Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer
Abstract
:1. Brief Overview of NSCLC Management and Importance of Biomarkers in Clinical Context
2. Interleukin-11: Member of the IL6 Family of Cytokines
3. Interleukin 11 Drives Pulmonary Fibrosis and Inflammation
4. Interleukin 11 Is a Tumor-Promoting Cytokine in NSCLC
5. Sources of Biomarkers in NSCLC
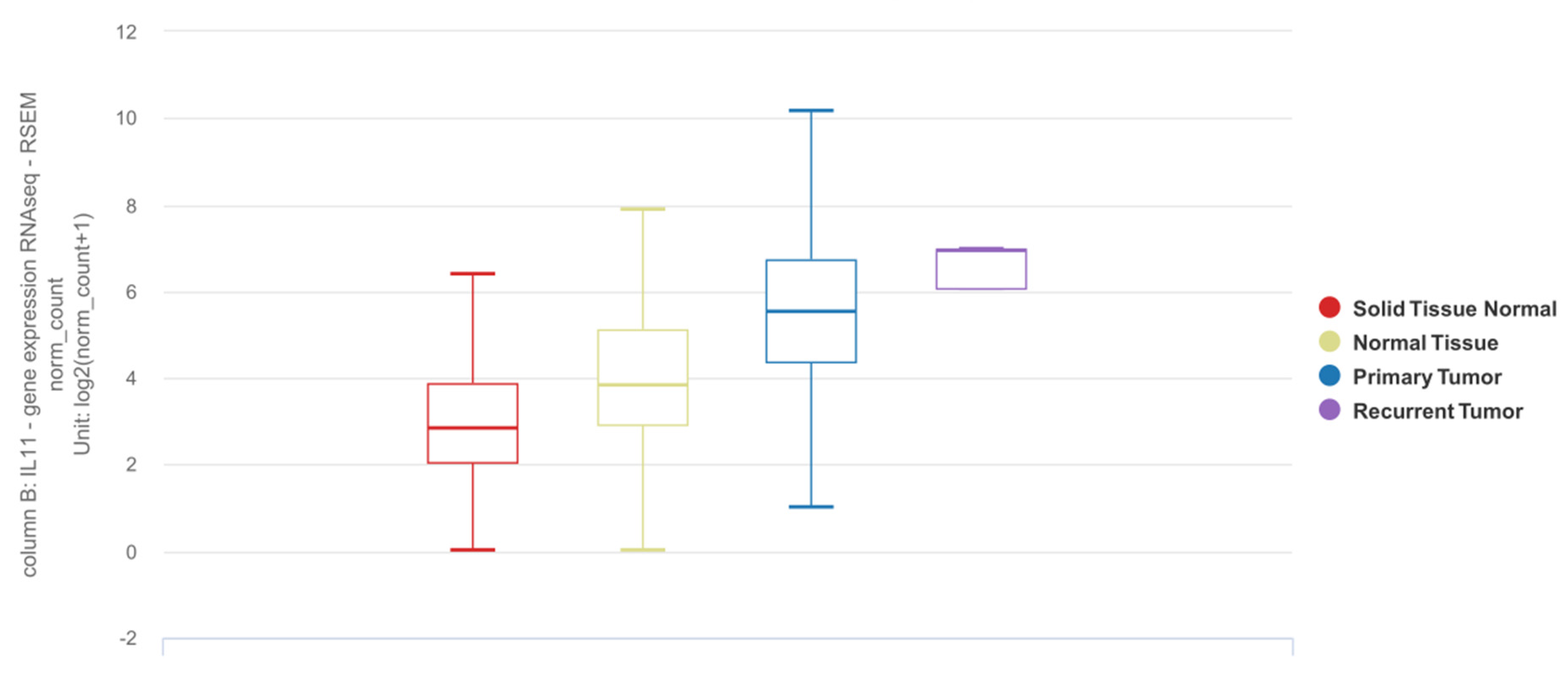
6. IL11 Differential Expression in NSCLC Tumor Tissue
7. Detection of IL11 for Diagnosis of NSCLC
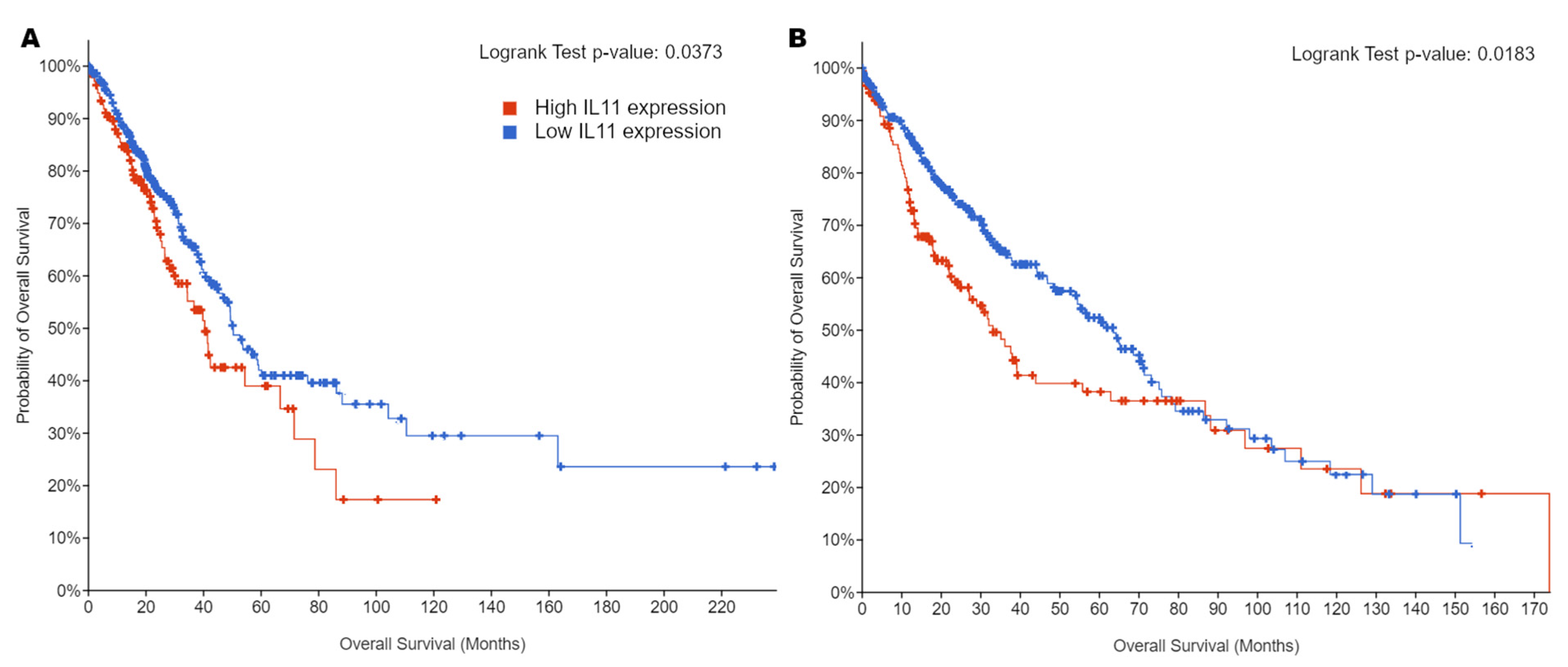
8. Quantification of IL11 Expression for Prognostication of NSCLC
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Gibson, C.J. Turning Gray: The Natural History of Lung Cancer over Time. J. Thorac. Oncol. 2008, 3, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Pirker, R. Adjuvant Chemotherapy in Patients with Completely Resected Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2014, 3, 305–310. [Google Scholar]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Taiwan Lung Cancer Screening Program for Never Smokers. Respirology 2018, 23, 69. [CrossRef]
- Huo, Y.R.; Chan, M.V.; Habib, A.-R.; Lui, I.; Ridley, L. Pneumothorax Rates in CT-Guided Lung Biopsies: A Comprehensive Systematic Review and Meta-Analysis of Risk Factors. Br. J. Radiol. 2020, 93, 20190866. [Google Scholar] [CrossRef]
- Metcalfe, R.D.; Putoczki, T.L.; Griffin, M.D.W. Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11. Front. Immunol. 2020, 11, 1424. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [Green Version]
- van Duijneveldt, G.; Griffin, M.D.W.; Putoczki, T.L. Emerging Roles for the IL-6 Family of Cytokines in Pancreatic Cancer. Clin. Sci. 2020, 134, 2091–2115. [Google Scholar] [CrossRef]
- Felcher, C.M.; Bogni, E.S.; Kordon, E.C. IL-6 Cytokine Family: A Putative Target for Breast Cancer Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 1809. [Google Scholar] [CrossRef] [PubMed]
- Unver, N.; McAllister, F. IL-6 Family Cytokines: Key Inflammatory Mediators as Biomarkers and Potential Therapeutic Targets. Cytokine Growth Factor Rev. 2018, 41, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent Insights into Targeting the IL-6 Cytokine Family in Inflammatory Diseases and Cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, Q.; Yue, C.; Lan, L.; Jiang, J.; Shen, Y.; Lu, B. Prognostic Value of IL-6R mRNA in Lung Adenocarcinoma and Squamous Cell Carcinoma. Oncol. Lett. 2018, 16, 2935–2948. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.M.; Mariano, V.S.; Pastrez, P.R.A.; Pinto, M.C.; Castro, A.G.; Syrjanen, K.J.; Longatto-Filho, A. High Systemic IL-6 Is Associated with Worse Prognosis in Patients with Non-Small Cell Lung Cancer. PLoS ONE 2017, 12, e0181125. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Yu, Z.; Guo, W.; Liu, Q.; Wu, Y.; Li, Y.; Bai, L. Prognostic Value of Circulating Inflammatory Factors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancer Biomark. 2014, 14, 469–481. [Google Scholar] [CrossRef] [PubMed]
- De Vita, F.; Orditura, M.; Auriemma, A.; Infusino, S.; Roscigno, A.; Catalano, G. Serum Levels of Interleukin-6 as a Prognostic Factor in Advanced Non-Small Cell Lung Cancer. Oncol. Rep. 1998, 5, 649–652. [Google Scholar] [CrossRef]
- Meaney, C.L.; Zingone, A.; Brown, D.; Yu, Y.; Cao, L.; Ryan, B.M. Identification of Serum Inflammatory Markers as Classifiers of Lung Cancer Mortality for Stage I Adenocarcinoma. Oncotarget 2017, 8, 40946–40957. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Smith, M.A.; Doshi, P.; Sasser, K.; Fulp, W.; Altiok, S.; Haura, E.B. Antitumor Efficacy of the Anti-Interleukin-6 (IL-6) Antibody Siltuximab in Mouse Xenograft Models of Lung Cancer. J. Thorac. Oncol. 2014, 9, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Keegan, A.; Ricciuti, B.; Garden, P.; Cohen, L.; Nishihara, R.; Adeni, A.; Paweletz, C.; Supplee, J.; Jänne, P.A.; Severgnini, M.; et al. Plasma IL-6 Changes Correlate to PD-1 Inhibitor Responses in NSCLC. J. Immunother Cancer 2020, 8, e000678. [Google Scholar] [CrossRef] [PubMed]
