arroyo, J. Differential expression of mTOR related molecules in the placenta from gestational diabetes mellitus (GDM), intrauterine growth restriction (IUGR) and preeclampsia patients. Reprod. Biol. 2021, 21, 100503. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.C. Placental function in development and disease. Reprod. Fertil. Dev. 2005, 18, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- O’Carrol, A.-M.; Lolai, S.L.; Harris, L.E.; Pope, G.R. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 2013, 219, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Ashley, E.A.; Den, D.X.F.; Tsalenko, A.; Deng, A.; Tabibiazar, R.; Ben-Dor, A.; Fenster, B.; Yang, E.; King, J.Y.; et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 2003, 108, 1432–1439. [Google Scholar] [CrossRef]
- Kawamata, Y.; Habata, Y.; Fukusumi, S.; Hosoya, M.; Fujii, R.; Hinuma, S.; Nishizawa, N.; Kitada, C.; Onda, H.; Nishimura, O.; et al. Molecular properties of apelin: Tissue distribution and receptor binding. Biochim. Biophys. Acta 2001, 1538, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Weir, R.A.P.; Chong, K.S.; Dalzell, J.R.; Petrie, C.J.; Murphy, C.A.; Steedman, T.; Mark, P.B.; McDonagh, T.A.; Dargie, H.J.; McMurray, J.J.V. Plasma apelin concentration is depressed following acute myocardial infarction in man. Eur. J. Heart. Fail. 2009, 11, 551–558. [Google Scholar] [CrossRef]
- Kuklińska, A.M.; Sobkowicz, B.; Sawicki, R.; Musiał, W.J.; Waszkiewicz, E.; Bolinska, S.; Małyszko, J. Apelin: A novel marker for the patients with first ST-elevation myocardial infarction. Heart Vessels 2010, 25, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, A.; Celebi, G.; Erdem, G.; Tapan, S.; Genc, H.; Tasci, I.; Ercin, C.N.; Dogru, T.; Kilic, S.; Uckaya, G.; et al. Plasma apelin and ADMA Levels in patients with essential hypertension. Clin. Exp. Hypertens. 2010, 32, 179–183. [Google Scholar] [CrossRef]
- Chen, D.; Lee, J.; Gu, X.; Wei, L.; Yu, S.P. Intranasal Delivery of Apelin-13 Is Neuroprotective and Promotes Angiogenesis After Ischemic Stroke in Mice. ASN Neuro. 2015, 7, 1759091415605114. [Google Scholar] [CrossRef]
- Sorli, S.C.; Le Gonidec, S.; Knibiehler, B.; Audigier, Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene 2007, 26, 7692–7699. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, L.; Qin, X.; Pan, W.; Feng, F.; Chen, F.; Zhu, B.; Liao, D.; Tanowitz, H.; Albanese, C.; et al. Apelin-induced vascular smooth muscle cell proliferation: The regulation of cyclin D1. Front. Biosci. 2008, 13, 3786–3792. [Google Scholar] [CrossRef] [Green Version]
- Shuang, L.; Jidong, W.; Hongjuan, P.; Zhenwei, Y. Effects of apelin on proliferation and apoptosis in rat ovarian granulosa cells. Clin. Exp. Obstet. Gynecol. 2016, 43, 409–413. [Google Scholar]
- Kurowska, P.; Barbe, A.; Różycka, M.; Chmielińska, J.; Dupont, J.; Rak, A. Apelin in reproductive physiology and pathology of different species: A critical review. Int. J. Endocrinol. 2018, 2018, 9170480. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Rak, A.; Froment, P.; Dupont, J. Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reproduction 2017, 153, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Soriguer, F.; Garrido-Sanchez, L.; Garcia-Serrano, S.; Garcia-Almeida, J.M.; Garcia-Arnes, J.; Tinahones, F.J.; Garcia-Fuentes, E. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes. Surg. 2009, 19, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tao, Y.; Feng, J.; Jiang, Y.R. Apelin Protects Primary Rat Retinal Pericytes from Chemical Hypoxia-Induced Apoptosis. J. Ophthalmol. 2015, 2015, 186946. [Google Scholar] [CrossRef]
- Yang, F.; Bai, Y.; Jiang, Y. Effects of apelin on RAW264.7 cells under both normal and hypoxic conditions. Peptides 2015, 69, 133–143. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.; Tsui, L.C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360. [Google Scholar] [CrossRef]
- Devic, E.; Rizzoti, K.; Bodin, S.; Knibiehler, B.; Audigier, Y. Amino acid sequence and embryonic expression of msr/apj, the mouse homolog of Xenopus X-msr and human APJ. Mech. Dev. 1999, 84, 199–203. [Google Scholar] [CrossRef]
- O’Carroll, A.M.; Selby, T.L.; Palkovits, M.; Lolait, S.J. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim. Biophys. Acta 2000, 1492, 72–80. [Google Scholar] [CrossRef]
- Wheatley, M.; Hawtin, S.R. Glycosylation of G-protein-coupled receptors for hormones central to normal reproductive functioning: Its occurrence and role. Hum. Reprod. Update 1999, 5, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Huynh, J.; Thomas, W.G.; Aguilar, M.I.; Pattenden, L.K. Role of helix 8 in G protein-coupled receptors based on structure–function studies on the type 1 angiotensin receptor. Mol. Cell Endocrinol. 2009, 302, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hidaka, K.; Akiho, H.; Tada, S.; Okada, M.; Yamaguchi, T. Low stringency hybridization study of the dopamine D4 receptor revealed D4-like mRNA distribution of the orphan seven-transmembrane receptor, APJ, in human brain. Neurosci. Lett. 1996, 219, 119–122. [Google Scholar] [CrossRef]
