Septic Cardiomyopathy: From Pathophysiology to the Clinical Setting
Abstract
:1. Introduction
2. Definition and Epidemiological Considerations
2.1. Challenges in Defining Septic Cardiomyopathy
2.2. Epidemiology of Septic Cardiomyopathy
3. Pathophysiology of Septic Cardiomyopathy
3.1. Inflammatory Pathways and Cardiomyocyte Dysfunction
3.2. Adrenergic System
3.3. Microvascular Dysfunction and Vasoactive Peptides
3.4. Energetic Dysmetabolism
4. Clinical Diagnosis
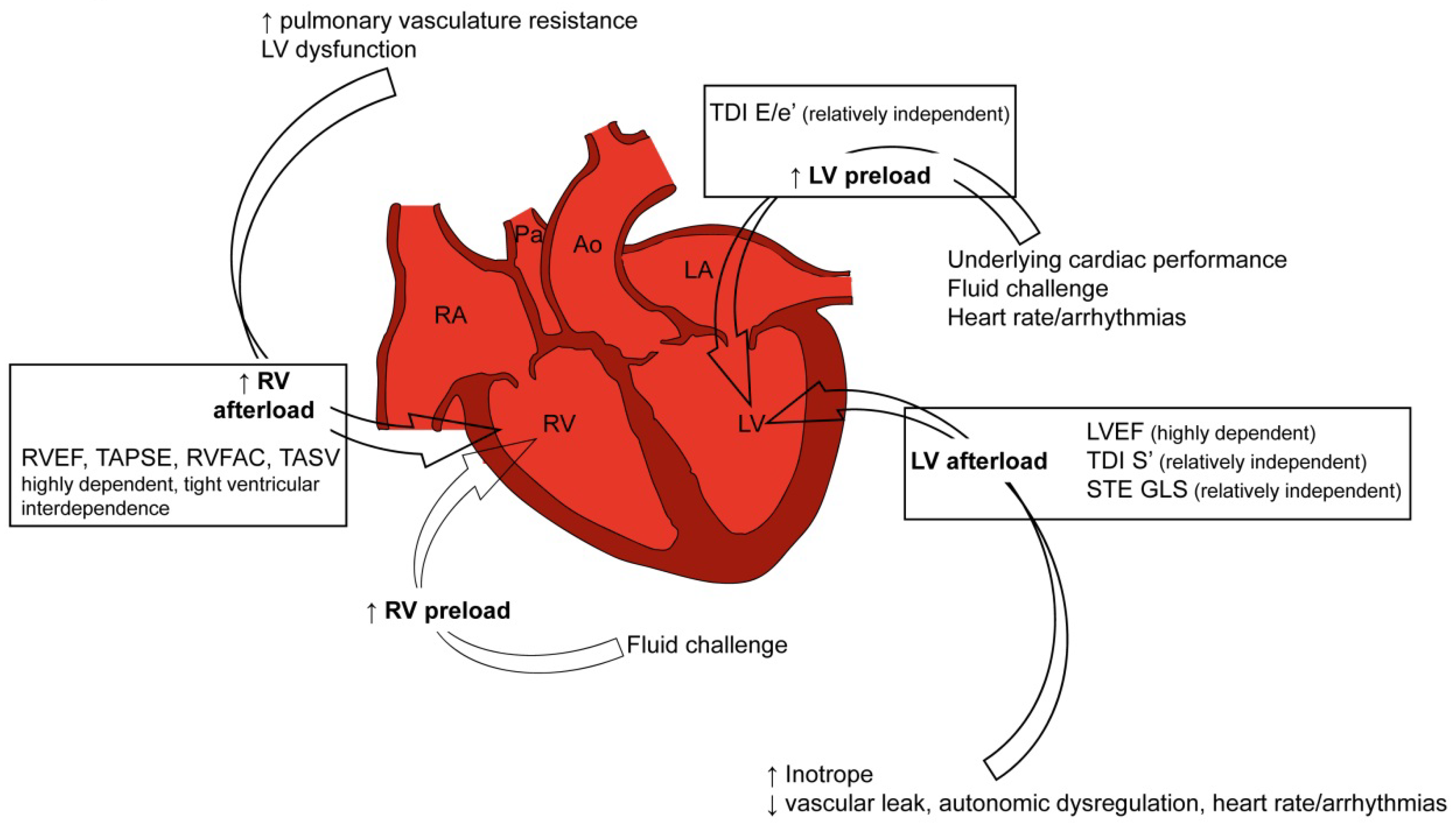
4.1. Doppler Echocardiography: From Classic to Innovative Assessment
4.2. Cardiac Performance
4.3. Biomarkers
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Ward, P.A. Sepsis-induced immune dysfunction: Can immune therapies reduce mortality? J. Clin. Investig. 2016, 126, 23–31. [Google Scholar] [CrossRef]
- Daulasim, A.; Vieillard-Baron, A.; Geri, G. Hemodynamic clinical phenotyping in septic shock. Curr. Opin. Crit. Care 2021, 27, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The septic heart: Current understanding of molecular mechanisms and clinical implications. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.L.; Charron, C.; Silva, S.; Repesse, X.; Vieillard-Baron, A. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 2019, 45, 657–667. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Lang, S.; Ansari, U.; Tulumen, E.; Schramm, K.; Fastner, C.; Zhou, X.; Hoffmann, U.; Borggrefe, M.; Akin, I. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace 2018, 20, 843–850. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Deshmukh, A.J.; Kashani, K.; Prasad, A.; Sakhuja, A. Tako-tsubo cardiomyopathy in severe sepsis: Nationwide trends, predictors, and outcomes. J. Am. Heart Assoc. 2018, 7, e009160. [Google Scholar] [CrossRef]