- Vicent, S.; Sayles, L.C.; Vaka, D.; Khatri, P.; Gevaert, O.; Chen, R.; Zheng, Y.; Gillespie, A.K.; Clarke, N.; Xu, Y.; et al. Cross-Species Functional Analysis of Cancer-Associated Fibroblasts Identifies a Critical Role for CLCF1 and IL-6 in Non-Small Cell Lung Cancer in Vivo. Cancer Res. 2012, 72, 5744–5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, L.; Chen, F.; Zhang, L.; Bu, H. The Role of Interleukin-31 Polymorphisms in Non-Small Cell Lung Cancer Genetic Susceptibility and Clinical Outcome. Genet. Test. Mol. Biomark. 2018, 22, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Si, S.N.; Jiang, M.; Chen, L.; Huang, K.; Yu, W. Leukemia Inhibitory Factor Is Involved in the Pathogenesis of NSCLC through Activation of the STAT3 Signaling Pathway. Oncol. Lett. 2021, 22, 663. [Google Scholar] [CrossRef]
- Chen, D.; Chu, C.-Y.; Chen, C.-Y.; Yang, H.-C.; Chiang, Y.-Y.; Lin, T.-Y.; Chiang, I.-P.; Chuang, D.-Y.; Yu, C.-C.; Chow, K.-C. Expression of Short-Form Oncostatin M Receptor as a Decoy Receptor in Lung Adenocarcinomas. J. Pathol. 2008, 215, 290–299. [Google Scholar] [CrossRef]
- Shien, K.; Papadimitrakopoulou, V.A.; Ruder, D.; Behrens, C.; Shen, L.; Kalhor, N.; Song, J.; Lee, J.J.; Wang, J.; Tang, X.; et al. JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2017, 16, 2234–2245. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Marquez, C.P.; Kostyrko, K.; Koehne, A.L.; Marini, K.; Simpson, D.R.; Lee, A.G.; Leung, S.G.; Sayles, L.C.; Shrager, J.; et al. Antitumor Activity of an Engineered Decoy Receptor Targeting CLCF1-CNTFR Signaling in Lung Adenocarcinoma. Nat. Med. 2019, 25, 1783–1795. [Google Scholar] [CrossRef]
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 Signaling Underlies Fibrosis, Parenchymal Dysfunction, and Chronic Inflammation of the Airway. Exp. Mol. Med. 2020, 52, 1871–1878. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Ko, N.S.J.; Tan, J.; Chothani, S.P.; Huang, B.; et al. Interleukin-11 Is a Therapeutic Target in Idiopathic Pulmonary Fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; Viswanathan, S.; Widjaja, A.A.; Paleja, B.S.; Adami, E.; Ko, N.S.J.; Wang, M.; Lim, S.; Tan, J.; et al. Fibroblast-Specific IL11 Signaling Drives Chronic Inflammation in Murine Fibrotic Lung Disease. FASEB J. 2020, 34, 11802–11815. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, E.; Takayanagi, N.; Takaku, Y.; Kagiyama, N.; Kanauchi, T.; Ishiguro, T.; Sugita, Y. Incidence and Predictive Factors of Lung Cancer in Patients with Idiopathic Pulmonary Fibrosis. ERJ Open Res. 2018, 4, 00111–2016. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.R.; Bennett, F.; Calvetti, J.A.; Kelleher, K.; Wood, C.R.; O’Hara, R.M., Jr.; Leary, A.C.; Sibley, B.; Clark, S.C.; Williams, D.A. Molecular Cloning of a cDNA Encoding Interleukin 11, a Stromal Cell-Derived Lymphopoietic and Hematopoietic Cytokine. Proc. Natl. Acad. Sci. USA 1990, 87, 7512–7516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepler, I.; Elias, L.; Smith, J.W., 2nd; Hussein, M.; Rosen, G.; Chang, A.Y.; Moore, J.O.; Gordon, M.S.; Kuca, B.; Beach, K.J.; et al. A Randomized Placebo-Controlled Trial of Recombinant Human Interleukin-11 in Cancer Patients with Severe Thrombocytopenia due to Chemotherapy. Blood 1996, 87, 3607–3614. [Google Scholar]
- Xu, Y.; Song, X.; Du, F.; Zhao, Q.; Liu, L.; Ma, Z.; Lu, S. A Randomized Controlled Study of rhTPO and rhIL-11 for the Prophylactic Treatment of Chemotherapy-Induced Thrombocytopenia in Non-Small Cell Lung Cancer. J. Cancer 2018, 9, 4718–4725. [Google Scholar] [CrossRef]
- Soda, H.; Raymond, E.; Sharma, S.; Lawrence, R.; Cerna, C.; Gomez, L.; Schaub, R.; Von Hoff, D.D.; Izbicka, E. Recombinant Human Interleukin-11 Is Unlikely to Stimulate the Growth of the Most Common Solid Tumors. Anticancer Drugs 1999, 10, 97–101. [Google Scholar] [CrossRef]