- Habata, Y.; Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Hinuma, S.; Kitada, C.; Nishizawa, N.; Murosaki, S.; Kurokawa, T.; et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta 1999, 1452, 25–35. [Google Scholar] [CrossRef] [Green Version]
- De Mota, N.; Lenkei, Z.; Llorens-Cortès, C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 2000, 72, 400–407. [Google Scholar] [CrossRef]
- Hosoya, M.; Kawamata, Y.; Fukusumi, S.; Fujii, R.; Habata, Y.; Hinuma, S.; Kitada, C.; Honda, S.; Kurokawa, T.; Onda, H.; et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 2000, 275, 21061–21067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.K.; Cheng, R.; Nguyen, T.; Fan, T.; Kariyawasam, A.P.; Liu, Y.; Osmond, D.H.; George, S.R.; O’Dowd, B.F. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 2000, 74, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Schilffarth, S.; Antoni, B.; Schams, D.; Meyer, H.H.D.; Berisha, B. The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary. Int. J. Biol. Sci. 2009, 5, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dray, C.; Debard, C.; Jager, J.; Disse, E.; Daviaud, D.; Martin, P.; Attané, C.; Wanecq, E.; Guigné, C.; Bost, F.; et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1161–E1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev. Cell 2013, 27, 672–680. [Google Scholar] [CrossRef] [Green Version]
- O’Carroll, A.-M.; Don, A.L.; Lolait, S.J. APJ receptor mRNA expression in the rat hypothalamic paraventricular nucleus: Regulation by stress and glucocorticoids. J. Neuroendocrinol. 2003, 15, 1095–1101. [Google Scholar] [CrossRef]
- Wang, G.; Kundu, R.; Han, S.; Qi, X.; Englander, E.W.; Quertermous, T.; Greeley, G.H., Jr. Ontogeny of apelin and its receptor in the rodent gastrointestinal tract. Regul. Pept. 2009, 158, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Kidoya, H.; Ueno, M.; Yamada, Y.; Mochizuki, N.; Nakata, M.; Yano, T.; Fujii, R.; Takakura, N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008, 27, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.-L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An embryonic signal that promotes cell movement via apelin receptors. Science 2014, 343, 1248636. [Google Scholar] [CrossRef]
- Yang, P.; Read, C.; Kuc, R.E.; Buonincontri, G.; Southwood, M.; Torella, R.; Upton, P.D.; Crosby, A.; Sawiak, S.J.; Carpenter, T.A.; et al. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Perjés, Á.; Kilpiö, T.; Ulvila, J.; Magga, J.; Alakoski, T.; Szabó, Z.; Vainio, L.; Halmetoja, E.; Vuolteenaho, O.; Petäjä-Repo, U.; et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res. Cardiol. 2016, 111, 2. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; van Dijk, M.; Chye, S.T.J.; Messerschmidt, D.M.; Chng, S.C.; Ong, S.; Yi, L.K.; Boussata, S.; Goh, G.H.-Y.; Afink, G.B.; et al. ELABELA Deficiency Promotes Preeclampsia and Cardiovascular Malformations in Mice. Science 2017, 357, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Camps, M.; Tian, J.; Chng, S.C.; Sem, K.P.; Sudhaharan, T.; Teh, C.; Wachsmuth, M.; Korzh, V.; Ahmed, S.; Reversade, B. Quantitative imaging reveals real-time Pou5f3-Nanog complexes driving dorsoventral mesendoderm patterning in zebrafish. eLife 2016, 5, e11475. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.L.; Ho, L.; Ford, G.H.; Chen, H.I.; Goldstone, A.B.; Woo, Y.J.; Quertermous, T.; Reversade, B.; Red-Horse, K. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and apj-deficient hearts. Dev. Cell 2017, 42, 655–666.e3. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yu, D.; Wang, M.; Wang, Q.; Kouznetsova, J.; Yang, R.; Qian, K.; Wu, W.; Shuldiner, A.; Sztalryd, C.; et al. Elabela-apelin receptor signaling pathway is functional in mammalian Systems. Sci. Rep. 2015, 5, 8170. [Google Scholar] [CrossRef] [Green Version]
- Helker, C.S.; Schuermann, A.; Pollmann, C.; Chng, S.C.; Kiefer, F.; Reversade, B.; Herzog, W. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. eLife 2015, 4, e06726. [Google Scholar] [CrossRef]
- Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Marx, P.; Besserer-Offroy, É.; Longpré, J.-M.; Lainé, J.; Reversade, B.; Salvail, D.; et al. Discovery and structure-activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J. Med. Chem. 2016, 59, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, D.; Ni, L.; Shi, L.; Xu, W.; Shi, M.; Chen, J.; Ai, Y.; Zhang, X. Serum Elabela/toddler levels are associated with albuminuria in patients with type 2 diabetes. Cell Physiol. Biochem. 2018, 48, 1347–1354. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, L.; Martin, S.; Zhang, Y.; Dong, Y.; Zhong, J.C.; Yang, X.C. Lower Plasma Elabela Levels in Hypertensive Patients With Heart Failure Predict the Occurrence of Major Adverse Cardiac Events: A Preliminary Study. Front. Cardiovasc. Med. 2021, 8, 638468. [Google Scholar] [CrossRef] [PubMed]
