Peroxisome Metabolism Contributes to PIEZO2-Mediated Mechanical Allodynia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Hot Plate Testing
2.3. Von Frey Filament Testing Mechanical Sensitivity
2.4. Brush Assay Test
2.5. Satellite Glial Cell Culture
2.6. Immortalized Rodent DRG Neuron Culture and Lipid Supplementation
2.7. Western Blotting
2.8. Immunofluorescence Staining and Confocal Microscopy Imaging
2.9. Lipid Analysis
2.10. Transcriptome Sequencing
2.11. Enrichment Analysis
2.12. Quantitative Real-Time Reverse-Transcription PCR
2.13. Intrathecal Injection of D-GsMTx4
2.14. Statistical Analysis
3. Results
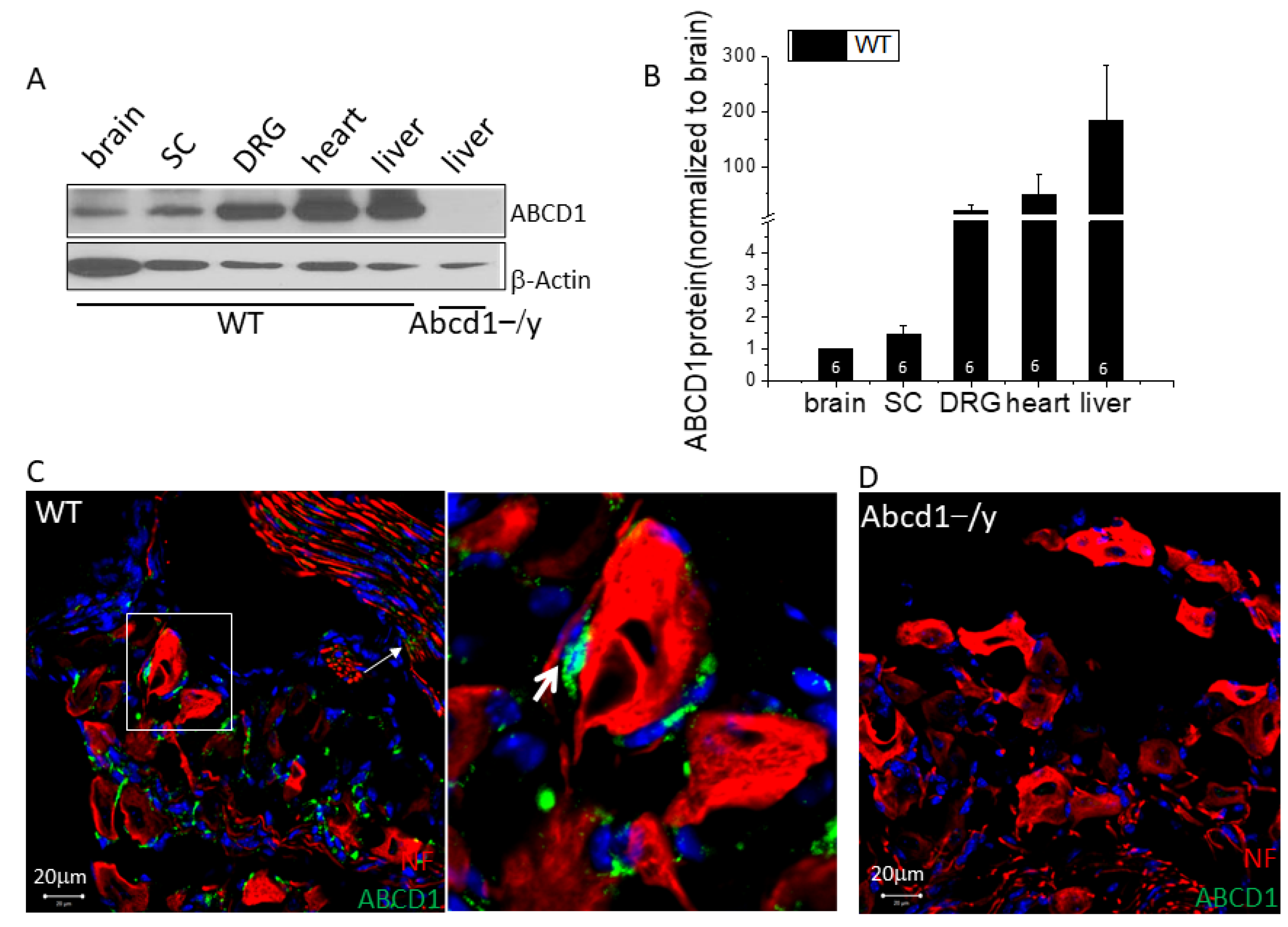
3.1. ABCD1 Expression in DRG Tissue
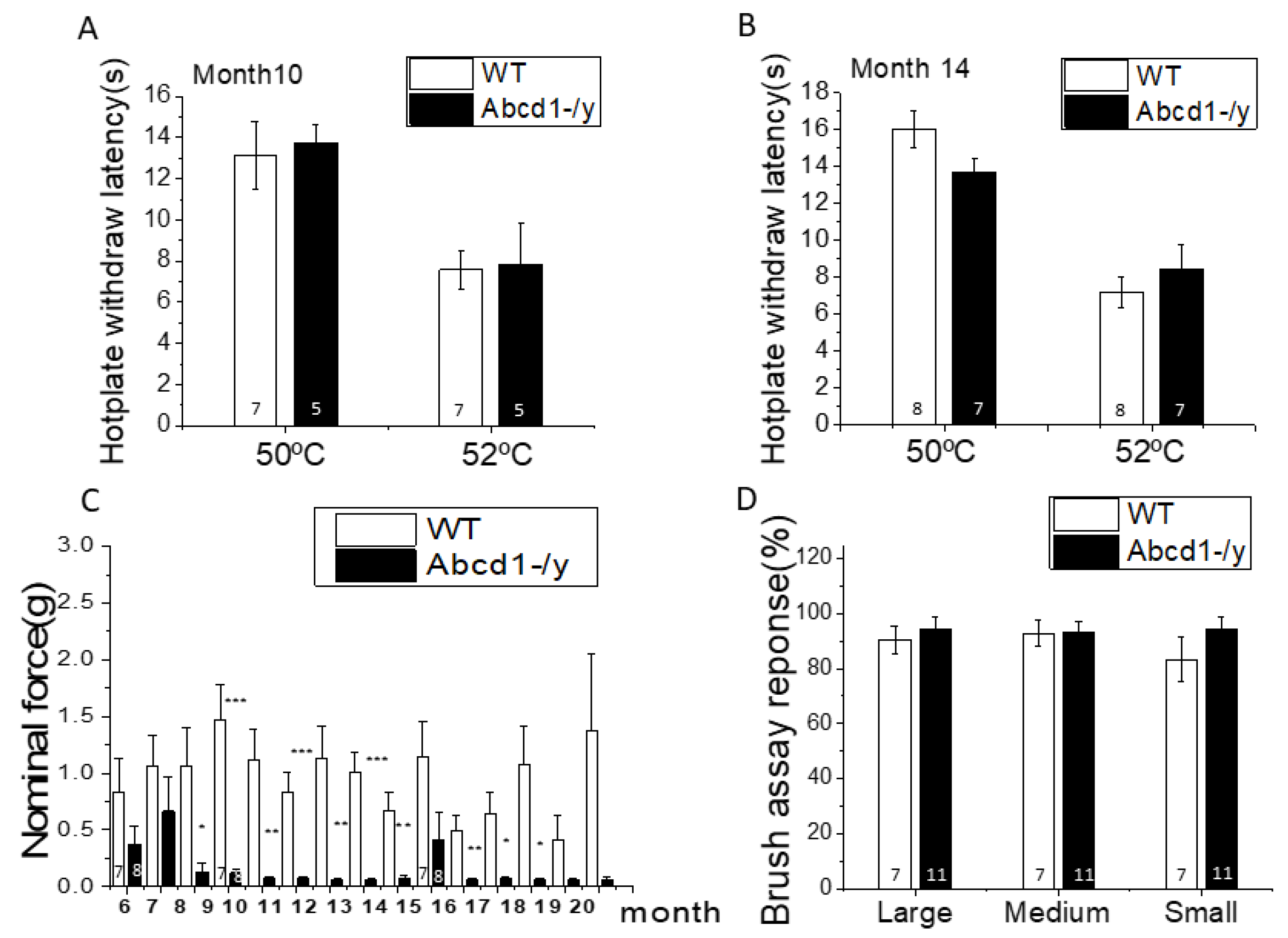
3.2. Nociceptive Behavior in Abcd1−/y Mice
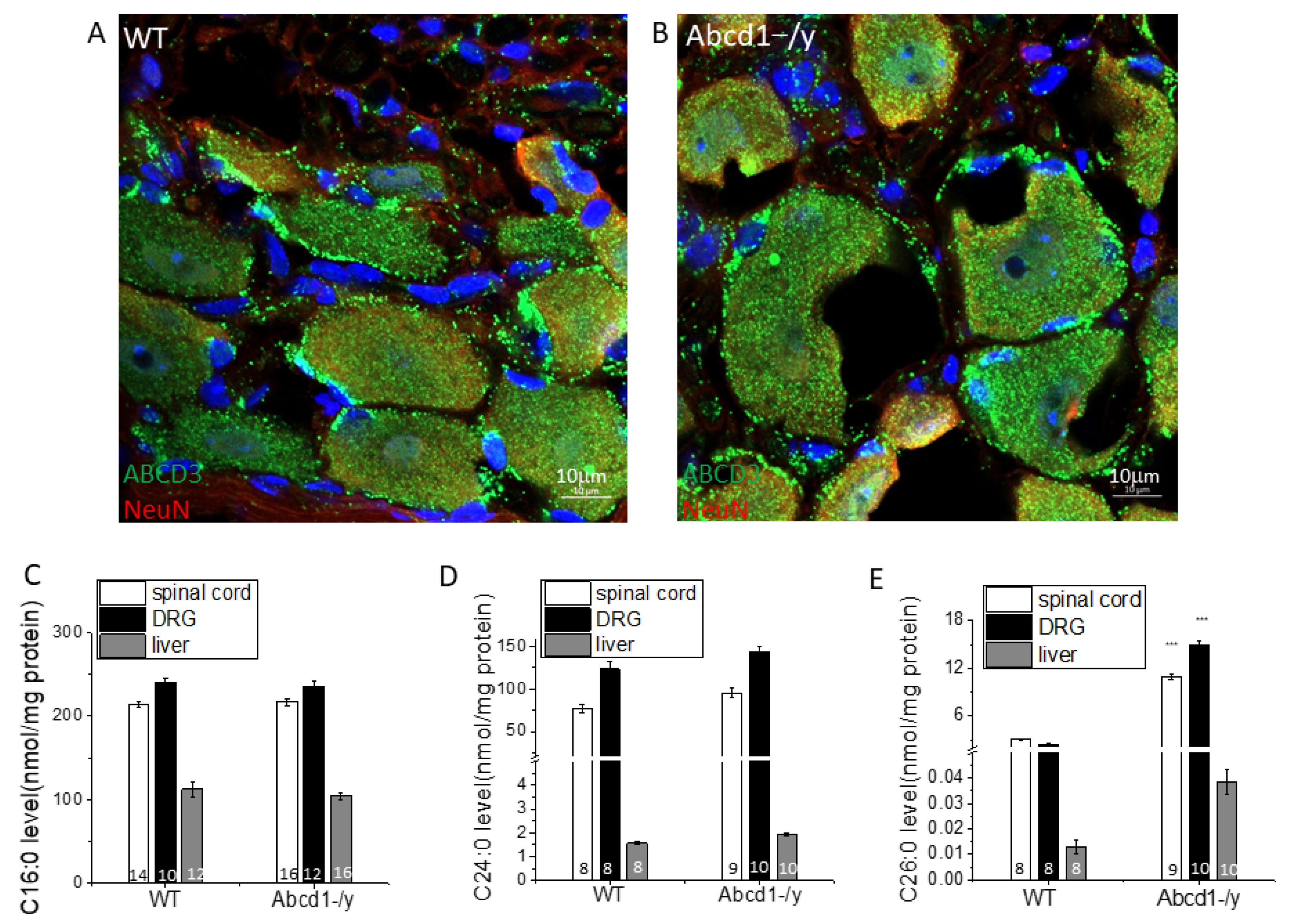
3.3. Peroxisomal Dysfunction in Abcd1−/y Mice
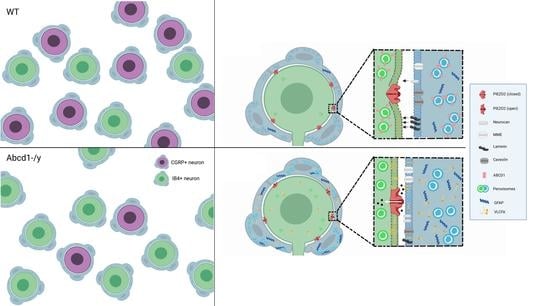
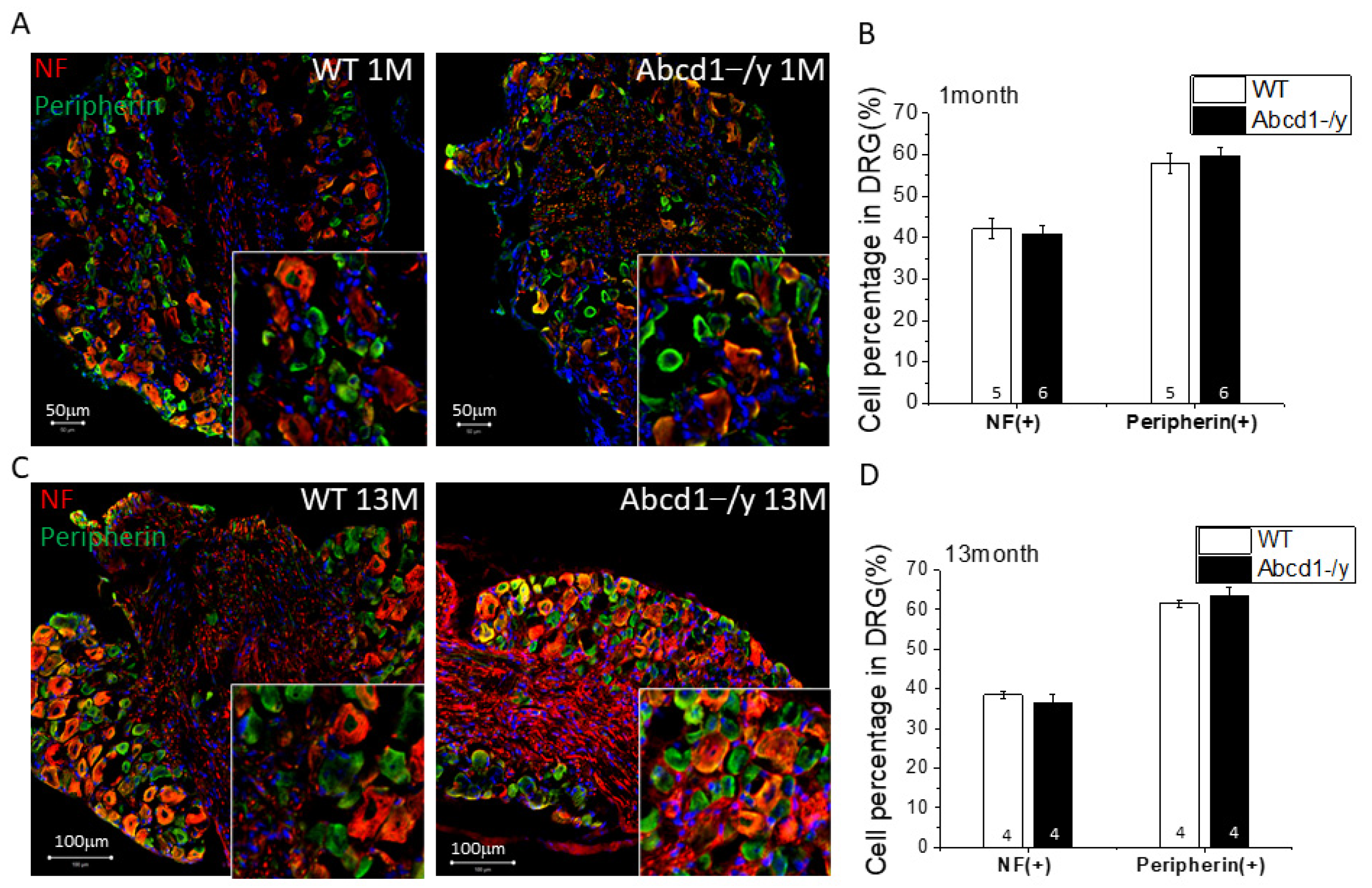
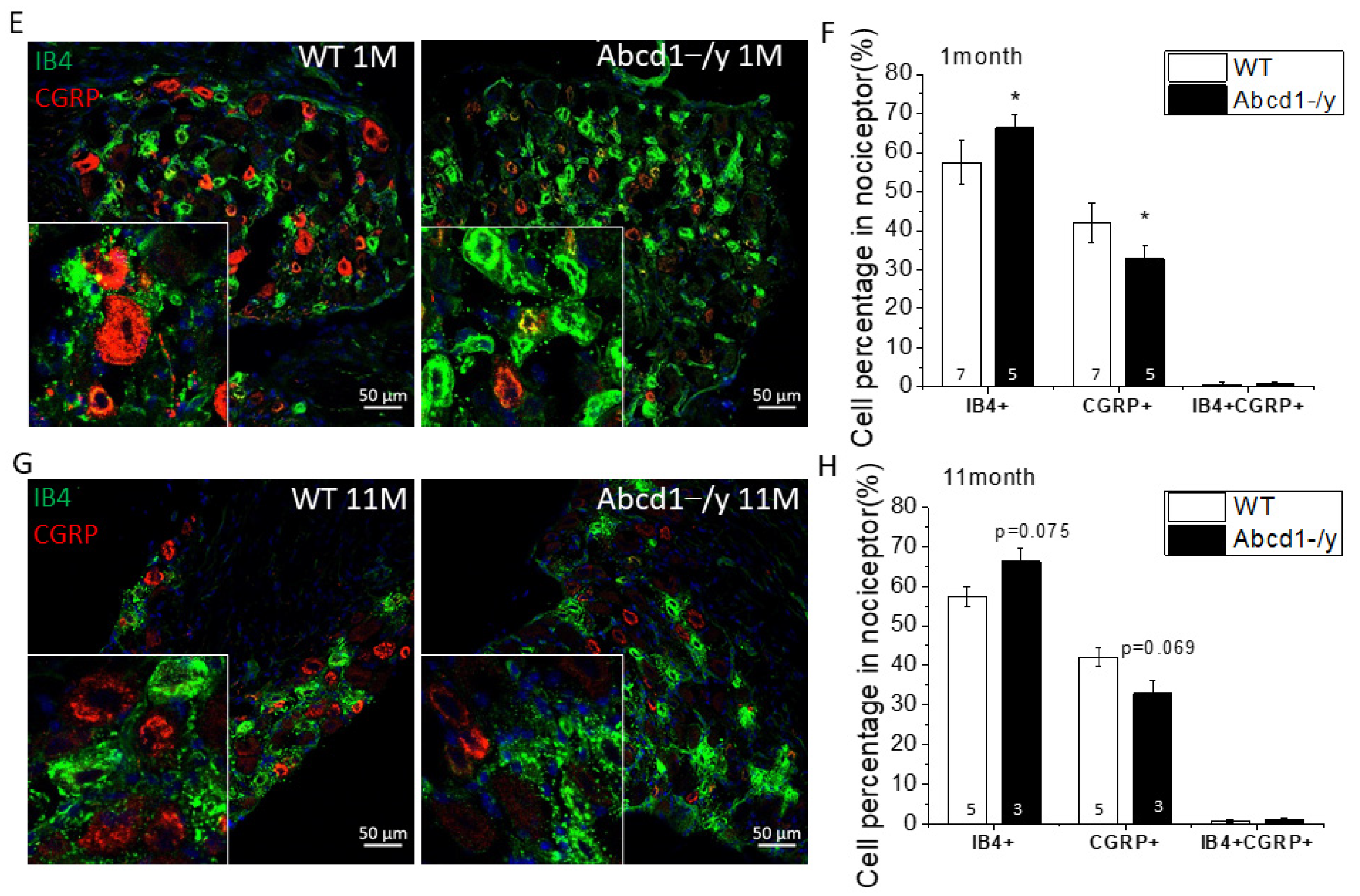
3.4. Neuron Subtype Distribution in DRG
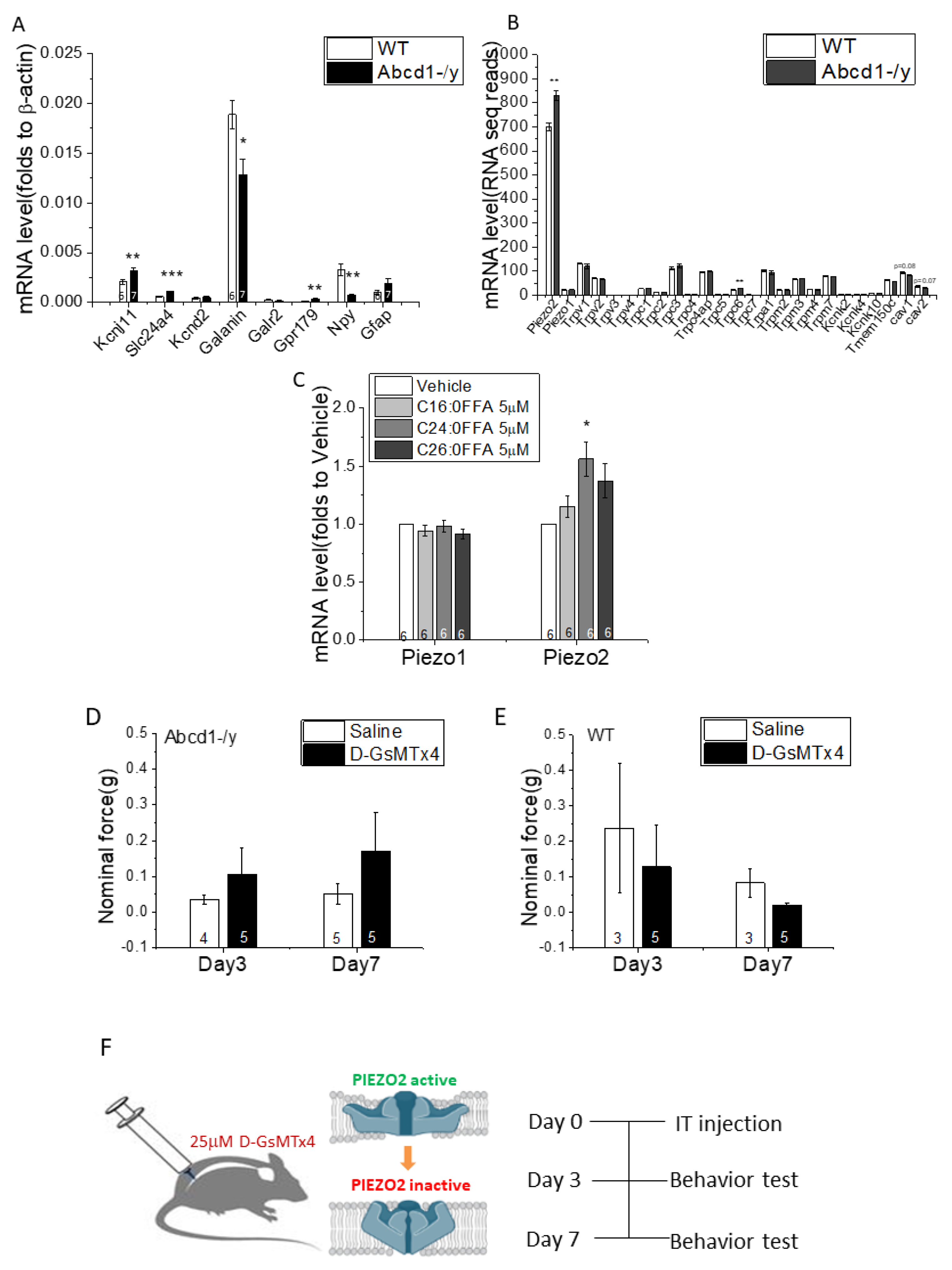
3.5. DRG Transcriptomic Analysis
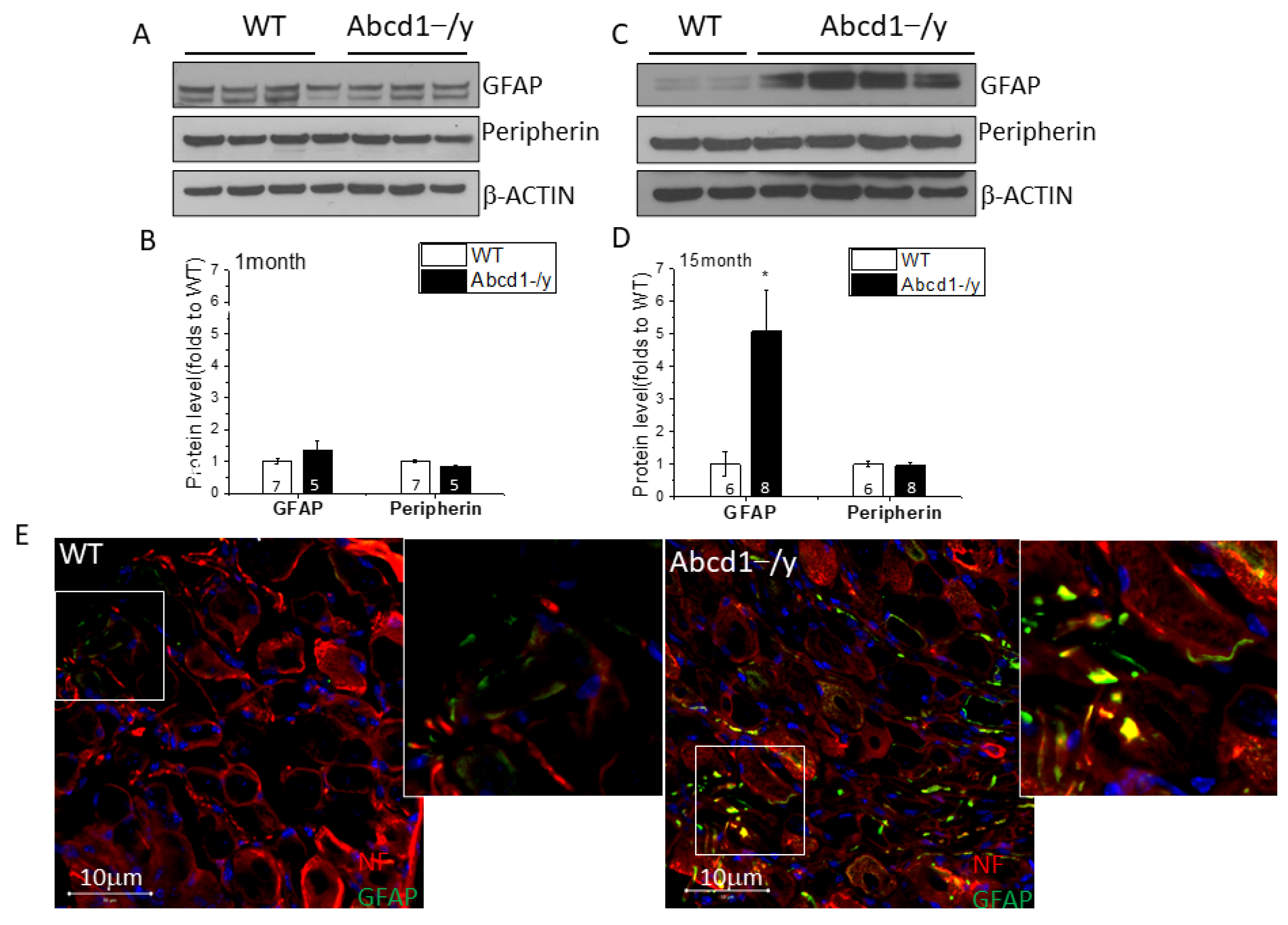
3.6. Increased GFAP Expression in DRG Tissue in the Absence of ABCD1
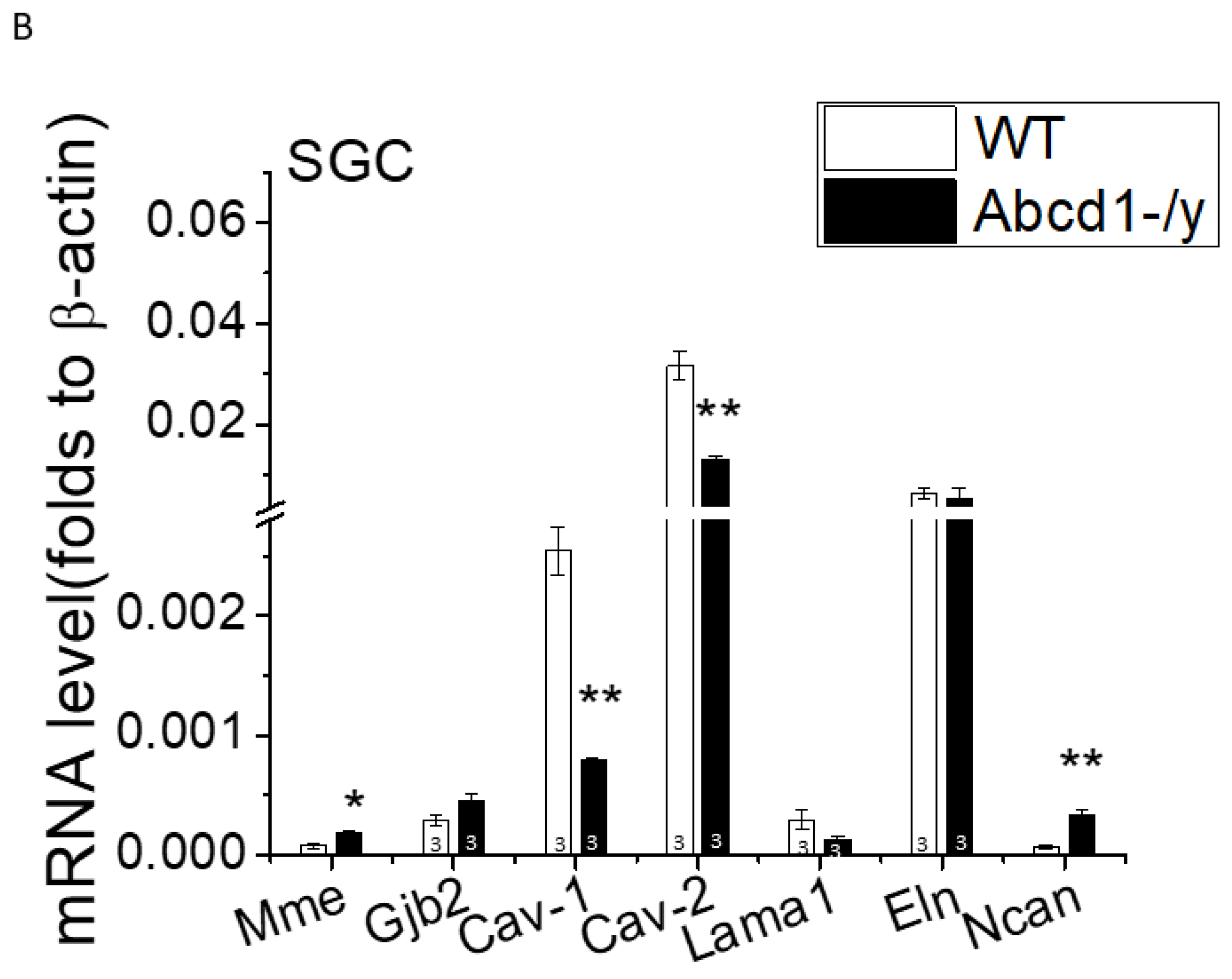
3.7. Increased GFAP Expression and VLCFA Accumulation in Abcd1−/y SGCs
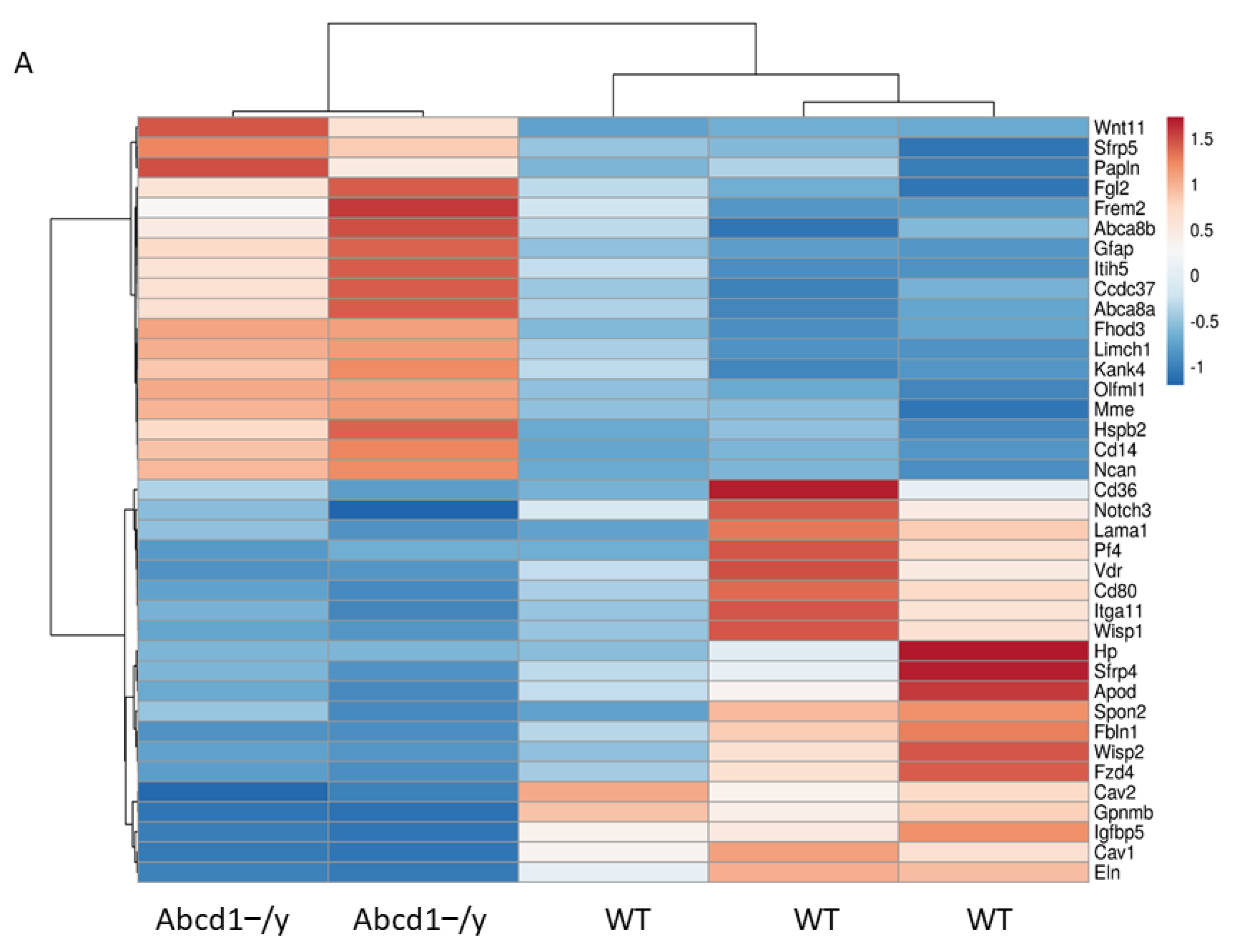
3.8. SGC Transcriptomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huffnagel, I.C.; Van Ballegoij, W.J.C.; Van Geel, B.M.; Vos, J.; Kemp, S.; Engelen, M. Progression of myelopathy in males with adrenoleukodystrophy: Towards clinical trial readiness. Brain 2019, 142, 334–343. [Google Scholar] [CrossRef]
- Huffnagel, I.C.; Dijkgraaf, M.G.W.; Janssens, G.E.; Van Weeghel, M.; Van Geel, B.M.; Poll-The, B.T.; Kemp, S.; Engelen, M. Disease progression in women with X-linked adrenoleukodystrophy is slow. Orphanet J. Rare Dis. 2019, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Bachiocco, V.; Cappa, M.; Petroni, A.; Salsano, E.; Bizzarri, C.; Ceccarelli, I.; Cevenini, G.; Pensato, V.; Aloisi, A.M. Pain study in x-linked adrenoleukodystrophy in males and females. Pain Ther. 2021, 10, 505–523. [Google Scholar] [CrossRef]