- Saitoh, M.; Taguchi, K.; Momose, K.; Suga, K.; Yamazaki, N.; Ono, C.; Suzuki, T.; Takeuchi, O.; Yasuda, S.; Miyata, K. Recombinant Human Interleukin-11 Improved Carboplatin-Induced Thrombocytopenia without Affecting Antitumor Activities in Mice Bearing Lewis Lung Carcinoma Cells. Cancer Chemother. Pharmacol. 2002, 49, 161–166. [Google Scholar] [CrossRef]
- Spence, M.J.; Streiff, R.; Day, D.; Ma, Y. Oncostatin M Induces Tissue-Type Plasminogen Activator and Plasminogen Activator Inhibitor-1 in Calu-1 Lung Carcinoma Cells. Cytokine 2002, 18, 26–34. [Google Scholar] [CrossRef]
- Howlett, M.; Giraud, A.S.; Lescesen, H.; Jackson, C.B.; Kalantzis, A.; Van Driel, I.R.; Robb, L.; Van der Hoek, M.; Ernst, M.; Minamoto, T.; et al. The Interleukin-6 Family Cytokine Interleukin-11 Regulates Homeostatic Epithelial Cell Turnover and Promotes Gastric Tumor Development. Gastroenterology 2009, 136, 967–977. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, X.; Fu, B.; Nian, Z.; Qian, Y.; Sun, R.; Tian, Z.; Wei, H. Hepatectomy Promotes Recurrence of Liver Cancer by Enhancing IL-11-STAT3 Signaling. EBioMedicine 2019, 46, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Song, X.; Xu, X.; Mou, Y. Cancer-Associated Fibroblasts Promote the Chemo-Resistance in Gastric Cancer through Secreting IL-11 Targeting JAK/STAT3/Bcl2 Pathway. Cancer Res. Treat. 2019, 51, 194–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Che, X.; Liu, C.; Fan, Y.; Bai, M.; Hou, K.; Shi, X.; Zhang, X.; Liu, B.; Zheng, C.; et al. Cancer-Associated Fibroblasts-Stimulated Interleukin-11 Promotes Metastasis of Gastric Cancer Cells Mediated by Upregulation of MUC1. Exp. Cell Res. 2018, 368, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Viswanathan, S.; Jinrui, D.; Singh, B.K.; Tan, J.; Wei Ting, J.G.; Lamb, D.; Shekeran, S.G.; George, B.L.; Schafer, S.; et al. Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front. Mol. Biosci. 2021, 8, 740650. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.A.; Schafer, S. Hiding in Plain Sight: Interleukin-11 Emerges as a Master Regulator of Fibrosis, Tissue Integrity, and Stromal Inflammation. Annu. Rev. Med. 2020, 71, 263–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, N.; Lu, M.; Kang, M.; Liu, X.; Li, B.; Dong, C. Recombinant Human IL-11 Promotes Lung Adenocarcinoma A549 Cell Growth and EMT through Activating STAT3/HIF-1α/EMT Signaling Pathway. Anticancer Agents Med. Chem. 2021, 21, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Y.; Liu, R.; Qi, J.; Hou, Y.; Chang, J.; Ren, L. Upregulation of IL-11, an IL-6 Family Cytokine, Promotes Tumor Progression and Correlates with Poor Prognosis in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2018, 45, 2213–2224. [Google Scholar] [CrossRef]
- Zhao, M.; Chang, J.; Liu, R.; Liu, Y.; Qi, J.; Wang, Y.; Zhang, X.; Qiao, L.; Jin, Y.; An, H.; et al. miR-495 and miR-5688 Are down-Regulated in Non-Small Cell Lung Cancer under Hypoxia to Maintain Interleukin-11 Expression. Cancer Commun. 2020, 40, 435–452. [Google Scholar] [CrossRef]
- Matsui, S.; Yamashita, N.; Mino, T.; Taki, H.; Sugiyama, E.; Hayashi, R.; Maruyama, M.; Kobayashi, M. Role of the Endogenous Prostaglandin E2 in Human Lung Fibroblast Interleukin-11 Production. Respir. Med. 1999, 93, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Nathanson, M.H.; Elias, J.A. Histamine Augments Cytokine-Stimulated IL-11 Production by Human Lung Fibroblasts. J. Immunol. 1994, 153, 4742–4752. [Google Scholar]
- Elias, J.A.; Wu, Y.; Zheng, T.; Panettieri, R. Cytokine- and Virus-Stimulated Airway Smooth Muscle Cells Produce IL-11 and Other IL-6-Type Cytokines. Am. J. Physiol. 1997, 273, L648–L655. [Google Scholar] [CrossRef]
- Elias, J.A.; Zheng, T.; Einarsson, O.; Landry, M.; Trow, T.; Rebert, N.; Panuska, J. Epithelial Interleukin-11. Regulation by Cytokines, Respiratory Syncytial Virus, and Retinoic Acid. J. Biol. Chem. 1994, 269, 22261–22268. [Google Scholar] [CrossRef]
- Jiang, C.-P.; Wu, B.-H.; Chen, S.-P.; Fu, M.-Y.; Yang, M.; Liu, F.; Wang, B.-Q. High COL4A3 Expression Correlates with Poor Prognosis after Cisplatin plus Gemcitabine Chemotherapy in Non-Small Cell Lung Cancer. Tumour Biol. 2013, 34, 415–420. [Google Scholar] [CrossRef]