- Sainsily, X.; Coquerel, D.; Giguère, H.; Dumont, L.; Tran, K.; Noll, C.; Ionescu, A.L.; Côté, J.; Longpré, J.-M.; Carpentier, A.; et al. Elabela Protects Spontaneously Hypertensive Rats From Hypertension and Cardiorenal Dysfunctions Exacerbated by Dietary High-Salt Intake. Front. Pharmacol. 2021, 12, 709467. [Google Scholar] [CrossRef]
- Freyer, L.; Hsu, C.-W.; Nowotschin, S.; Pauli, A. Loss of Apela peptide in mice causes low penetrance embryonic lethality and defects in early mesodermal derivatives. Cell Rep. 2017, 20, 2116–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Chen, H.; Ju, H.; Sun, M. Circulating chemerin levels and gestational diabetes mellitus: A systematic review and meta-analysis. Lipids Health Dis. 2018, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Tang, J.; Liu, H.; Chen, J.; Li, Y.; Song, W. Apelin-13 induces ERK1/2 but not p38 MAPK activation through coupling of the human apelin receptor to the Gi2 pathway. Acta Biochim. Biophys. Sin. 2008, 40, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Choe, W.; Albright, A.; Sulcove, J. Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J. Neurovirol. 2000, 6, 61–69. [Google Scholar]
- Masri, B.; Morin, N.; Pedebernade, L.; Knibiehler, B.; Audigier, Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 2006, 281, 18317–18326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Zhang, P.; Li, C.; Si, J.; Wang, Y.; Zhang, X.; Zhang, D.; Zhang, H.; Lin, C. Apelin-13 promotes cell proliferation in the H9c2 cardiomyoblast cell line by triggering extracellular signal-regulated kinase 1/2 and protein kinase B phosphorylation. Mol. Med. Rep. 2018, 17, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.J.; Yu, S.P.; Zhang, L.; Wei, L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp. Cell Res. 2010, 316, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, Y.; Sumino, A.; Kajioka, D.; Shibagaki, F.; Yamamuro, A.; Yoshioka, Y.; Maeda, S. Apelin protects against NMDA-induced retinal neuronal death via an APJ receptor by activating Akt and ERK1/2, and suppressing TNF-α expression in mice. J. Pharmacol. Sci. 2017, 133, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Jin, H.; Xu, S.; Aillaud, M.; Deng, A.C.; Azuma, J.; Kundu, R.K.; Reaven, G.M.; Quertermous, T.; Tsao, P.S. Apelin decreases lipolysis via G(q), G(i), and AMPK-Dependent Mechanisms. Endocrinology 2011, 152, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, X.-J.; Li, L.T. Apelin-13 protects against apoptosis by activating AMP-activated protein kinase pathway in ischemia stroke. Peptides 2016, 75, 96–100. [Google Scholar] [CrossRef]
- Boyd, J.D.; Hamilton, W.J. The Human Placenta; Heffer: Cambridge, UK, 1970; p. 365. [Google Scholar]
- Richart, R. Studies of Placental Morphogenesis I. Radioautographic Studies of Human Placenta Utilizing Tritiated Thymidine. Proc. Soc. Exp. Biol. Med. 1961, 106, 829–831. [Google Scholar] [CrossRef]
- Aplin, J.D.; Lewis, R.M.; Jones, C.J.P. Development of the human placental villus. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; p. 90964811. [Google Scholar]
- Pötgens, A.J.G.; Schmitz, U.; Bose, P.; Versmold, A.; Kaufmann, P.; Frank, H.-G. Mechanisms of syncytial fusion: A review. Placenta 2002, 23, 107–113. [Google Scholar] [CrossRef]
- Huppertz, B.; Gauster, M. Trophoblast fusion. Adv. Exp. Med. Biol. 2011, 713, 81–95. [Google Scholar]
- Arnholdt, H.; Diebold, J.; Kuhlmann, B.; Löhrs, U. Receptor-mediated processing of epidermal growth factor in the trophoblast of the human placenta. Virchows. Arch. B Cell Pathol. Incl. Mol. Pathol. 1991, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Lao, T.T.; Cheung, A.N. Apoptotic and proliferative activities in first trimester placentae. Placenta 1999, 20, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Ghosh, D.; Sengupta, J. Histochemical and morphological examination of proliferation and apoptosis in human first trimester villous trophoblast. Hum. Reprod. 2007, 22, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Maruo, T.; Ladines-Llave, C.A.; Matsuo, H.; Manalo, A.S.; Mochizuki, M. A novel change in cytologic localization of human chorionic gonadotropin and human placental lactogen in first-trimester placenta in the course of gestation. Am. J. Obstet. Gynecol. 1992, 167, 217–222. [Google Scholar] [CrossRef]