- Mosser, J.; Douar, A.M.; Sarde, C.O.; Kioschis, P.; Feil, R.; Moser, H.; Poustka, A.M.; Mandel, J.L.; Aubourg, P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993, 361, 726–730. [Google Scholar] [CrossRef]
- Moser, A.B.; Kreiter, N.; Bezman, L.; Lu, S.E.; Raymond, G.V.; Naidu, S.; Moser, H.W. Plasma very long chain fatty acids in 3000 peroxisome disease patients and 29,000 controls. Ann. Neurol. 1999, 45, 100–110. [Google Scholar] [CrossRef]
- Valianpour, F.; Selhorst, J.J.; Van Lint, L.E.; Van Gennip, A.H.; Wanders, R.J.; Kemp, S. Analysis of very long-chain fatty acids using electrospray ionization mass spectrometry. Mol. Genet. Metab. 2003, 79, 189–196. [Google Scholar] [CrossRef]
- Engelen, M.; Kemp, S.; De Visser, M.; van Geel, B.M.; Wanders, R.J.; Aubourg, P. X-linked adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J. Rare Dis. 2012, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Forss-Petter, S.; Eichler, F.S. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 2014, 98, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, S.; Huffnagel, I.C.; Linthorst, G.E.; Wanders, R.J.; Engelen, M. Adrenoleukodystrophy—Neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 2016, 12, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.K.; Moser, H.; Kishimoto, Y.; Hamilton, J.A. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J. Clin. Invest. 1995, 96, 1455–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoftberger, R.; Kunze, M.; Weinhofer, I.; Aboul-Enein, F.; Voigtländer, T.; Oezen, I.; Amann, G.; Bernheimer, H.; Budka, H.; Berger, J. Bernheimer Distribution and cellular localization of adrenoleukodystrophy protein in human tissues: Implications for X-linked adrenoleukodystrophy. Neurobiol. Dis. 2007, 28, 165–174. [Google Scholar] [CrossRef]