- Alcaraz, J.; Carrasco, J.L.; Millares, L.; Luis, I.-C.; Fernández-Porras, F.J.; Martínez-Romero, A.; Diaz-Valdivia, N.; De Cos, J.S.; Rami-Porta, R.; Seijo, L.; et al. Stromal Markers of Activated Tumor Associated Fibroblasts Predict Poor Survival and Are Associated with Necrosis in Non-Small Cell Lung Cancer. Lung Cancer 2019, 135, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Irvine, A.F.; Waise, S.; Green, E.W.; Stuart, B.; Thomas, G.J. Characterising Cancer-Associated Fibroblast Heterogeneity in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 3727. [Google Scholar] [CrossRef]
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-Associated Fibroblasts Treated with Cisplatin Facilitates Chemoresistance of Lung Adenocarcinoma through IL-11/IL-11R/STAT3 Signaling Pathway. Sci. Rep. 2016, 6, 38408. [Google Scholar] [CrossRef]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype Molding of Stromal Cells in the Lung Tumor Microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Ratajczak, M.Z. Lung Cancer Secreted Microvesicles: Underappreciated Modulators of Microenvironment in Expanding Tumors. Int. J. Cancer 2009, 125, 1595–1603. [Google Scholar] [CrossRef] [Green Version]
- Cardó-Vila, M.; Marchiò, S.; Sato, M.; Staquicini, F.I.; Smith, T.L.; Bronk, J.K.; Yin, G.; Zurita, A.J.; Sun, M.; Behrens, C.; et al. Interleukin-11 Receptor Is a Candidate Target for Ligand-Directed Therapy in Lung Cancer: Analysis of Clinical Samples and BMTP-11 Preclinical Activity. Am. J. Pathol. 2016, 186, 2162–2170. [Google Scholar] [CrossRef] [Green Version]
- Lokau, J.; Nitz, R.; Agthe, M.; Monhasery, N.; Aparicio-Siegmund, S.; Schumacher, N.; Wolf, J.; Möller-Hackbarth, K.; Waetzig, G.H.; Grötzinger, J.; et al. Proteolytic Cleavage Governs Interleukin-11 Trans-Signaling. Cell Rep. 2016, 14, 1761–1773. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.D.; McLeod, L.; Alhayyani, S.; Miller, A.; Russell, P.A.; Ferlin, W.; Rose-John, S.; Ruwanpura, S.; Jenkins, B.J. IL6 Trans-Signaling Promotes KRAS-Driven Lung Carcinogenesis. Cancer Res. 2016, 76, 866–876. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Najdovska, M.; Grail, D.; Lundgren-May, T.; Buchert, M.; Tye, H.; Matthews, V.B.; Armes, J.; Bhathal, P.S.; Hughes, N.R.; et al. STAT3 and STAT1 Mediate IL-11-Dependent and Inflammation-Associated Gastric Tumorigenesis in gp130 Receptor Mutant Mice. J. Clin. Investig. 2008, 118, 1727–1738. [Google Scholar] [CrossRef]
- Ernst, M.; Putoczki, T.L. Molecular Pathways: IL11 as a Tumor-Promoting Cytokine-Translational Implications for Cancers. Clin. Cancer Res. 2014, 20, 5579–5588. [Google Scholar] [CrossRef] [Green Version]
- Heavey, S.; O’Byrne, K.J.; Gately, K. Strategies for Co-Targeting the PI3K/AKT/mTOR Pathway in NSCLC. Cancer Treat. Rev. 2014, 40, 445–456. [Google Scholar] [CrossRef]
- Al-Saad, S.; Donnem, T.; Al-Shibli, K.; Persson, M.; Bremnes, R.M.; Busund, L.-T. Diverse Prognostic Roles of Akt Isoforms, PTEN and PI3K in Tumor Epithelial Cells and Stromal Compartment in Non-Small Cell Lung Cancer. Anticancer Res. 2009, 29, 4175–4183. [Google Scholar]
- Tang, J.-M.; He, Q.-Y.; Guo, R.-X.; Chang, X.-J. Phosphorylated Akt Overexpression and Loss of PTEN Expression in Non-Small Cell Lung Cancer Confers Poor Prognosis. Lung Cancer 2006, 51, 181–191. [Google Scholar] [CrossRef]
- Tong, M.; Wang, J.; Jiang, N.; Pan, H.; Li, D. Correlation between P-STAT3 Overexpression and Prognosis in Lung Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0182282. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhao, Q.; He, X.-P.; Wang, Z.; Liu, X.-Y.; Zhang, Z.-P. Signal Transducer and Activator of Transcription 3 Overexpression Promotes Lymph Node Micrometastasis in Early-Stage Non-Small Cell Lung Cancer. Thorac. Cancer 2018, 9, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Widjaja, A.A.; Ting, J.G.W.; Viswanathan, S.; Tan, J.; Shekeran, S.G.; Carling, D.; Wen, L.W.; Cook, S.A. IL11 Stimulates ERK/P90RSK to Inhibit LKB1/AMPK and Activate mTOR in Hepatocytes, the Stroma and Cancer Cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Mograbi, B.; Heeke, S.; Hofman, P. The Importance of STK11/LKB1 Assessment in Non-Small Cell Lung Carcinomas. Diagnostics 2021, 11, 196. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Lugat, A.; Fontenau, J.-F.; Denis, M.G.; Bennouna, J. STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact. Cells 2021, 10, 3129. [Google Scholar] [CrossRef]