- Kolahi, K.S.; Valent, A.M.; Thornburg, K.L. Cytotrophoblast, Not Syncytiotrophoblast, Dominates Glycolysis and Oxidative Phosphorylation in Human Term Placenta. Sci. Rep. 2017, 7, 42941. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.J.; Fox, H. Ultrastructure of the normal human placenta. Electron. Microsc. Rev. 1991, 4, 129–178. [Google Scholar] [CrossRef]
- Mayhew, T.M. Villous trophoblast of human placenta: A coherent view of its turnover, repair and contributions to villous development and maturation. Histol. Histopathol. 2001, 16, 1213–1224. [Google Scholar]
- Jones, C.J.; Fox, H. Ultrastructure of the placenta in prolonged pregnancy. J. Pathol. 1978, 126, 173–179. [Google Scholar] [CrossRef]
- Evain-Brion, D.; Malassine, A. Human placenta as an endocrine organ. Growth Horm. IGF Res. 2003, 13, S34–S37. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Thrower, S.; Wells, M. Expression of epidermal growth factor receptor and transferrin receptor by human trophoblast populations. Am. J. Reprod. Immunol. 1989, 21, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hayati, A.R.; Cheah, F.C.; Tan, A.E.; Tan, G.C. Insulin-like growth factor-1 receptor expression in the placentae of diabetic and normal pregnancies. Early Hum. Dev. 2007, 83, 41–46. [Google Scholar] [CrossRef]
- Naing, Z.; Hamilton, S.T.; van Zuylen, W.J.; Scott, G.M.; Rawlinson, W.D. Differential Expression of PDGF Receptor-α in Human Placental Trophoblasts Leads to Different Entry Pathways by Human Cytomegalovirus Strains. Sci. Rep. 2020, 10, 1082. [Google Scholar] [CrossRef] [Green Version]
- Ellery, P.M.; Cindrova-Davies, T.; Jauniaux, E.; Ferguson-Smith, A.C.; Burton, G.J. Evidence for transcriptional activity in the syncytiotrophoblast of the human placenta. Placenta 2009, 30, 329–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertig, A.T.; Rock, J.; Adams, E.C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 1956, 98, 435–493. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [Green Version]
- Proud, J.; Grant, A.M. Third trimester placental grading by ultrasonography as a test of fetal wellbeing. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 1641–1644. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, O.R.; Powell, T.L.; Jansson, T. Apelin is a novel regulator of human trophoblast amino acid transport. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E810–E816. [Google Scholar] [CrossRef]
- Cobellis, L.; De Falco, M.; Mastrogiacomo, A.; Giraldi, D.; Dattilo, D.; Scaffa, C.; Colacurci, N.; De Luca, A. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol. Histopathol. 2007, 22, 1–8. [Google Scholar]
- Van Mieghem, T.; van Bree, R.; Van Herck, E.; Pijnenborg, R.; Deprest, J.; Verhaeghe, J. Maternal apelin physiology during rat pregnancy: The role of the placenta. Placenta 2010, 31, 725–730. [Google Scholar] [CrossRef]
- Kourtis, A.; Gkiomisi, A.; Mouzaki, M.; Makedou, K.; Anastasilakis, A.D.; Toulis, K.A.; Gerou, S.; Gavana, E.; Agorastos, T. Apelin levels in normal pregnancy. Clin. Endocrinol. 2011, 75, 367–371. [Google Scholar] [CrossRef]
- Yamaleyeva, L.M.; Chappell, M.C.; Brosnihan, K.B.; Anton, L.; Caudell, D.L.; Shi, S.; McGee, C.; Pirro, N.; Gallagher, P.E.; Taylor, R.N.; et al. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2015, 15, E852–E860. [Google Scholar] [CrossRef] [Green Version]
- Mlyczyńska, E.; Kurowska, P.; Drwal, E.; Opydo-Chanek, M.; Tworzydło, W.; Kotula-Balak, M.; Rak, A. Apelin and apelin receptor in human placenta: Expression, signalling pathway and regulation of trophoblast JEG-3 and BeWo cells proliferation and cell cycle. Int. J. Mol. Med. 2020, 45, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Devic, E.; Paquereau, L.; Vernier, P.; Knibiehler, B.; Audigier, Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech. Dev. 1996, 59, 129–140. [Google Scholar] [CrossRef]
- Zeng, X.-X.I.; Wilm, T.P.; Sepich, D.S.; Solnica-Krezel, L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev. Cell 2007, 12, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLoia, J.A.; Burlingame, J.M.; Krasnow, J.S. Differential expression of G1 cyclins during human placentogenesis. Placenta 1997, 18, 9–16. [Google Scholar] [CrossRef]
- Genbacev, O.; McMaster, M.T.; Fisher, S.J. A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am. J. Pathol. 2000, 157, 1337–1351. [Google Scholar] [CrossRef] [Green Version]
- Bamberger, A.; Sudahl, S.; Bamberger, C.M.; Schulte, H.M.; Löning, T. Expression patterns of the cell-cycle inhibitor p27 and the cell-cycle promoter cyclin E in the human placenta throughout gestation: Implications for the control of proliferation. Placenta 1999, 20, 401–406. [Google Scholar] [CrossRef]
