Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Dissecting and Mounting of Microvessels
2.3. Experimental Procedures for the Functional Assays
2.4. Measurement of Superoxide Production by Chemiluminescence
2.5. Quantitative Rt-PCR
2.6. Immunofluorescence Staining
2.7. Data Presentation and Statistical Analysis
3. Results
3.1. Endothelial Dysfunction in Renal Preglomerular Arteries Is Associated to Augmented Oxidative Stress in Renal Cortex of Hyperoxaluric Rats
3.2. Role of COX-2 and Xanthin Oxidase in Endothelial Dysfunction of Preglomerular Arteries of Hyperoxaluric Rats
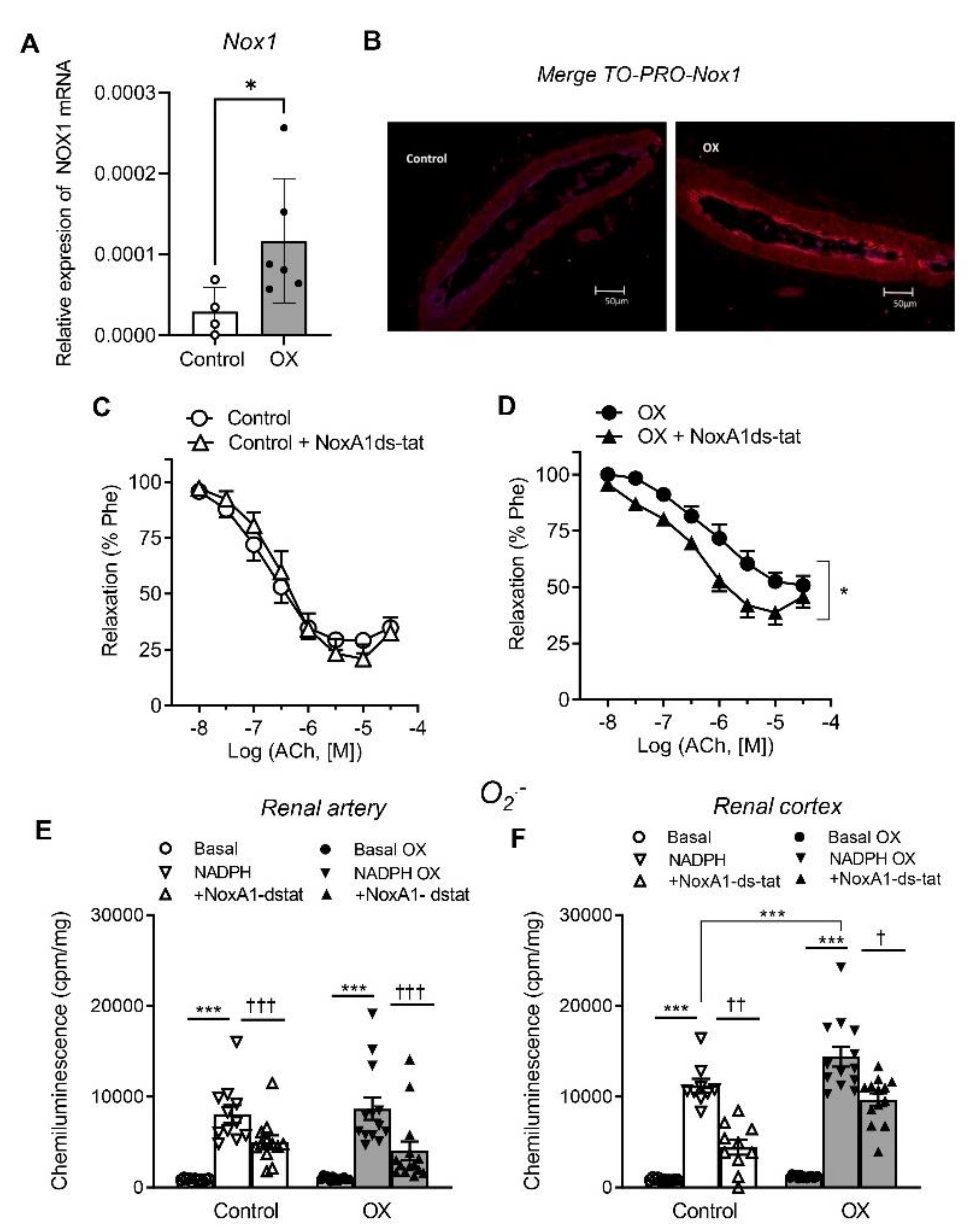
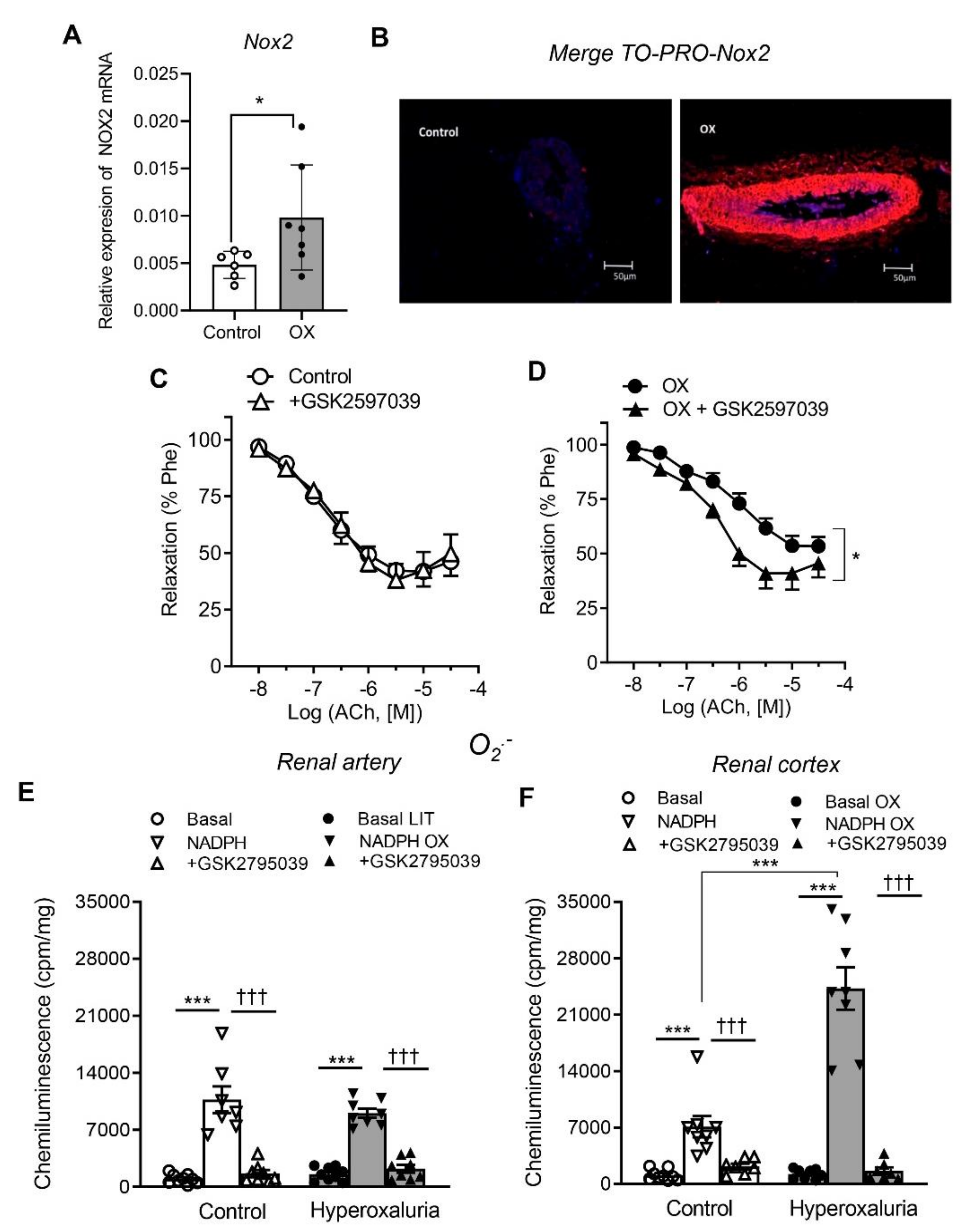
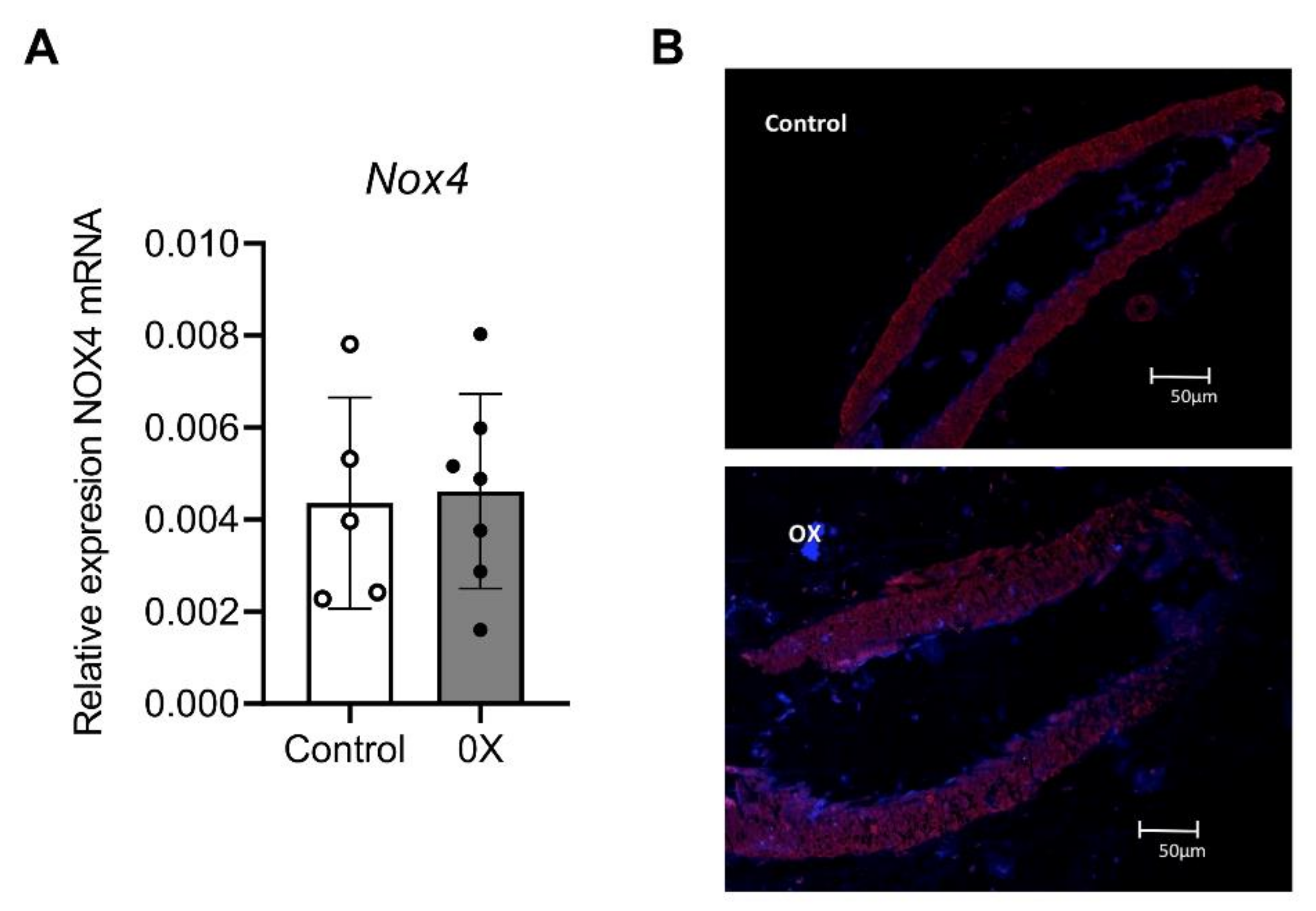
3.3. Role of Nox1, Nox2 and Nox4 in Endothelial Dysfunction and ROS Generation of Renal Preglomerular Arteries from Hyperoxaluric Rats
3.4. Vascular Inflammatory Response in Preglomerular Arteries from Hyperoxaluric Rats (NFκB1, MCP1, TNFα)
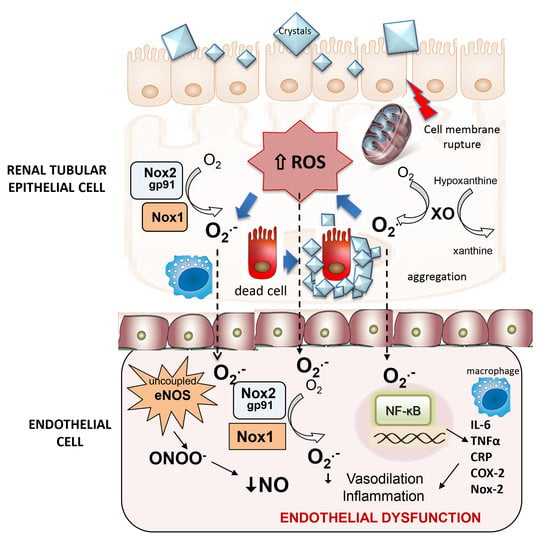
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lotan, Y.; Jiménez, I.B.; Lenoir-Wijnkoop, I.; Daudon, M.; Molinier, L.; Tack, I.; Nuijten, M.J. Primary prevention of nephrolithiasis is cost-effective for a national healthcare system. Br. J. Urol. 2012, 110, E1060–E1067. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome. Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; van Hinsbergh, V.W.M. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2008, 335, 165–189. [Google Scholar] [CrossRef] [Green Version]
- de Vries, A.P.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Barlovic, D.P.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Muñoz, M.; López-Oliva, M.E.; Rodríguez, C.; Martínez, M.P.; Sáenz-Medina, J.; Sánchez, A.; Climent, B.; Benedito, S.; García-Sacristán, A.; Rivera, L.; et al. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2019, 28, 101330. [Google Scholar] [CrossRef]
- Prieto, D.; Contreras, C.; Sanchez, A. Endothelial dysfunction, obesity and insulin resistance. Curr. Vasc. Pharmacol. 2014, 12, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Zhe, M.; Hang, Z. Nephrolithiasis as a risk factor of chronic kidney disease: A meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis 2017, 45, 441–448. [Google Scholar] [CrossRef]
