Centrosome Positioning in Migrating Dictyostelium Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Chemotaxis Conditions
2.3. Micropipette Chemotaxis Assay
2.4. Migration within Microfabricated Polydimethylsiloxane (PDMS)-Based Microchannels or Pillar Arrays
2.5. Migration in 3D Hydrogel and Collagen Matrices
2.6. Live-Cell Imaging of Migrating Cells
2.7. Statistics
2.8. Determination of Centrosome and Nucleus Distance
2.9. Analysis of the Centrosome Position Nucleus/Centrosome Centroids Relative to Cell Center
3. Results
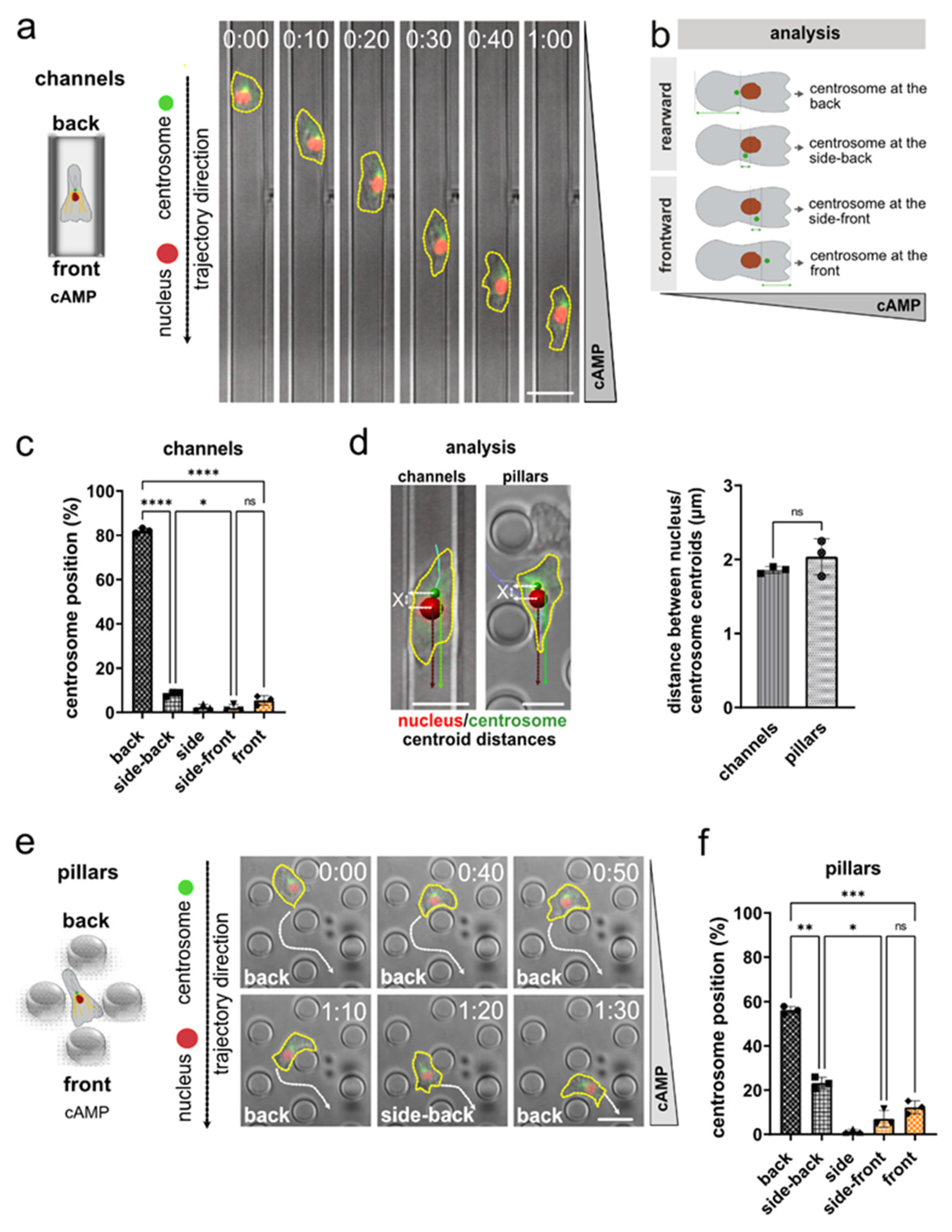
3.1. Nucleus and Centrosome Positioning in Dictyostelium Cells Migrating in Confined Environments
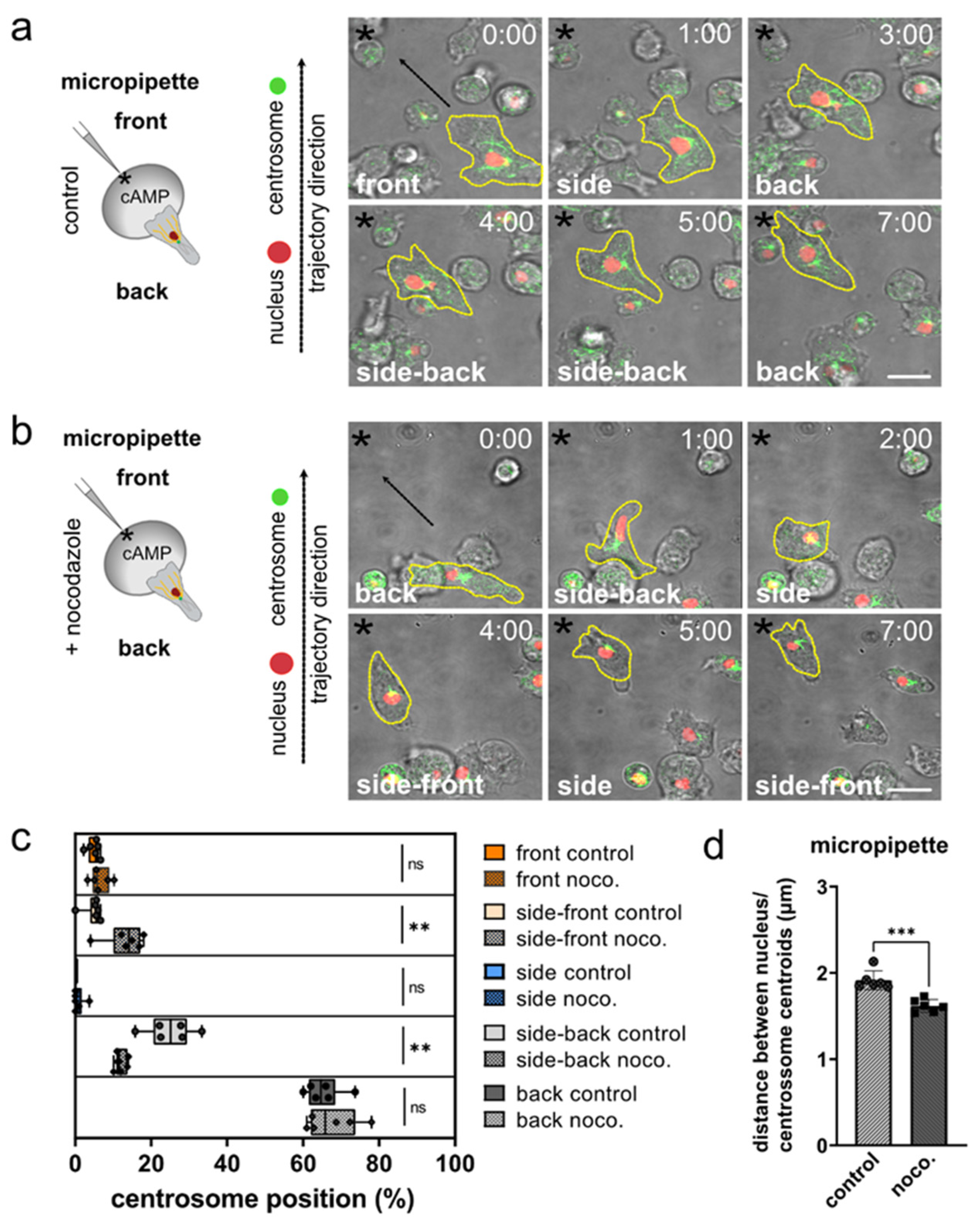
3.2. The Role of Microtubules for Centrosome Positioning in Dictyostelium Cells Migrating in 2D Confined Environments
3.3. Nucleus and Centrosome Positioning in Dictyostelium Cells Migrating in 3D Confined Environments
3.4. Nucleus and Centrosome Positioning in Dictyostelium Cells Migrating in Confined Environments toward Folate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SenGupta, S.; Parent, C.A.; Bear, J.E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 2021, 22, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, Y.; Lampert, T.J.; Devreotes, P.N. Moving towards a paradigm: Common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell Mol. Life Sci. 2014, 71, 3711–3747. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef]
- Kameritsch, P.; Renkawitz, J. Principles of Leukocyte Migration Strategies. Trends Cell Biol. 2020, 30, 818–832. [Google Scholar] [CrossRef]
- Lämmermann, T.; Bader, B.L.; Monkley, S.J.; Worbs, T.; Wedlich-Söldner, R.; Hirsch, K.; Keller, M.; Förster, R.; Critchley, D.R.; Fässler, R.; et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 2008, 453, 51–55. [Google Scholar] [CrossRef]
- Zatulovskiy, E.; Tyson, R.; Bretschneider, T.; Kay, R.R. Bleb-driven chemotaxis of Dictyostelium cells. J. Cell Biol. 2014, 204, 1027–1044. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Soldati, T. Dissection of amoeboid movement into two mechanically distinct modes. J. Cell Sci. 2006, 119, 3833–3844. [Google Scholar] [CrossRef] [Green Version]
- Renkawitz, J.; Schumann, K.; Weber, M.; Lämmermann, T.; Pflicke, H.; Piel, M.; Polleux, J.; Spatz, J.P.; Sixt, M. Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol. 2009, 11, 1438–1443. [Google Scholar] [CrossRef]