- Powers, J.M.; Deciero, D.P.; Cox, C.; Richfield, E.K.; Ito, M.; Moser, A.B.; Moser, H.W. The dorsal root ganglia in adrenomyeloneuropathy: Neuronal atrophy and abnormal mitochondria. J. Neuropathol. Exp. Neurol. 2001, 60, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Forss-Petter, S.; Werner, H.; Berger, J.; Lassmann, H.; Molzer, B.; Schwab, M.H.; Bernheimer, H.; Zimmermann, F.; Nave, K.A. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci. Res. 1997, 50, 829–843. [Google Scholar] [CrossRef]
- Broom, D.C.; Samad, T.A.; Kohno, T.; Tegeder, I.; Geisslinger, G.; Woolf, C.J. Cyclooxygenase 2 expression in the spared nerve injury model of neuropathic pain. Neuroscience 2004, 124, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.N.; Larsen, F.; Duroux, M.; Gazerani, P. Primary culture of trigeminal satellite glial cells: A cell-based platform to study morphology and function of peripheral glia. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 1–12. [Google Scholar]
- Chen, W.; Mi, R.; Haughey, N.; Oz, M.; Hoke, A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J. Peripher. Nerv. Syst. 2007, 12, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, S.; Wei, H.M.; Lu, J.F.; Bratierman, L.T.; McGuinness, M.C.; Moser, A.B.; Watkins, P.A.; Smith, K.D. Gene redundancy and pharmacological gene therapy: Implications for X-linked adrenoleukodystrophy. Nat. Med. 1998, 4, 1261–1268. [Google Scholar] [CrossRef]
- Pujol, A.; Ferrer, I.; Camps, C.; Metzger, E.; Hindel, C.; Callizot, N.; Ruiz, M.; Pàmpols, T.; Giròs, M.; Louis Mandel, J. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: A therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 2004, 13, 2997–3006. [Google Scholar] [CrossRef] [Green Version]
- Sleigh, J.N.; Dawes, J.M.; West, S.J.; Wei, N.; Spaulding, E.L.; Gómez-Martín, A.; Zhang, Q.; Burgess, R.W.; Zameel Cader, M.; Talbot, K.; et al. Trk receptor signaling and sensory neuron fate are perturbed in human neuropathy caused by Gars mutations. Proc. Natl. Acad. Sci. USA 2017, 114, E3324–E3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, Y.; Geng, J.; Zhou, S.; Xiao, B. Mechanically activated piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep. 