- Korgun, E.T.; Celik-Ozenci, C.; Acar, N.; Cayli, S.; Desoye, G.; Demir, R. Location of cell cycle regulators cyclin B1, cyclin A, PCNA, Ki67 and cell cycle inhibitors p21, p27 and p57 in human first trimester placenta and deciduas. Histochem. Cell Biol. 2006, 125, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Danihel, L.; Gomolcák, P.; Korbel, M.; Pruzinec, J.; Vojtassák, J.; Janík, P.; Babál, P. Expression of proliferation and apoptotic markers in human placenta during pregnancy. Acta Histochem. 2002, 104, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, S.; Gao, J.; Meng, T.; Xing, X.; Chen, C.; Chen, H.; Luo, Y. Cyclin G2 Is Involved in the Proliferation of Placental Trophoblast Cells and Their Interactions with Endothelial Cells. Med. Sci. Monit. 2020, 26, e926414. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Lu, H.; Wang, H.; Shi, X.; Shao, X.; Li, Y.-X.; Zhao, Y.; Wang, Y.-L. miR-518b Enhances Human Trophoblast Cell Proliferation Through Targeting Rap1b and Activating Ras-MAPK Signal. Front. Endocrinol. 2018, 9, 100. [Google Scholar] [CrossRef]
- Georgiadou, D.; Boussata, S.; Willemijn, H.; Ranzijn, M.; Leah, E.; Root, A.; Hillenius, S.; Jeske, M.; de Weg, B.; Carolien, N.; et al. Peptide hormone ELABELA enhances extravillous trophoblast differentiation, but placenta is not the major source of circulating ELABELA in pregnancy. Sci. Rep. 2019, 9, 19077. [Google Scholar] [CrossRef]
- Ka, H.; Hunt, J.S. Temporal and spatial patterns of expression of inhibitors of apoptosis in human placentas. Am. J. Pathol. 2003, 163, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Black, S.; Kadyrov, M.; Kaufmann, P.; Ugele, B.; Emans, N.; Huppertz, B. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2004, 11, 90–98. [Google Scholar] [CrossRef]
- Soni, S.; Rath, G.; Prasad, C.P.; Salhan, S.; Saxena, S.; Jain, A.K. Apoptosis and Bcl-2 protein expression in human placenta over the course of normal pregnancy. Anat. Histol. Embryol. 2010, 39, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Mlyczyńska, E.; Myszka, M.; Kurowska, P.; Dawid, M.; Milewicz, T.; Bałajewicz-Nowak, M.; Kowalczyk, P.; Rak, A. Anti-Apoptotic Effect of Apelin in Human Placenta: Studies on BeWo Cells and Villous Explants from Third-Trimester Human Pregnancy. Int. J. Mol. Sci. 2021, 22, 2760. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef] [Green Version]
- Pidoux, G.; Gerbaud, P.; Tsatsaris, V.; Marpeau, O.; Ferreira, F.; Meduri, G.; Guibourdenche, J.; Badet, J.; Evain-Brion, D.; Frendo, J.L. Biochemical characterization and modulation of LH/CG-receptor during human trophoblast differentiation. J. Cell Physiol. 2007, 212, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coya, R.; Martul, P.; Algorta, J.; Aniel-Quiroga, M.A.; Busturia, M.A.; Señarís, R. Progesterone and human placental lactogen inhibit leptin secretion on cultured trophoblast cells from human placentas at term. Gynecol. Endocrinol. 2005, 21, 27–32. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Zhang, Y.; Hu, X.; Gao, G.; Ye, Y.; Peng, W.; Zhou, J. Human placental lactogen mRNA in maternal plasma play a role in prenatal diagnosis of abnormally invasive placenta: Yes or no? Gynecol. Endocrinol. 2019, 35, 631–634. [Google Scholar] [CrossRef]
- Morel, Y.; Roucher, F.; Plotton, I.; Goursaud, C.; Tardy, V.; Mallet, D. Evolution of steroids during pregnancy: Maternal, placental and fetal synthesis. Ann. Endocrinol. 2016, 77, 82–89. [Google Scholar] [CrossRef]
- Voutilainen, R.; Miller, W.L. Developmental expression of genes for the stereoidogenic enzymes P450scc (20,22-desmolase), P450c17 (17 alpha-hydroxylase/17,20-lyase), and P450c21 (21-hydroxylase) in the human fetus. J. Clin. Endocrinol. Metab. 1986, 63, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Karahoda, R.; Kallol, S.; Groessl, M.; Ontsouka, E.; Anderle, P.; Fluck, C.; Staud, F.; Albrecht, C. Revisiting Steroidogenic Pathways in the Human Placenta and Primary Human Trophoblast Cells. Int. J. Mol. Sci. 2021, 22, 1704. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, S.C.; Park, M.N.; Jeong, J.S.; Yang, S.Y.; Lee, Y.J.; Bae, O.N.; Yang, H.S.; Seo, S.; Lee, K.S.; et al. Expression of steroidogenic enzymes in human placenta according to the gestational age. Mol. Med. Rep. 2019, 19, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; Li, T.; Liu, H.; Tang, L.; Li, Y.-C.; Hu, H.-T.; Su, Y.-F.; Lin, Y.; Wang, Y.-Y.; Li, C.; et al. Circulating levels of Elabela and Apelin in the second and third trimesters of pregnancies with gestational diabetes mellitus. Gynecol. Endocrinol. 2020, 36, 890–894. [Google Scholar] [CrossRef]
- Dawid, M.; Mlyczynska, E.; Kurowska, P.; Sierpowski, M.; Rak, A. Apelin decreased placental hormone secretion by human trophoblast BeWo cells via apelin receptor, protein kinase A and extracellular signal-regulated kinases 1/2 activation. J. Physiol. Pharmacol. 2019, 70, 211246102. [Google Scholar]