- Tyson, R.A.; Zatulovskiy, E.; Kay, R.R.; Bretschneider, T. How blebs and pseudopods cooperate during chemotaxis. Proc. Natl. Acad. Sci. USA 2014, 111, 11703–11708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, N.; Traynor, D.; Piel, M.; Kabla, A.J.; Kay, R.R. Pressure sensing through Piezo channels controls whether cells migrate with blebs or pseudopods. Proc. Natl. Acad. Sci. USA 2020, 117, 2506–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Taubenberger, A.; Kortholt, A.; Eichinger, L. Simple system-substantial share: The use of Dictyostelium in cell biology and molecular medicine. Eur. J. Cell Biol. 2013, 92, 45–53. [Google Scholar] [CrossRef]
- Storey, C.L.; Williams, R.S.B.; Fisher, P.R.; Annesley, S.J. Dictyostelium discoideum: A Model System for Neurological Disorders. Cells 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xu, X.; Fang, J.; Isik, N.; Yan, J.; Brzostowski, J.A.; Hereld, D. How human leukocytes track down and destroy pathogens: Lessons learned from the model organism Dictyostelium discoideum. Immunol. Res. 2009, 43, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Devreotes, P.N.; Zigmond, S.H. Chemotaxis in eukaryotic cells: A focus on leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 1988, 4, 649–686. [Google Scholar] [CrossRef]
- Swaney, K.F.; Huang, C.H.; Devreotes, P.N. Eukaryotic chemotaxis: A network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 2010, 39, 265–289. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Devreotes, P.N. Moving in the right direction: How eukaryotic cells migrate along chemical gradients. Semin Cell Dev Biol 2011, 22, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Van Haastert, P.J.; Devreotes, P.N. Chemotaxis: Signalling the way forward. Nat. Rev. Mol. Cell Biol. 2004, 5, 626–634. [Google Scholar] [CrossRef]
- Insall, R.H. Understanding eukaryotic chemotaxis: A pseudopod-centred view. Nat. Rev. Mol. Cell Biol. 2010, 11, 453–458. [Google Scholar] [CrossRef]
- Pan, P.; Hall, E.M.; Bonner, J.T. Folic acid as second chemotactic substance in the cellular slime moulds. Nat. New. Biol. 1972, 237, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Konijn, T.M.; Van De Meene, J.G.; Bonner, J.T.; Barkley, D.S. The acrasin activity of adenosine-3’,5’-cyclic phosphate. Proc. Natl. Acad. Sci. USA 1967, 58, 1152–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, P.S.; Sun, T.J.; Saxe, C.L., 3rd; Kimmel, A.R.; Johnson, R.L.; Devreotes, P.N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science 1988, 241, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Xu, X.; Chen, Y.; Jin, T. Identification of a Chemoattractant G-Protein-Coupled Receptor for Folic Acid that Controls Both Chemotaxis and Phagocytosis. Dev. Cell 2016, 36, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, A.; Kapitein, L.C. Mechanisms of microtubule organization in differentiated animal cells. Nat. Rev. Mol. Cell Biol. 2022, 1–18. [Google Scholar] [CrossRef]