2019, 26, 1419–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelman, J.W.; Grant, N.R.; Molay, F.; Stephen, C.D.; Sadjadi, R.; Eichler, F.S. Restless legs syndrome in X-linked adrenoleukodystrophy. Sleep Med. 2022, 91, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, R.; Yang, J.; Zhao, Y.; Qi, C.; Bian, G.; Wang, M.; Shan, J.; Wang, C.; Wang, D.; et al. Chronic pain induces nociceptive neurogenesis in dorsal root ganglia from Sox2-positive satellite cells. Glia 2019, 67, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.D.; Suter, M.R.; Ji, R.R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154, S10–S28. [Google Scholar] [CrossRef] [PubMed]
- Van de Beek, M.C.; Ofman, R.; Dijkstra, I.; Wijburg, F.; Engelen, M.; Wanders, R.; Kemp, S. Lipid-induced endoplasmic reticulum stress in X-linked adrenoleukodystrophy. Biochim. Et Biophys. Acta Mol. Basis Disease 2017, 1863, 2255–2265. [Google Scholar] [CrossRef]
- Du, X.; Gamper, N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013, 11, 621–640. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.; Sun, K.; Meng, Z.; Chen, L. The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J. Mol. Cell. Biol. 2019, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229. [Google Scholar] [CrossRef]
- Szczot, M.; Liljencrantz, J.; Ghitani, N.; Barik, A.; Lam, R.; Thompson, J.H.; Bharucha-Goebel, D.; Saade, D.; Necaise, A.; Donkervoort, S.; et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 2018, 10(462), eaat9892. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016, 7, 10366. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bae, C.; Sachs, F.; Suchyna, T.M. Caveolae regulation of mechanosensitive channel function in myotubes. PLoS ONE. 2013, 8, e72894. [Google Scholar] [CrossRef]