- Sáenz-Medina, J.; Jorge, E.; Corbacho, C.; Santos, M.; Sánchez, A.; Soblechero, P.; Virumbrales, E.; Ramil, E.; Coronado, M.J.; Castillón, I.; et al. Metabolic syndrome contributes to renal injury mediated by hyperoxaluria in a murine model of nephrolithiasis. Urolithiasis 2018, 46, 179–186. [Google Scholar] [CrossRef]
- Sáenz-Medina, J.; Muñoz, M.; Sanchez, A.; Rodriguez, C.; Jorge, E.; Corbacho, C.; Izquierdo, D.; Santos, M.; Donoso, E.; Virumbrales, E.; et al. Nox1-derived oxidative stress as a common pathogenic link between obesity and hyperoxaluria-related kidney injury. Urolithiasis 2020, 48, 481–492. [Google Scholar] [CrossRef]
- Sáenz-Medina, J.; Martinez, M.; Rosado, S.; Durán, M.; Prieto, D.; Carballido, J. Urolithiasis Develops Endothelial Dysfunction as a Clinical Feature. Antioxidants 2021, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Yazici, O.; Narter, F.; Erbin, A.; Aydin, K.; Kafkasli, A.; Sarica, K. Effect of endothelial dysfunction on the pathogenesis of urolithiasis in patients with metabolic syndrome. Aging Male 2019, 23, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.P.; Zheng, H. Kidney stones may increase the risk of coronary heart disease and stroke: A PRISMA-Compliant meta-analysis. Medicine 2018, 96, e7898. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Mao, M.; O′Corragain, O.; Edmonds, P.; Erickson, S.; Thongprayoon, C. The risk of coronary heart disease in patients with kidney stones: A systematic review and meta-analysis. N. Am. J. Med Sci. 2014, 6, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, S.; Zeng, Z.; Wang, J.; Xie, L.; Li, T.; He, Y.; Qin, X.; Zhao, J. Kidney Stones and Cardiovascular Risk: A Meta-analysis of Cohort Studies. Am. J. Kidney Dis. 2014, 64, 402–410. [Google Scholar] [CrossRef]
- Aydın, H.; Yencilek, F.; Mutlu, N.; Çomunoğlu, N.; Koyuncu, H.H.; Sarıca, K. Ethylene Glycol Induced Hyperoxaluria Increases Plasma and Renal Tissue Asymmetrical Dimethylarginine in Rats: A New Pathogenetic Link in Hyperoxaluria Induced Disorders. J. Urol. 2010, 183, 759–764. [Google Scholar] [CrossRef]