- Kaverina, I.; Straube, A. Regulation of cell migration by dynamic microtubules. Semin. Cell Dev. Biol. 2011, 22, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.K.; Lam, P.Y.; Eichelberg, M.R.; Zasadil, L.; Bement, W.M.; Huttenlocher, A. The role of microtubules in neutrophil polarity and migration in live zebrafish. J. Cell Sci. 2012, 125, 5702–5710. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Manneville, S. Actin and microtubules in cell motility: Which one is in control? Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Van Keymeulen, A.; Rentel, M.; Bourne, H.R. Neutrophil microtubules suppress polarity and enhance directional migration. Proc. Natl. Acad. Sci. USA 2005, 102, 6884–6889. [Google Scholar] [CrossRef] [Green Version]
- Euteneuer, U.; Schliwa, M. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature 1984, 310, 58–61. [Google Scholar] [CrossRef]
- Ganguly, A.; Yang, H.; Sharma, R.; Patel, K.D.; Cabral, F. The role of microtubules and their dynamics in cell migration. J. Biol. Chem. 2012, 287, 43359–43369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopf, A.; Kiermaier, E. Dynamic Microtubule Arrays in Leukocytes and Their Role in Cell Migration and Immune Synapse Formation. Front. Cell Dev. Biol. 2021, 9, 635511. [Google Scholar] [CrossRef] [PubMed]
- Luxton, G.W.; Gundersen, G.G. Orientation and function of the nuclear-centrosomal axis during cell migration. Curr. Opin. Cell Biol. 2011, 23, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundersen, G.G.; Worman, H.J. Nuclear positioning. Cell 2013, 152, 1376–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros-Becker, F.; Lam, P.Y.; Fisher, R.; Huttenlocher, A. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. J. Cell Sci. 2017, 130, 3801–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudima, G.O.; Vorobjev, I.A.; Chentsov Yu, S. Centriolar location during blood cell spreading and motion in vitro: An ultrastructural analysis. J. Cell Sci. 1988, 89, 225–241. [Google Scholar] [CrossRef]
- Gudima, G.O.; Vorob’ev, I.A.; Chentsov Iu, S. Cell center of macrophages, granulocytes and lymphocytes during in vitro cell spreading, polarization and movement. Tsitologiia 1984, 26, 1002–1007. [Google Scholar]
- Kopf, A.; Renkawitz, J.; Hauschild, R.; Girkontaite, I.; Tedford, K.; Merrin, J.; Thorn-Seshold, O.; Trauner, D.; Hacker, H.; Fischer, K.D.; et al. Microtubules control cellular shape and coherence in amoeboid migrating cells. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Renkawitz, J.; Kopf, A.; Stopp, J.; de Vries, I.; Driscoll, M.K.; Merrin, J.; Hauschild, R.; Welf, E.S.; Danuser, G.; Fiolka, R.; et al. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature 2019, 568, 546–550. [Google Scholar] [CrossRef]