- Diem, K.; Fauler, M.; Fois, G.; Hellmann, A.; Winokurow, N.; Schumacher, S.; Kranz, C.; Frick, M. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 2020, 34, 12785–12804. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Ghatak, C.; Yasmann, A.; Nishizawa, K.; Sachs, F.; Ladokhin, A.S.; Sukharev, S.I.; Suchyna, T.M. GsMTx4: Mechanism of inhibiting mechanosensitive ion channels. Biophys. J. 2017, 112, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, T.; Cranfield, C.G.; Deplazes, E.; Owen, D.M.; Macmillan, A.; Battle, A.R.; Constantine, M.; Sokabe, M.; Martinac, B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc. Natl. Acad. Sci. USA 2012, 109, 8770–8775. [Google Scholar] [CrossRef] [Green Version]
- Kassmann, C.M.; Lappe-Siefke, C.; Baes, M.; Brügger, B.; Mildner, A.; Werner, H.B.; Natt, O.; Michaelis, T.; Prinz, M.; Grahm, J. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007, 39, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Hanani, M. Role of satellite glial cells in gastrointestinal pain. Front. Cell. Neurosci. 2015, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Ohara, P.T.; Vit, J.P.; Bhargava, A.; Romero, M.; Sundberg, C.; Charles, A.C.; Jasmin, L. Gliopathic pain: When satellite glial cells go bad. Neuroscientist 2009, 15, 450–463. [Google Scholar] [CrossRef] [Green Version]
- Avraham, O.; Den, P.Y.; Jones, S.; Kuruvilla, R.; Semenkovich, C.F.; Klyachko, V.A.; Cavalli, V. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 2020, 11, 4891. [Google Scholar] [CrossRef]
- Christie, K.; Koshy, D.; Cheng, C.; Guo, G.; Martinez, J.A.; Duraikannu, A.; Zochodne, D.W. Intraganglionic interactions between satellite cells and adult sensory neurons. Mol. Cell. Neurosci. 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef]
- Goodman, M.B. Mechanosensation. In WormBook: The Online Review of C Elegans Biology; WormBook: Pasadena, CA, USA, 2006; pp. 1–14. [Google Scholar]
- Nourse, J.L.; Pathak, M.M. How cells channel their stress: Interplay between Piezo1 and the cytoskeleton. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2017; Volume 71, pp. 3–12. [Google Scholar]