- Sarıca, K.; Aydin, H.; Yencilek, F.; Telci, D.; Yilmaz, B. Human umbilical vein endothelial cells accelerate oxalate-induced apoptosis of human renal proximal tubule epithelial cells in co-culture system which is prevented by pyrrolidine dithiocarbamate. Urol. Res. 2012, 40, 461–466. [Google Scholar] [CrossRef]
- Khan, S.R.; Kok, D.J. Modulators of urinary stone formation. Front Biosci. 2004, 9, 1450–1482. [Google Scholar] [CrossRef]
- Muñoz, M.; Martínez, M.P.; López-Oliva, M.E.; Rodríguez, C.; Corbacho, C.; Carballido, J.; García-Sacristán, A.; Hernández, M.; Rivera, L.; Sáenz-Medina, J.; et al. Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries. Redox Biol. 2018, 19, 92–104. [Google Scholar] [CrossRef]
- Muñoz, M.; Sánchez, A.; Martínez, M.P.; Benedito, S.; López-Oliva, M.-E.; García-Sacristán, A.; Hernández, M.; Prieto, D. COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic. Biol. Med. 2015, 84, 77–90. [Google Scholar] [CrossRef]
- Khan, S.R. Animal models of kidney stone formation: An analysis. World J. Urol. 1997, 15, 236–243. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease-a 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Sánchez, A.; Sáenz-Medina, J.; Muñoz, M.; Hernández, M.; López, M.; Rivera, L.; Contreras, C.; Prieto, D. Activation of AMP kinase ameliorates kidney vascular dysfunction, oxidative stress and inflammation in rodent models of obesity. Br. J. Pharmacol. 2021, 178, 4085–4103. [Google Scholar] [CrossRef]
- Yencilek, E.; Sarı, H.; Yencilek, F.; Yeşil, E.; Aydın, H. Systemic endothelial function measured by flow-mediated dilation is impaired in patients with urolithiasis. Urolithiasis 2017, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. Nitric Oxide and the Vascular Endothelium. In The Vascular Endothelium I. Handbook of Experimental Pharmacology; Moncada, S., Higgs, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Yuan, S.Y.; Breslin, J.W.; Perrin, R.; Gaudreault, N.; Guo, M.; Kargozaran, H.; Wu, M.H. Microvascular Permeability in Diabetes and Insulin Resistance. Microcirculation 2007, 14, 363–373. [Google Scholar] [CrossRef]