- Uzbekov, R.E.; Vorob’ev, I.A.; Drachev, V.A. The effect of the laser microirradiation of the cell center on neutrophil motility. Tsitologiia 1989, 31, 874–881. [Google Scholar]
- Eddy, R.J.; Pierini, L.M.; Maxfield, F.R. Microtubule asymmetry during neutrophil polarization and migration. Mol. Biol. Cell 2002, 13, 4470–4483. [Google Scholar] [CrossRef] [Green Version]
- Gräf, R.; Batsios, P.; Meyer, I. Evolution of centrosomes and the nuclear lamina: Amoebozoan assets. Eur. J. Cell Biol. 2015, 94, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Gräf, R.; Grafe, M.; Meyer, I.; Mitic, K.; Pitzen, V. The Dictyostelium Centrosome. Cells 2021, 10, 2657. [Google Scholar] [CrossRef] [PubMed]
- Schulz, I.; Baumann, O.; Samereier, M.; Zoglmeier, C.; Gräf, R. Dictyostelium Sun1 is a dynamic membrane protein of both nuclear membranes and required for centrosomal association with clustered centromeres. Eur. J. Cell Biol. 2009, 88, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Rivero, F.; Euteneuer, U.; Mondal, S.; Mana-Capelli, S.; Larochelle, D.; Vogel, A.; Gassen, B.; Noegel, A.A. Dictyostelium Sun-1 connects the centrosome to chromatin and ensures genome stability. Traffic 2008, 9, 708–724. [Google Scholar] [CrossRef]
- Mana-Capelli, S.; Gräf, R.; Larochelle, D.A. Dictyostelium discoideum CenB is a bona fide centrin essential for nuclear architecture and centrosome stability. Eukaryot Cell 2009, 8, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Kuhnert, O.; Baumann, O.; Meyer, I.; Gräf, R. Functional characterization of CP148, a novel key component for centrosome integrity in Dictyostelium. Cell Mol. Life Sci. 2012, 69, 1875–1888. [Google Scholar] [CrossRef]
- Tikhonenko, I.; Magidson, V.; Gräf, R.; Khodjakov, A.; Koonce, M.P. A kinesin-mediated mechanism that couples centrosomes to nuclei. Cell Mol. Life Sci. 2013, 70, 1285–1296. [Google Scholar] [CrossRef] [Green Version]
- Sameshima, M. The orientation of nucleus, nucleus-associated body and protruding nucleolus in aggregating Dictyostelium discoideum. Exp. Cell Res. 1985, 156, 341–350. [Google Scholar] [CrossRef]
- Sameshima, M.; Imai, Y.; Hashimoto, Y. The position of the microtubule-organizing center relative to the nucleus is independent of the direction of cell migration in Dictyostelium discoideum. Cell Motil. Cytoskelet. 1988, 9, 111–116. [Google Scholar] [CrossRef]