- Zoga, V.; Kawano, T.; Liang, M.Y.; Bienengraeber, M.; Weihrauch, D.; McCallum, B.; Gemes, G.; Hogan, Q.; Sarantopoulos, C. KATP channel subunits in rat dorsal root ganglia: Alterations by painful axotomy. Mol. Pain 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu, W.; Bjork, J.; Salo, E.; Entenmann, N.; Jurgenson, T.; Fisher, C.; Klein, A.H. Modulation of SUR1 KATP channel subunit activity in the peripheral nervous system reduces mechanical hyperalgesia after nerve injury in mice. Int. J. Mol. Sci. 2019, 20, 2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemel, B.M.; Ritter, D.M.; Covarrubias, M.; Muqeem, T. A-Type KV channels in dorsal root ganglion neurons: Diversity, function, and dysfunction. Front. Mol. Neurosci. 2018, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ch’ng, J.L.; Christofides, N.D.; Anand, P.; Gibson, S.J.; Allen, Y.S.; Su, H.C.; Tatemoto, K.; Morrison, J.F.B.; Polak, J.M.; Bloom, S.R. Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience 1985, 16, 343–354. [Google Scholar] [CrossRef]
- Skofitsch, G.; Jacobowitz, D.M. Galanin-like immunoreactivity in capsaicin sensitive sensory neurons and ganglia. Brain Res. Bull. 1985, 15, 191–195. [Google Scholar] [CrossRef]
- Liu, H.X.; Hokfelt, T. The participation of galanin in pain processing at the spinal level. Trends Pharm. Sci. 2002, 10, 468–474. [Google Scholar] [CrossRef]
- Li, S.Y.; Huo, M.L.; Wu, X.Y.; Huang, Y.Q.; Wang, L.; Zhang, X.; Jiang, Y.M.; Zhang, M.L.; Wang, L.L.; Yu, L.C. Involvement of galanin and galanin receptor 1 in nociceptive modulation in the central nucleus of amygdala in normal and neuropathic rats. Sci. Rep. 2017, 7, 15317. [Google Scholar] [CrossRef] [Green Version]


| # | Networks | p-Value | Network Objects |
|---|---|---|---|
| 1 | Muscle contraction | 1.601 × 10−4 | GALR2, K(+) channel, subfamily J, EDNRA, Galpha(i)-specific peptide GPCRs, MyHC, nAChR alpha, Galanin, Galpha(q)-specific peptide GPCRs |
| 2 | Inflammation_Interferon signaling | 3.178 × 10−3 | TIMP1, IRF7, IFI56, IL21R, IFI44 |
| 3 | Signal transduction_Neuropeptide signaling pathways | 1.326 × 10−2 | NPY, Galpha(i)-specific peptide GPCRs, Secretogranin V, Galanin, Galpha(q)-specific peptide GPCRs |
| 4 | Signal transduction_Leptin signaling | 1.619 × 10−2 | NPY, Kir6.2, TIMP1, Galanin |