- Reynolds, L.P.; Redmer, D.A. Angiogenesis in the placenta. Biol. Reprod. 2001, 64, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Klagsbrun, M.D.A.P.; D’amore, P.A. Regulators of angiogenesis. Annu. Rev. Physiol. 1991, 53, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Singh, M.; Ebaugh, M.J.; Brace, R.A. Vascular endothelial growth factor gene expression in ovine placenta and fetal membranes. Am. Obstet. Gynecol. 1995, 173, 753–759. [Google Scholar] [CrossRef]
- Cooper, J.C.; Sharkey, A.M.; McLaren, J.; Charnock-Jones, D.S.; Smith, S.K. Localization of vascular endothelial growth factor and its receptor, flt, in human placenta and decidua by immunohistochemistry. J. Reprod. Fertil. 1995, 105, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Dumont, D.J.; Fong, G.H.; Puri, M.C.; Gradwohl, G.; Alitalo, K.; Breitman, M.L. Vascularization of the mouse embryo: A study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn. 1995, 203, 80–92. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Babaei, S.; Teichert-Kuliszewska, K.; Monge, J.C.; Mohamed, F.; Bendeck, M.P.; Stewart, D.J. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ. Res. 1998, 82, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patan, S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 1998, 56, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Huang, W.Q.; Mallah, J.; Yang, D.; Lippman, M.E.; Li, L.Y. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc. Res. 1999, 58, 224–237. [Google Scholar] [CrossRef]
- Meegdes, B.H.; Ingenhoes, R.; Peeters, L.L.; Exalto, N. Early pregnancy wastage: Relationship between chorionic vascularization and embryonic development. Fertil. Steril. 1988, 49, 216–220. [Google Scholar] [CrossRef]
- Bassil, S.; Magritte, J.P.; Roth, J.; Nisolle, M.; Donnez, J.; Gordts, S. Uterine vascularity during stimulation and its correlation with implantation in in-vitro fertilization. Hum. Reprod. 1995, 10, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, H.; Nishizawa, H.; Inagaki, A.; Suzuki, M.; Ota, S.; Miyamura, H.; Miyazaki, J.; Sekiya, T.; Kurahashi, H.; Udagawa, Y. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens. Pregnancy 2013, 32, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Eberlé, D.; Marousez, L.; Hanssens, S.; Knauf, C.; Breton, C.; Deruelle, P.; Lesage, J. Elabela and Apelin actions in healthy and pathological pregnancies. Cytokine Growth Factor Rev. 2019, 46, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-Y.; Franklyn, J.A.; Pemberton, H.N.; Bulmer, J.N.; Visser, T.J.; McCabe, C.J.; Kilby, M.D. Monocarboxylate transporter 8 expression in the human placenta: The effects of severe intrauterine growth restriction. J. Endocrinol. 2006, 189, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.; Kachilele, S.; Hobbs, E.; Bulmer, J.N.; Boelaert, K.; McCabe, C.J.; Driver, P.M.; Bradwell, A.R.; Kester, M.; Visser, T.J.; et al. Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J. Clin. Endocrinol. Metab. 2003, 88, 4488–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beall, M.H.; van den Wijngaard, J.P.; van Gemert, M.J.; Ross, M.G. Regulation of amniotic fluid volume. Placenta 2007, 28, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L.A. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Molina, R.D.; Meschia, G.; Battaglia, F.C.; Hay, W.W., Jr. Gestational maturation of placental glucose transfer capacity in sheep. Am. J. Physiol. 1991, 261, R697–R704. [Google Scholar] [CrossRef]
- Duggan, C.; Watkins, J.B.; Koletzko, B.; Walker, W.A. Nutrition in Pediatrics: Basic Science, Clinical Applications; PMPH-USA Limited: New Haven, CT, USA, 2016; p. 1328. [Google Scholar]
- Herrera, E.; Amusquivar, E.; López-Soldado, I.; Ortega, H. Maternal lipid metabolism and placental lipid transfer. Horm. Res. 2006, 65, 59–64. [Google Scholar] [CrossRef]
- Mayeur, S.; Wattez, J.-S.; Lukaszewski, M.-A.; Lecoutre, S.; Butruille, L.; Drougard, A.; Eberlé, D.; Bastide, B.; Laborie, C.; Storme, L.; et al. Apelin Controls Fetal and Neonatal Glucose Homeostasis and Is Altered by Maternal Undernutrition. Diabetes 2016, 65, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Tomimatsu, T.; Mimura, K.; Endo, M.; Kumasawa, K.; Kimura, T. Pathophysiology of preeclampsia: An angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens. Res. 2017, 40, 305–310. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef]
- Simsek, Y.; Celen, S.; Ustun, Y.; Danisman, N.; Bayramoglu, H. Severe preeclampsia and fetal virilization in a spontaneous singleton pregnancy complicated by hyperreactio luteinalis. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 118–121. [Google Scholar] [PubMed]