- Ueda, M.; Gräf, R.; MacWilliams, H.K.; Schliwa, M.; Euteneuer, U. Centrosome positioning and directionality of cell movements. Proc. Natl. Acad. Sci. USA 1997, 94, 9674–9678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neujahr, R.; Albrecht, R.; Köhler, J.; Matzner, M.; Schwartz, J.M.; Westphal, M.; Gerisch, G. Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J. Cell Sci. 1998, 111, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Koonce, M.P.; Tikhonenko, I. Centrosome Positioning in Dictyostelium: Moving beyond Microtubule Tip Dynamics. Cells 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odell, J.; Sikirzhytski, V.; Tikhonenko, I.; Cobani, S.; Khodjakov, A.; Koonce, M. Force balances between interphase centrosomes as revealed by laser ablation. Mol Biol Cell 2019, 30, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Bindl, J.; Molnar, E.S.; Ecke, M.; Prassler, J.; Müller-Taubenberger, A.; Gerisch, G. Unilateral Cleavage Furrows in Multinucleate Cells. Cells 2020, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Renkawitz, J.; Reversat, A.; Leithner, A.; Merrin, J.; Sixt, M. Micro-engineered “pillar forests” to study cell migration in complex but controlled 3D environments. Methods Cell Biol. 2018, 147, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Ruiz-Fernandez, M.J.A.; Braun, M.B.; Merrin, J.; Renkawitz, J. Quantifying the Probing and Selection of Microenvironmental Pores by Motile Immune Cells. Curr. Protoc. 2022, 2, e407. [Google Scholar] [CrossRef]
- Roth, H.; Samereier, M.; Trommler, G.; Noegel, A.A.; Schleicher, M.; Müller-Taubenberger, A. Balanced cortical stiffness is important for efficient migration of Dictyostelium cells in confined environments. Biochem. Biophys. Res. Commun. 2015, 467, 730–735. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gerisch, G. Imaging actin cytoskeleton dynamics in Dictyostelium chemotaxis. Methods Mol. Biol. 2009, 571, 385–400. [Google Scholar] [CrossRef]
- Gräf, R.; Euteneuer, U.; Ho, T.H.; Rehberg, M. Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 2003, 14, 4067–4074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornens, M. Organelle positioning and cell polarity. Nat. Rev. Mol. Cell Biol. 2008, 9, 874–886. [Google Scholar] [CrossRef] [PubMed]
- McGregor, A.L.; Hsia, C.R.; Lammerding, J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 2016, 40, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luxton, G.W.; Gomes, E.R.; Folker, E.S.; Vintinner, E.; Gundersen, G.G. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 2010, 329, 956–959. [Google Scholar] [CrossRef] [Green Version]
- Janota, C.S.; Calero-Cuenca, F.J.; Costa, J.; Gomes, E.R. SnapShot: Nucleo-cytoskeletal Interactions. Cell 2017, 169, 970–971. [Google Scholar] [CrossRef]