- Ekman, S.; Wynes, M.W.; Hirsch, F.R. The mTOR Pathway in Lung Cancer and Implications for Therapy and Biomarker Analysis. J. Thorac. Oncol. 2012, 7, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, S.; Petti, F.; Sujka-Kwok, I.; Mercado, P.; Bean, J.; Monaghan, M.; Seymour, S.L.; Argast, G.M.; Epstein, D.M.; Haley, J.D. A Systems View of Epithelial-Mesenchymal Transition Signaling States. Clin. Exp. Metastasis 2011, 28, 137–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Tagawa, T.; Takada, K.; Toyokawa, G.; Shimokawa, M.; Kozuma, Y.; Akamine, T.; Haro, A.; Osoegawa, A.; Mori, M. Clinical and Prognostic Significance of the Epithelial-Mesenchymal Transition in Stage IA Lung Adenocarcinoma: A Propensity Score-Matched Analysis. Clin. Lung Cancer 2019, 20, e504–e513. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA, 2016. [Google Scholar]
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- GTEx Consortium The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 2013, 45, 580–585. [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and Interpreting Cancer Genomics Data via the Xena Platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.; Rao, A.A.; Nothaft, F.A.; Ketchum, C.; Armstrong, J.; Novak, A.; Pfeil, J.; Narkizian, J.; Deran, A.D.; Musselman-Brown, A.; et al. Toil Enables Reproducible, Open Source, Big Biomedical Data Analyses. Nat. Biotechnol. 2017, 35, 314–316. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, M.-S.; Wang, Y.; Huang, Y.-Q.; Shi, J.-P.; Ding, Z.-L.; Wang, W.-J. Prognostic Value of Immune Related Genes in Lung Adenocarcinoma. Oncol. Lett. 2020, 20, 259. [Google Scholar] [CrossRef]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.-W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e35. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Zhang, C.; Wang, X.; Zhai, L.; Ma, Y.; Mao, Y.; Qian, K.; Sun, C.; Liu, Z.; Jiang, S.; et al. Integrative Proteomic Characterization of Human Lung Adenocarcinoma. Cell 2020, 182, 245–261.e17. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Roumeliotis, T.I.; Chang, Y.-H.; Chen, C.-T.; Han, C.-L.; Lin, M.-H.; Chen, H.-W.; Chang, G.-C.; Chang, Y.-L.; Wu, C.-T.; et al. Proteogenomics of Non-Smoking Lung Cancer in East Asia Delineates Molecular Signatures of Pathogenesis and Progression. Cell 2020, 182, 226–244.e17. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Hsu, C.-W.; Hsueh, C.; Wang, C.-L.; Wu, Y.-C.; Wu, C.-C.; Liu, C.-C.; Yu, J.-S.; Chang, Y.-S.; Yu, C.-J. Identification and Characterization of Potential Biomarkers by Quantitative Tissue Proteomics of Primary Lung Adenocarcinoma. Mol. Cell. Proteom. 2016, 15, 2396–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okayama, A.; Miyagi, Y.; Oshita, F.; Nishi, M.; Nakamura, Y.; Nagashima, Y.; Akimoto, K.; Ryo, A.; Hirano, H. Proteomic Analysis of Proteins Related to Prognosis of Lung Adenocarcinoma. J. Proteome Res. 2014, 13, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Ao, M.; Gabrielson, E.; Askin, F.; Zhang, H.; Li, Q.K. Aberrant Mucin5B Expression in Lung Adenocarcinomas Detected by iTRAQ Labeling Quantitative Proteomics and Immunohistochemistry. Clin. Proteom. 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Pernemalm, M.; De Petris, L.; Branca, R.M.; Forshed, J.; Kanter, L.; Soria, J.-C.; Girard, P.; Validire, P.; Pawitan, Y.; van den Oord, J.; et al. Quantitative Proteomics Profiling of Primary Lung Adenocarcinoma Tumors Reveals Functional Perturbations in Tumor Metabolism. J. Proteome Res. 2013, 12, 3934–3943. [Google Scholar] [CrossRef]