- Simionescu, M.; Gafencu, A.; Antohe, F. Transcytosis of plasma macromolecules in endothelial cells: A cell biological survey. Microsc. Res. Tech. 2002, 57, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Maric, C.; Kaesemeyer, W.H.; Zaharis, C.Z.; Stewart, J.; Pollock, J.S.; Imig, J.D. Rofecoxib decreases renal injury in obese Zucker rats. Clin. Sci. 2004, 107, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Erdei, N.; Tóth, A.; Pásztor, E.T.; Papp, Z.; Édes, I.; Koller, A.; Bagi, Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: Role of xanthine oxidase-derived superoxide anion. Am. J. Physiol. Circ. Physiol. 2006, 291, H2107–H2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.R.; Darley-Usmar, V.; Berrington, W.R.; McAdams, M.; Gore, J.Z.; Thompson, J.A.; Parks, D.A.; Tarpey, M.M.; Freeman, B.A. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc. Natl. Acad. Sci. USA 1996, 93, 8745–8749. [Google Scholar] [CrossRef] [Green Version]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Allopurinol protects human glomerular endothelial cells from high glucose-induced reactive oxygen species generation, p53 overexpression and endothelial dysfunction. Int. Urol. Nephrol. 2018, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, C.; Kilcoyne, C.M.; Cannon, R.O.; Quyyumi, A.A.; Panza, J.A. Xanthine Oxidase Inhibition With Oxypurinol Improves Endothelial Vasodilator Function in Hypercholesterolemic but Not in Hypertensive Patients. Hypertension 1997, 30, 57–63. [Google Scholar] [CrossRef]
- Houston, M.; Estevez, A.; Chumley, P.; Aslan, M.; Marklund, S.; Parks, D.A.; Freeman, B.A. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999, 274, 4985–4994. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Man, R.Y.; Vanhoutte, P.M. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol. Sin. 2008, 29, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Vanhoutte, P.M. Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br. J. Pharmacol. 2008, 154, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Revelles, S.; Avendaño, M.S.; García-Redondo, A.B.; Alvarez, Y.; Aguado, A.; Pérez-Girón, J.V.; García-Redondo, L.; Esteban, V.; Redondo, J.M.; Alonso, M.J.; et al. Reciprocal Relationship Between Reactive Oxygen Species and Cyclooxygenase-2 and Vascular Dysfunction in Hypertension. Antioxidants Redox Signal. 