| 5 | Development_Blood vessel morphogenesis | 1.695 × 10−2 | Tissue kallikreins, EDNRA, Galpha(i)-specific peptide GPCRs, RBP-J kappa (CBF1), Galpha(q)-specific peptide GPCRs, EDNRB |
| 6 | Cardiac development_FGF_ErbB signaling | 2.625 × 10−2 | Kv4.2 channel, EDNRA, MyHC, BARX2 |
| 7 | Transport_Potassium transport | 3.153 × 10−2 | K(+) channel, subfamily J, Kir6.2, Kv4.2 channel, SLC24A4, SERT |
| 8 | Apoptosis_Apoptotic mitochondria | 3.513 × 10−2 | Bcl-G, HSP70, HRK |
| 9 | Proliferation_Positive regulation cell proliferation | 5.056 × 10−2 | EDNRA, Galpha(i)-specific peptide GPCRs, TIMP1, ALK, CCKBR |
| 10 | Protein folding_ER and cytoplasm | 6.076 × 10−2 | HSC70, HSP70 |
| # | Networks | p-Value | Network Objects |
|---|---|---|---|
| 1 | Cell adhesion_Amyloid proteins | 1.453 × 10−5 | Frizzled, NOTCH3 (3ICD), SFRP4, WNT11, NOTCH3, FZD4, SR-BI, Notch, Actin cytoskeletal, WNT, Caveolin-1, Actin |
| 2 | Development_Blood vessel morphogenesis | 2.888 × 10−4 | Galpha(q)-specific amine GPCRs, Galpha(i)-specific amine GPCRs, Galpha(i)-specific peptide GPCRs, FOXC1/2, PF4, Alpha-1B adrenergic receptor, ANGPTL4, Notch, Galpha(q)-specific peptide GPCRs, FOXC1, Transferrin |
| 3 | Development_Ossification and bone remodeling | 1.463 × 10−3 | Frizzled, Follistatin, SFRP4, FOXC1/2, FOXC1, IGF-1, IBP, WNT |
| 4 | Signal transduction_ Androgen receptor signaling cross-talk | 3.145 × 10−3 | Frizzled, IL-6, IGF-1, WNT, Caveolin-1 |
| 5 | Cell adhesion_Cadherins | 3.809 × 10−3 | WISP1, Frizzled, WISP2, SFRP4, Actin cytoskeletal, WNT, F-Actin, Actin |
| 6 | Proliferation_Negative regulation of cell proliferation | 3.938 × 10−3 | IBP2, WISP2, Galpha(i)-specific peptide GPCRs, IBP5, IL-6, GPNMB (Osteoactivin), IGF-1, IBP |
| 7 | Cell adhesion_ Glycoconjugates | 7.156 × 10−3 | Fibulin-1, PF4, Elastin, HP, Actin cytoskeletal, Neurocan, Actin |
| 8 | Cell adhesion_Cell-matrix interactions | 8.849 × 10−3 | WISP1, MMP-12, Fibulin-1, Elastin, ITGA11, Mindin, LAMA1, Neurocan |
| 9 | Signal transduction_WNT signaling | 1.137 × 10−2 | WISP1, Frizzled, WISP2, SFRP4, WNT11, FOXC1, WNT |