- Infante, E.; Castagnino, A.; Ferrari, R.; Monteiro, P.; Aguera-Gonzalez, S.; Paul-Gilloteaux, P.; Domingues, M.J.; Maiuri, P.; Raab, M.; Shanahan, C.M.; et al. LINC complex-Lis1 interplay controls MT1-MMP matrix digest-on-demand response for confined tumor cell migration. Nat. Commun. 2018, 9, 2443. [Google Scholar] [CrossRef] [Green Version]
- Marks, P.C.; Petrie, R.J. Push or pull: How cytoskeletal crosstalk facilitates nuclear movement through 3D environments. Phys. Biol. 2022, 19, 021003. [Google Scholar] [CrossRef]
- Calero-Cuenca, F.J.; Janota, C.S.; Gomes, E.R. Dealing with the nucleus during cell migration. Curr. Opin. Cell Biol. 2018, 50, 35–41. [Google Scholar] [CrossRef]
- Barzilai, S.; Yadav, S.K.; Morrell, S.; Roncato, F.; Klein, E.; Stoler-Barak, L.; Golani, O.; Feigelson, S.W.; Zemel, A.; Nourshargh, S.; et al. Leukocytes Breach Endothelial Barriers by Insertion of Nuclear Lobes and Disassembly of Endothelial Actin Filaments. Cell Rep. 2017, 18, 685–699. [Google Scholar] [CrossRef] [Green Version]
- Willard, S.S.; Devreotes, P.N. Signaling pathways mediating chemotaxis in the social amoeba, Dictyostelium discoideum. Eur J Cell Biol. 2006, 85, 897–904. [Google Scholar] [CrossRef]
- King, J.S.; Insall, R.H. Chemotaxis: Finding the way forward with Dictyostelium. Trends Cell Biol. 2009, 19, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.R.; Langridge, P.; Traynor, D.; Hoeller, O. Changing directions in the study of chemotaxis. Nat. Rev. Mol Cell Biol. 2008, 9, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Veltman, D.; Kay, R.R. Chemotaxis of a model organism: Progress with Dictyostelium. Curr. Opin. Cell Biol. 2015, 36, 7–12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa-Ankerhold, H.; Kroll, J.; Heuvel, D.v.d.; Renkawitz, J.; Müller-Taubenberger, A. Centrosome Positioning in Migrating Dictyostelium Cells. Cells 2022, 11, 1776. https://doi.org/10.3390/cells11111776
Ishikawa-Ankerhold H, Kroll J, Heuvel Dvd, Renkawitz J, Müller-Taubenberger A. Centrosome Positioning in Migrating Dictyostelium Cells. Cells. 2022; 11(11):1776. https://doi.org/10.3390/cells11111776
Chicago/Turabian StyleIshikawa-Ankerhold, Hellen, Janina Kroll, Dominic van den Heuvel, Jörg Renkawitz, and Annette Müller-Taubenberger. 2022. "Centrosome Positioning in Migrating Dictyostelium Cells" Cells 11, no. 11: 1776. https://doi.org/10.3390/cells11111776
APA StyleIshikawa-Ankerhold, H., Kroll, J., Heuvel, D. v. d., Renkawitz, J., & Müller-Taubenberger, A. (2022). Centrosome Positioning in Migrating Dictyostelium Cells. Cells, 11(11), 1776. https://doi.org/10.3390/cells11111776