2013, 18, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.-M.; Breyer, M.D. Physiological Regulation of Prostaglandins in the Kidney. Annu. Rev. Physiol. 2008, 70, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Quilley, J.; Santos, M.; Pedraza, P. Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. Am. J. Physiol. Circ. Physiol. 2011, 300, H2316–H2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghamohammadzadeh, R.; Withers, S.; Lynch, F.; Greenstein, A.; Malik, R.; Heagerty, A. Perivascular adipose tissue from human systemic and coronary vessels: The emergence of a new pharmacotherapeutic target. Br. J. Pharmacol. 2012, 165, 670–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Booth, F.W.; Lee, S.; Laye, M.J.; Zhang, C. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J. Physiol. 2012, 590, 4255–4268. [Google Scholar] [CrossRef]
- Youn, J.Y.; Gao, L.; Cai, H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 2012, 55, 2069–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picchi, A.; Gao, X.; Belmadani, S.; Potter, B.J.; Focardi, M.; Chilian, W.M.; Zhang, C. Tumor Necrosis Factor-α Induces Endothelial Dysfunction in the Prediabetic Metabolic Syndrome. Circ. Res. 2006, 99, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Sud, A.; Kaur, T.; Tandon, C.; Singla, S.K. N-acetylcysteine with apocynin prevents hyperoxaluria-induced mitochondrial protein perturbations in nephrolithiasis. Free Radic. Res. 2016, 50, 1032–1044. [Google Scholar] [CrossRef]
- Babelova, A.; Avaniadi, D.; Jung, O.; Fork, C.; Beckmann, J.; Kosowski, J.; Weissmann, N.; Anilkumar, N.; Shah, A.M.; Schaefer, L.; et al. Role of Nox4 in murine models of kidney disease. Free. Radic. Biol. Med. 2012, 53, 842–853. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Read, M.A.; Whitley, M.Z.; Williams, A.J.; Collins, T. NF-kappa B and I kappa B alpha: An inducible regulatory system in endothelial activation. J. Exp. Med. 1994, 179, 503–512. [Google Scholar] [CrossRef]