| 10 | Development_Regulation of angiogenesis | 1.152 × 10−2 | Ephrin-A receptors, Galpha(i)-specific peptide GPCRs, Leptin receptor, AGTR2, PF4, IL-6, ANGPTL4, Galpha(q)-specific peptide GPCRs |
| 11 | Development_Skeletal muscle development | 1.436 × 10−2 | Actin muscle, Elastin, ITGA11, ACTG2, IGF-1, Actin |
| 12 | Development_Neurogenesis_Axonal guidance | 1.439 × 10−2 | Ephrin-A receptor 3, Ephrin-A receptors, NckAP1, PACAP receptor 1, Mindin, Actin cytoskeletal, DARPP-32, Actin |
| 13 | Signal transduction NOTCH signaling | 1.620 × 10−2 | Frizzled, NOTCH3 (3ICD), SFRP4, WNT11, NOTCH3, FZD4, WNT, PDGF-B |
| 14 | Development_Neurogenesis in general | 1.761 × 10−2 | Frizzled, Galpha(q)-specific amine GPCRs, Galpha(i)-specific amine GPCRs, NOTCH3, FZD4, Notch, WNT |
| 15 | Cell adhesion_Integrin-mediated cell-matrix adhesion | 7.696 × 10−2 | RHG7, Caveolin-2, ITGA11, Actin cytoskeletal, Caveolin-1, Actin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Laheji, F.; Berenson, A.; Qian, A.; Park, S.-O.; Kok, R.; Selig, M.; Hahn, R.; Sadjadi, R.; Kemp, S.; et al. Peroxisome Metabolism Contributes to PIEZO2-Mediated Mechanical Allodynia. Cells 2022, 11, 1842. https://doi.org/10.3390/cells11111842
Gong Y, Laheji F, Berenson A, Qian A, Park S-O, Kok R, Selig M, Hahn R, Sadjadi R, Kemp S, et al. Peroxisome Metabolism Contributes to PIEZO2-Mediated Mechanical Allodynia. Cells. 2022; 11(11):1842. https://doi.org/10.3390/cells11111842
Chicago/Turabian StyleGong, Yi, Fiza Laheji, Anna Berenson, April Qian, Sang-O Park, Rene Kok, Martin Selig, Ryan Hahn, Reza Sadjadi, Stephan Kemp, and et al. 2022. "Peroxisome Metabolism Contributes to PIEZO2-Mediated Mechanical Allodynia" Cells 11, no. 11: 1842. https://doi.org/10.3390/cells11111842
APA StyleGong, Y., Laheji, F., Berenson, A., Qian, A., Park, S.-O., Kok, R., Selig, M., Hahn, R., Sadjadi, R., Kemp, S., & Eichler, F. (2022). Peroxisome Metabolism Contributes to PIEZO2-Mediated Mechanical Allodynia. Cells, 11(11), 1842. https://doi.org/10.3390/cells11111842