- Kikuchi, T.; Hassanein, M.; Amann, J.M.; Liu, Q.; Slebos, R.J.C.; Rahman, S.M.J.; Kaufman, J.M.; Zhang, X.; Hoeksema, M.D.; Harris, B.K.; et al. In-Depth Proteomic Analysis of Nonsmall Cell Lung Cancer to Discover Molecular Targets and Candidate Biomarkers. Mol. Cell. Proteom. 2012, 11, 916–932. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yang, Y.C. Heparin Inhibits the Expression of Interleukin-11 and Granulocyte-Macrophage Colony-Stimulating Factor in Primate Bone Marrow Stromal Fibroblasts through mRNA Destabilization. Blood 1995, 86, 2526–2533. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Williams, D.A. Interleukin-11: Review of Molecular, Cell Biology, and Clinical Use. Blood 1997, 89, 3897–3908. [Google Scholar] [CrossRef]
- Pastor, M.D.; Nogal, A.; Molina-Pinelo, S.; Quintanal-Villalonga, Á.; Meléndez, R.; Ferrer, I.; Romero-Romero, B.; De Miguel, M.J.; López-Campos, J.L.; Corral, J.; et al. IL-11 and CCL-1: Novel Protein Diagnostic Biomarkers of Lung Adenocarcinoma in Bronchoalveolar Lavage Fluid (BALF). J. Thorac. Oncol. 2016, 11, 2183–2192. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Lv, X.; Yang, Q.; Yao, S.; Zhang, D.; Chen, J. Clinical Value of Serum and Exhaled Breath Condensate Inflammatory Factor IL-11 Levels in Non-Small Cell Lung Cancer: Clinical Value of IL-11 in Non-Small Cell Lung Cancer. Int. J. Biol. Markers 2021, 36, 64–76. [Google Scholar] [CrossRef]
- Horvatovich, P.L.; Bischoff, R. Current Technological Challenges in Biomarker Discovery and Validation. Eur. J. Mass Spectrom. 2010, 16, 101–121. [Google Scholar] [CrossRef] [PubMed]
- McKeown, D.J.; Brown, D.J.F.; Kelly, A.; Wallace, A.M.; McMillan, D.C. The Relationship between Circulating Concentrations of C-Reactive Protein, Inflammatory Cytokines and Cytokine Receptors in Patients with Non-Small-Cell Lung Cancer. Br. J. Cancer 2004, 91, 1993–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomej, P.; Bauer, K.; Bitterlich, N.; Hui, D.S.C.; Chan, K.S.; Gosse, H.; Schauer, J.; Hoheisel, G.; Sack, U. Differential Diagnosis of Pleural Effusions by Fuzzy-Logic-Based Analysis of Cytokines. Respir. Med. 2004, 98, 308–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitter, E.-M.; Ay, C.; Kaider, A.; Pirker, R.; Zielinski, C.; Zlabinger, G.; Pabinger, I. Interleukin Levels and Their Potential Association with Venous Thromboembolism and Survival in Cancer Patients. Clin. Exp. Immunol. 2014, 177, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Ortuño, M.T.; Gómez-Martín, Ó.; Ponce, S.; Iglesias, L.; Ojeda, L.; Ferrer, I.; García-Ruiz, I.; Paz-Ares, L.; Pardo-Marqués, V. Blood Predictive Biomarkers for Patients With Non-Small-Cell Lung Cancer Associated With Clinical Response to Nivolumab. Clin. Lung Cancer 2020, 21, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Deo, P.; Sachdeva, M.U.S.; Bose, P.; Lad, D.; Prakash, G.; Khadwal, A.; Varma, N.; Varma, S.; Malhotra, P. Aberrant Expression of Cytokines in Polycythemia Vera Correlate with the Risk of Thrombosis. Blood Cells Mol. Dis. 2021, 89, 102565. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, G.; Ren, Q.; Wu, J.; Gu, B.; Su, D.; Shen, M. Increased Interleukin-11 Associated with Disease Activity and Development of Interstitial Lung Disease in Patients with Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2021. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Z.; Ye, D.; Wang, Y.; Wang, M.; Ji, Q.; Huang, Y.; Liu, L.; Shi, Y.; Shi, L.; et al. Increased Interleukin-11 Levels Are Correlated with Cardiac Events in Patients with Chronic Heart Failure. Mediat. Inflamm. 2019, 2019, 1575410. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Wang, S.S.; Lu, R.H.; Chang, F.Y.; Lee, S.D. Serum Interleukin 10 and Interleukin 11 in Patients with Acute Pancreatitis. Gut 1999, 45, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Wang, X.; Dong, Z.; Liu, J.; Zhang, S. Bone Metastasis from Breast Cancer Involves Elevated IL-11 Expression and the gp130/STAT3 Pathway. Med. Oncol. 2013, 30, 634. [Google Scholar] [CrossRef]