- Ungvari, Z.; Orosz, Z.; Labinskyy, N.; Rivera, A.; Xiangmin, Z.; Smith, K.; Csiszar, A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H37–H47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralisch, S.; Sommer, G.; Stangl, V.; Köhler, U.; Kratzsch, J.; Stepan, H.; Fasshauer, M. Secretory products from human adipocytes impair endothelial function via nuclear factor kappaB. Atherosclerosis 2008, 196, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.L.; Lesniewski, L.A.; Lawson, B.R.; Beske, S.D.; Seals, D.R. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 2009, 119, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonassen, J.A.; Kohjimoto, Y.; Scheid, C.R.; Schmidt, M. Oxalate toxicity in renal cells. Urol. Res. 2005, 33, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, H.; Li, Q.; Wang, Y.; Xu, Q. The expression of monocyte chemoattractant protein-1 and C–C chemokine receptor 2 in post-kidney transplant patients and the influence of simvastatin treatment. Clin. Chim. Acta 2006, 373, 44–48. [Google Scholar] [CrossRef]
- Knight, S.F.; Yuan, J.; Roy, S.; Imig, J.D. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am. J. Physiol. Physiol. 2010, 298, F86–F94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonla, C.; Hunapathed, C.; Bovornpadungkitti, S.; Poonpirome, K.; Tungsanga, K.; Sampatanukul, P.; Tosukhowong, P. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int. 2008, 101, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, P.J.; Mariappan, N.; Elks, C.M.; Haque, M.; Francis, J. Diet-induced Renal Changes in Zucker Rats Are Ameliorated by the Superoxide Dismutase Mimetic TEMPOL. Obesity 2009, 17, 1994–2002. [Google Scholar] [CrossRef] [Green Version]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet–induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| CONTROL | Relaxation | OX | ||||||
|---|---|---|---|---|---|---|---|---|
| pEC50 | Emax (%) | l1 | n | pEC50 | Emax (%) | l1 | n | |
| Ach | 6.84 ± 0.07 | 69 ± 3 | 295 ± 13 | 22 | 6.25 ± 0.12 *** | 50 ± 3 *** | 360 ± 14 ** | 30 |
| Contraction | ||||||||
| pEC50 | Emax (Nm−1) | l1 | n | pEC50 | Emax (Nm−1) | l1 | n | |
| Phe | 6.80 ± 0.13 | 3.09 ± 0.50 | 299 ± 14 | 12 | 6.73 ± 0.03 | 3.30 ± 0.38 | 360 ± 26 * | 15 |