- Kucur, M.; Tuten, A.; Oncul, M.; Acikgoz, A.S.; Yuksel, M.A.; Imamoglu, M.; Balci Ekmekci, O.; Yilmaz, N.; Madazli, R. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens. Pregnancy 2014, 33, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Colcimen, N.; Bulut, G.; Ergul Erkec, O.; Ragbetli, M.C. Investigation of role of vascular endothelial growth factor, Annexin A5 and Apelin by immunohistochemistry method in placenta of preeclampsia patients. Cell Mol. Biol. 2017, 63, 42–45. [Google Scholar] [CrossRef]
- Bortoff, K.D.; Qiu, C.; Runyon, S.; Williams, M.A.; Maitra, R. Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens. Pregnancy 2012, 31, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Zahraei, Z.; Al-Hakeim, H.K. Serum apelin and galectin-3 in preeclampsia in Iraq. Hypertens. Pregnancy 2020, 39, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, S.; Khodadadi, I.; Alizadeh, N.; Shafiee, G. Association of glutamate cystein ligase (GCL) activity Peroxiredoxin 4 (prxR4) and apelin levels in women with preeclampsia. Pregnancy Hypertens. 2021, 23, 163–168. [Google Scholar] [CrossRef]
- Temur, M.; Yilmaz, Ö.; Taşgöz, F.N.; Kume, T. The evaluation of serum apelin levels in patients complicated with preeclampsia. J. Matern. Fetal. Neonatal. Med. 2020, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gürlek, B.; Yılmaz, A.; Durakoğlugil, M.E.; Karakaş, S.; Kazaz, İ.M.; Önal, Ö.; Şatıroğlu, Ö. Evaluation of serum apelin-13 and apelin-36 concentrations in preeclamptic pregnancies. J. Obstet. Gynaecol. Res. 2020, 46, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Gandham, R.; Dayanand, C.D.; Sheela, S.R.; Kiranmayee, P. Maternal serum Apelin 13 and APLN gene promoter variant -1860T > C in preeclampsia. J. Matern. Fetal. Neonatal. Med. 2021, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Kong, D.; Qin, X.; Li, Y.; Teng, X.; Huang, X. Apelin as a novel drug for treating preeclampsia. Exp. Ther. Med. 2017, 14, 5917–5923. [Google Scholar] [CrossRef] [PubMed]
- Yamaleyeva, L.M.; Brosnihan, K.B.; Elsangeedy, E.; McGee, C.; Shi, S.; Caudell, D.; Miller, C.; Varagic, J.; Bader, M.; Dechend, R.; et al. Systemic Outcomes of (Pyr 1)-Apelin-13 Infusion at Mid-Late Pregnancy in a Rat Model with Preeclamptic Features. Sci. Rep. 2019, 9, 8579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, R.Z.; Diab, A.A.A.; Zahra, M.H.; Asalah, A.K.; Attia, M.S.; Moursi, S.M. Ameliorative effect of apelin-13 against renal complications in L-NAME-induced preeclampsia in rats. PeerJ. 2021, 9, e11110. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, B.; Romero, R.; Gomez-Lopez, N.; Pacora, P.; Erez, O.; Vadillo-Ortega, F.; Yeo, L.; Hassan, S.S.; Hsu, C.D. ELABELA plasma concentrations are increased in women with late-onset preeclampsia. J. Matern. Fetal. Neonatal. Med. 2020, 33, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Para, R.; Romero, R.; Gomez-Lopez, N.; Tarca, A.L.; Panaitescu, B.; Done, B.; Hsu, R.; Pacora, P.; Hsu, C.-D. Maternal circulating concentrations of soluble Fas and Elabela in early- and late-onset preeclampsia. J. Matern. Fetal. Neonatal. Med. 2020, 1–14. [Google Scholar] [CrossRef]
- Pritchard, N.; Kaitu’u-Lino, T.J.; Gong, S.; Dopierala, J.; Smith, G.C.S.; Charnock-Jones, D.S.; Tong, S. ELABELA/APELA Levels Are Not Decreased in the Maternal Circulation or Placenta among Women with Preeclampsia. Am. J. Pathol. 2018, 188, 1749–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniz, R.; Baykus, Y.; Ustebay, S.; Ugur, K.; Yavuzkir, Ş.; Aydin, S. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre-eclampsia, severe pre-eclampsia and umbilical arteries and venules of newborns. J. Obstet. Gynaecol. 2019, 39, 907–912. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, H.; Cheng, R.; Fan, X.; Lai, S.; Deng, C. ELABELA, as a potential diagnostic biomarker of preeclampsia, regulates abnormally shallow placentation via APJ. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E773–E781. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, J.; Zhang, L.; Hua, X.; Ye, W.; Chen, C.; Sun, K.; Wang, W.; Feng, L.; Zhang, J. Is ELABELA a reliable biomarker for hypertensive disorders of pregnancy? Pregnancy Hypertens. 2019, 17, 226–232. [Google Scholar] [CrossRef]
- Ma, J.; Hu, H.; Lin, M.; Chen, L.; Liu, M.; Li, H.; Quan, S. ELABELA alleviates syncytiotrophoblast hypoxia/reoxygenation injury and preeclampsia-like symptoms in mice by reducing apoptosis. Placenta 2021, 106, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Diab, A.A.A.; Zahra, M.H.; Asalah, A.K.; Moursi, S.M.; Al-Baqami, N.M.; Al-Salmi, F.A.; Attia, M.S. Correlation between Apelin and Some Angiogenic Factors in the Pathogenesis of Preeclampsia: Apelin-13 as Novel Drug for Treating Preeclampsia and Its Physiological Effects on Placenta. Int. J. Endocrinol. 2021, 2021, 5017362. [Google Scholar] [CrossRef] [PubMed]