- Ren, C.; Chen, Y.; Han, C.; Fu, D.; Chen, H. Plasma Interleukin-11 (IL-11) Levels Have Diagnostic and Prognostic Roles in Patients with Pancreatic Cancer. Tumor Biol. 2014, 35, 11467–11472. [Google Scholar] [CrossRef]
- Wang, D.Q.; Ding, X.P.; Yin, S.; Mao, Y.D. Role of the IL-11/STAT3 Signaling Pathway in Human Chronic Atrophic Gastritis and Gastric Cancer. Genet. Mol. Res. 2016, 15, gmr.15027358. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Koga, K.; Menkhorst, E.; Van Sinderen, M.; Rainczuk, K.; Nagai, M.; Cuman, C.; Yap, J.; Zhang, J.-G.; Simmons, D.; et al. Interleukin-11 Alters Placentation and Causes Preeclampsia Features in Mice. Proc. Natl. Acad. Sci. USA 2015, 112, 15928–15933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Ye, J.; Wang, M.; Wang, Y.; Ji, Q.; Huang, Y.; Zeng, T.; Wang, Z.; Ye, D.; Jiang, H.; et al. Increased Interleukin-11 Levels in Thoracic Aorta and Plasma from Patients with Acute Thoracic Aortic Dissection. Clin. Chim. Acta 2018, 481, 193–199. [Google Scholar] [CrossRef]
- Haro, G.J.; Sheu, B.; Cook, N.R.; Woodard, G.A.; Mann, M.J.; Kratz, J.R. Comparison of Conventional TNM and Novel TNMB Staging Systems for Non-Small Cell Lung Cancer. JAMA Netw. Open 2019, 2, e1917062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratz, J.R.; He, J.; Van Den Eeden, S.K.; Zhu, Z.-H.; Gao, W.; Pham, P.T.; Mulvihill, M.S.; Ziaei, F.; Zhang, H.; Su, B.; et al. A Practical Molecular Assay to Predict Survival in Resected Non-Squamous, Non-Small-Cell Lung Cancer: Development and International Validation Studies. Lancet 2012, 379, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Watza, D.; Lusk, C.M.; Dyson, G.; Purrington, K.S.; Chen, K.; Wenzlaff, A.S.; Ratliff, V.; Neslund-Dudas, C.; Bepler, G.; Schwartz, A.G. Prognostic Modeling of the Immune-Centric Transcriptome Reveals Interleukin Signaling Candidates Contributing to Differential Patient Outcomes. Carcinogenesis 2018, 39, 1447–1454. [Google Scholar] [CrossRef]
- Fan, T.; Pan, S.; Yang, S.; Hao, B.; Zhang, L.; Li, D.; Geng, Q. Clinical Significance and Immunologic Landscape of a Five-IL(R)-Based Signature in Lung Adenocarcinoma. Front. Immunol. 2021, 12, 693062. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Tang, Y.; Qu, W.; Zuo, J.; Ke, X.; Song, Y. Screening and Evaluation of the Role of Immune Genes of Brain Metastasis in Lung Adenocarcinoma Progression Based on the TCGA and GEO Databases. J. Thorac. Dis. 2021, 13, 5016–5034. [Google Scholar] [CrossRef]
- Peng, L.; Tao, Y.; Wu, R.; Su, J.; Sun, M.; Cheng, Y.; Xie, Z.; Mao, J.; Zhan, X.; Liu, G. NFAT as a Biomarker and Therapeutic Target in Non-Small Cell Lung Cancer-Related Brain Metastasis. Front. Oncol. 2021, 11, 781150. [Google Scholar] [CrossRef]
- Győrffy, B.; Surowiak, P.; Budczies, J.; Lánczky, A. Online Survival Analysis Software to Assess the Prognostic Value of Biomarkers Using Transcriptomic Data in Non-Small-Cell Lung Cancer. PLoS ONE 2013, 8, e82241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
| Cytokine | Receptors | References |
|---|---|---|
| IL6 | IL6R, gp130/IL6ST | [15,16,17,18,19,20,21,22] |
| IL-31 | IL31Rα, OSMR | [23] |
| LIF | LIFR/LIFRα, gp130/IL6ST | [24] |
| OSM | OSMR/OSMRβ, gp130/IL6ST, LIFR | [25,26] |
| CLCF1 | CNTFR, LIFR, gp130/IL6ST | [22,27] |
| Study | Recruited Population | Comparison | Sample Type | Diagnostic Biomarker | Assay | Receiver Operator Curve and Test Metrics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Cutoff (pg/mL) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||||||
| Pastor et al. [90] | Age > 40 yrs, current or ex-smokers of 30 pack-years, evaluated for hemoptysis or pulmonary nodule or mass, excluding those with prior diagnosis of malignancy, active tuberculosis, history of drug abuse or other inflammatory disease apart from COPD | LUAD vs. non-LUAD—First validation cohort (n = 149) | BALF | IL11 protein | ELISA | 0.93 (0.90–0.97) | 42.0 | 90.2 (79–95.7) | 88.7 (90.6–93.5) | 80.7 (68.7–88.9) | 94.5 (87.8–97.6) |