| KPSS | ----- | 2.36 ± 0.33 | 297 ± 14 | 14 | ----- | 2.14 ± 0.26 | 360 ± 26 * | 15 |
| CONTROL | OX | |||||||
|---|---|---|---|---|---|---|---|---|
| pEC50 | Emax (%) | l1 | n | pEC50 | Emax (%) | l1 | n | |
| Control | 7.02 ± 0.10 | 65 ± 6 | 286 ± 16 | 7 | 6.98 ± 0.11 | 44 ± 5 ‡‡ | 367 ± 43 | 9 |
| +tempol | 6.96 ± 0.11 | 71 ± 6 | 7 | 7.09 ± 0.13 | 56 ± 6 ** | 9 | ||
| Control | 7.02 ± 0.10 | 65 ± 6 | 286 ± 16 | 7 | 6.92 ± 0.12 | 45 ± 6 ‡‡ | 376 ± 48 | 8 |
| +NS-398 | 7.19 ± 0.21 | 84 ± 3 ** | 7 | 7.07 ± 0.15 | 62 ± 10 ** | 8 | ||
| Control | 6.76 ± 0.13 | 74 ± 5 | 267 ± 23 | 9 | 6,01 ± 0.15 ‡‡ | 55 ± 4 ‡‡ | 354 ± 13 | 13 |
| +allopurinol | 6.58 ± 0.12 | 78 ± 6 | 9 | 6,69 ± 0.18 ** | 59 ± 5 | 13 | ||
| Control | 6.69 ± 0.16 | 74 ± 6 | 259 ± 24 | 7 | 5.99 ± 0.16‡‡ | 53 ± 5 ‡‡ | 354 ± 13 | 12 |
| +NoxA1ds-tat | 6.50 ± 0.13 | 80 ± 7 | 7 | 6.44 ± 0.13 * | 62 ± 5 | 12 | ||
| Control | 6.80 ± 0.09 | 65 ± 7 | 345 ± 14 | 6 | 6.01 ± 0.21 ‡‡ | 48 ± 4 ‡ | 324 ± 24 | 7 |
| +GSK2795039 | 6.82 ± 0.15 | 66 ± 8 | 6 | 6.54 ± 0.10 * | 67 ± 8 * | 7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenz-Medina, J.; Muñoz, M.; Rodriguez, C.; Contreras, C.; Sánchez, A.; Coronado, M.J.; Ramil, E.; Santos, M.; Carballido, J.; Prieto, D. Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress. Cells 2022, 11, 2306. https://doi.org/10.3390/cells11152306
Saenz-Medina J, Muñoz M, Rodriguez C, Contreras C, Sánchez A, Coronado MJ, Ramil E, Santos M, Carballido J, Prieto D. Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress. Cells. 2022; 11(15):2306. https://doi.org/10.3390/cells11152306
Chicago/Turabian StyleSaenz-Medina, Javier, Mercedes Muñoz, Claudia Rodriguez, Cristina Contreras, Ana Sánchez, María José Coronado, Elvira Ramil, Martin Santos, Joaquín Carballido, and Dolores Prieto. 2022. "Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress" Cells 11, no. 15: 2306. https://doi.org/10.3390/cells11152306
APA StyleSaenz-Medina, J., Muñoz, M., Rodriguez, C., Contreras, C., Sánchez, A., Coronado, M. J., Ramil, E., Santos, M., Carballido, J., & Prieto, D. (2022). Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress. Cells, 11(15), 2306. https://doi.org/10.3390/cells11152306