- Simsek, Y.; Celik, O.; Yilmaz, E.; Karaer, A.; Dogan, C.; Aydin, S.; Ozer, A. Serum levels of apelin, salusin-alpha, and salusin-beta in normal pregnancy and preeclampsia. J. Matern. Fetal. Neonatal. Med. 2015, 2015, 1–32. [Google Scholar]
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr. Endocrinol. Rev. 2009, 3, 332–336. [Google Scholar]
- Dötsch, J. Perinatal programming—myths, fact, and future of research. Mol. Cell Pedriatr. 2014, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine growth restriction: Antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, A. The IUGR newborn. Semin. Perinatol. 2008, 32, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Malamitsi-Puchner, A.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Briana, D.D. Circulating apelin concentrations in mother/infant pairs at term. Acta Paediatr. 2007, 96, 1751–1754. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod. Sci. 2009, 16, 921–937. [Google Scholar] [CrossRef]
- Van Mieghem, T.; Doherty, A.; Baczyk, D.; Drewlo, S.; Baud, D.; Carvalho, J.; Kingdom, J. Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reprod. Sci. 2016, 23, 1037–1043. [Google Scholar] [CrossRef]
- Behram, M.; Oğlak, S.C.; Dağ, İ. Circulating levels of Elabela in pregnant women complicated with intrauterine growth restriction. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102127. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Retnakaran, R.; Ye, C.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B. Impact of Changes Over Time in Adipokines and Inflammatory Proteins on Changes in Insulin Sensitivity, Beta-Cell Function, and Glycemia in Women with Previous Gestational Dysglycemia. Diabetes Care 2017, 40, e101–e102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasshauer, M.; Blüher, M.; Stumvoll, M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014, 2, 488–499. [Google Scholar] [CrossRef]
- Telejko, B.; Kuzmicki, M.; Wawrusiewicz-Kurylonek, N.; Szamatowicz, J.; Nikolajuk, A.; Zonenberg, A.; Zwierz-Gugala, D.; Jelski, W.; Laudański, P.; Wilczynski, J.; et al. Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 87, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Oncul, M.; Tuten, A.; Erman, H.; Gelisgen, R.; Benian, A.; Uzun, H. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva Med. 2013, 104, 527–535. [Google Scholar]
- Aslan, M.; Celik, O.; Celik, N.; Turkcuoglu, I.; Yilmaz, E.; Karaer, A.; Simsek, Y.; Celik, E.; Aydin, S. Cord blood nesfatin-1 and apelin-36 levels in gestational diabetes mellitus. Endocrine 2012, 41, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Mierzyński, R.; Poniedziałek-Czajkowska, E.; Dłuski, D.; Kamiński, M.; Mierzyńska, A.; Leszczyńska-Gorzelak, B. The Potential Role of Chemerin, Lipocalin 2, and Apelin in the Diagnosis and Pathophysiology of Gestational Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 5547228. [Google Scholar] [CrossRef]



| Apelinergic System | Apelin | ELABELA | APJ |
|---|---|---|---|
| Approved gene symbol | APLN | APELA | APLNR |
| Approved name | Apelin | Apelin receptor early endogenous ligand | Apelin receptor |
| Chromosomal location | Xq26.1 | 4q32.3 | 11q12.1 |
| Gene groups | Neuropeptides | Receptor ligands | Receptors |
| First isolation | Bovine stomach extracts | ESC in zebrafish | Human blood |
| Function in organisms | ↑ Carbohydrate disorders treatment ↓ Pericytes apoptosis ↑ Proliferation of Gc/VSMC ↓ Proinflammatory cytokines production ↑ Cancer neoangiogenesis | ↓ Gastrulation disorders ↑ Blood vessel angiogenesis/vasculogenesis ↓ Renal dysfunctions ↓ Blood pressure ↓ Cardiovascular disorders | ↑ Signal transmission pathway from apelin/ELABELA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawid, M.; Mlyczyńska, E.; Jurek, M.; Respekta, N.; Pich, K.; Kurowska, P.; Gieras, W.; Milewicz, T.; Kotula-Balak, M.; Rak, A. Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells 2022, 11, 99. https://doi.org/10.3390/cells11010099
Dawid M, Mlyczyńska E, Jurek M, Respekta N, Pich K, Kurowska P, Gieras W, Milewicz T, Kotula-Balak M, Rak A. Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells. 2022; 11(1):99. https://doi.org/10.3390/cells11010099
Chicago/Turabian StyleDawid, Monika, Ewa Mlyczyńska, Małgorzata Jurek, Natalia Respekta, Karolina Pich, Patrycja Kurowska, Wiktoria Gieras, Tomasz Milewicz, Małgorzata Kotula-Balak, and Agnieszka Rak. 2022. "Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview" Cells 11, no. 1: 99. https://doi.org/10.3390/cells11010099
APA StyleDawid, M., Mlyczyńska, E., Jurek, M., Respekta, N., Pich, K., Kurowska, P., Gieras, W., Milewicz, T., Kotula-Balak, M., & Rak, A. (2022). Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells, 11(1), 99. https://doi.org/10.3390/cells11010099