| LUAD vs. non-LUAD—Second validation cohort (n = 160) | BALF | IL11 protein | ELISA | 0.95 (0.92–0.98) | 42.0 | 90.6 (79.7–95.9) | 83.0 (86.8–87.7) | 60.8 (49.7–70.8) | 96.8 (92.7–98.6) | ||
| Wu et al. [91] | NSCLC patients with no history of radiochemotherapy, immune-targeted therapy or surgery (n = 91 for serum, of which 63 have LUAD and 28 have SCC; 64 for EBC) | Healthy volunteers without acute or chronic infectious diseases, vital organ diseases, or genetic family tumor history (n = 72 for serum; 63 for EBC) | Serum | IL11 protein | ELISA | 0.93 (0.88–0.97) | 126.1 | 75.0 | 100.0 | NR | NR |
| EBC | IL11 protein | ELISA | 0.78 (0.69–0.86) | 21.5 | 78.1 | 79.4 | NR | NR | |||
| Pathology | Comparison | Source | Findings | Reference |
|---|---|---|---|---|
| Polycythemia vera | Healthy | Plasma | Increased | [97] |
| Rheumatoid arthritis with or without interstitial lung disease | Healthy | Serum | Increased in rheumatoid arthritis, more so with concomitant interstitial lung disease | [98] |
| Congestive heart failure | Healthy | Plasma | Increased | [99] |
| Severe pancreatitis | Mild pancreatitis | Serum | Increased | [100] |
| Breast cancer metastatic to bone | Primary breast cancer and healthy controls | Serum | Increased compared to healthy controlsCorrelated with shorter disease-free survival | [101] |
| Pancreatic cancer | Healthy | Plasma | Increased Correlated with survival. | [102] |
| Gastric cancer | Chronic superficial gastritis and chronic atrophic Gastritis | Serum | Increased in gastric cancer > chronic atrophic gastritis > chronic superficial gastritis | [103] |
| Preeclampsia | Normal pregnant gestation-matched control | Serum | Increased | [104] |
| Thoracic aortic dissection | Non-aortic dissection patients presenting with chest pain | Plasma | Increased | [105] |
| Study | Year | Training Cohort | Validation Cohort (s) | Cancer Type | Prognostic Signature | Findings |
|---|---|---|---|---|---|---|
| Kratz et al. [107] | 2012 | Non-squamous NSCLC (n = 361) | Stage I non-squamous NSCLC (n = 433), and stage I-III non-squamous NSCLC (n = 1006) | Non-squamous NSCLC | 11 Target genes (BAG1, BRCA1, CDC6, CDK2AP1, ERBB3, FUT3, IL11, LCK) and 3 reference genes (ESD, TBP, YAP1) |
|
| Watza et al. [108] | 2018 | NSCLC patients without history of bronchiectasis or cystic fibrosis (n = 280) | TCGA Lung SCC and TCGA LUAD datasets (n = 1026) | NSCLC | 23 genes involved in the interleukin signaling pathway, including IL11 |
|
| Wang et al. [79] | 2020 | TCGA LUAD dataset (497 LUAD tissues, 54 normal lung tissues) | n/a | LUAD | 6 genes (CRABP1, IGKV4-1, IL11, INHA, LGR4, VIPR1) |
|
| Fan et al. [109] | 2021 | TGCA LUAD dataset (n = 464)(majority stage I and II) | GSE13213 (n = 117), GSE30219 (n = 85), GSE31210 (n = 226), GSE72094 (n = 420)(majority stage I and II) | LUAD | 5 genes (IL7R, IL5RA, IL20RB, IL11, IL22RA1) |
|
| Chen et al. [110] | 2021 | TCGA LUAD (535 LUAD tissues, 59 normal lung tissues)GSE161116 (9 LUAD tissues, 9 LUAD brain metastasis tissues) | n/a | LUAD | 6 genes (TNFRSF11A, MS4A2, IL11, CAMP, MS4A1, F2RL1) |
|
| Peng et al. [111] | 2021 | GSE161116 (13 lung tumor tissues, 15 brain tissues), GSE747706 (18 lung tumor tissues, 18 normal tissues), GSE21933 (21 lung tumor tissues, 21 normal tissues) datasets | n/a | NSCLC andBrain tumor | n/a |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, J.H.; Ng, B.; Lim, W.-W. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells 2022, 11, 2257. https://doi.org/10.3390/cells11142257
Leung JH, Ng B, Lim W-W. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells. 2022; 11(14):2257. https://doi.org/10.3390/cells11142257
Chicago/Turabian StyleLeung, Jason Hongting, Benjamin Ng, and Wei-Wen Lim. 2022. "Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer" Cells 11, no. 14: 2257. https://doi.org/10.3390/cells11142257