- Dias, A.; Nunez Gil, I.J.; Santoro, F.; Madias, J.E.; Pelliccia, F.; Brunetti, N.D.; Salmoirago-Blotcher, E.; Sharkey, S.W.; Eitel, I.; Akashi, Y.J.; et al. Takotsubo syndrome: State-of-the-art review by an expert panel—Part 1. Cardiovasc. Revasc. Med. 2019, 20, 70–79. [Google Scholar] [CrossRef]
- Dias, A.; Nunez Gil, I.J.; Santoro, F.; Madias, J.E.; Pelliccia, F.; Brunetti, N.D.; Salmoirago-Blotcher, E.; Sharkey, S.W.; Eitel, I.; Akashi, Y.J.; et al. Takotsubo syndrome: State-of-the-art review by an expert panel—Part 2. Cardiovasc. Revasc. Med. 2019, 20, 153–166. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Santoro, F.; Stiermaier, T.; Moller, C.; Guastafierro, F.; Novo, G.; Novo, S.; Santangelo, A.; Mariano, E.; Romeo, F.; et al. Prevalence, management, and outcome of adverse rhythm disorders in takotsubo syndrome: Insights from the international multicenter geist registry. Heart Fail. Rev. 2020, 25, 505–511. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Santoro, F.; Stiermaier, T.; Moller, C.; Guastafierro, F.; Novo, G.; Novo, S.; Mariano, E.; Romeo, F.; Romeo, F.; et al. Incidence and clinical impact of right ventricular involvement (biventricular ballooning) in takotsubo syndrome: Results from the geist registry. Chest 2021, 160, 1433–1441. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Cammann, V.L.; Kato, K.; Szawan, K.A.; Di Vece, D.; Rossi, A.; Wischnewsky, M.; Hermes-Laufer, J.; Gili, S.; Citro, R.; et al. Impact of atrial fibrillation on outcome in takotsubo syndrome: Data from the international takotsubo registry. J. Am. Heart Assoc. 2021, 10, e014059. [Google Scholar] [CrossRef] [PubMed]
- Schmittinger, C.A.; Dunser, M.W.; Torgersen, C.; Luckner, G.; Lorenz, I.; Schmid, S.; Joannidis, M.; Moser, P.; Hasibeder, W.R.; Halabi, M.; et al. Histologic pathologies of the myocardium in septic shock: A prospective observational study. Shock 2013, 39, 329–335. [Google Scholar] [CrossRef]
- Liu, R.; Greenstein, J.L.; Granite, S.J.; Fackler, J.C.; Bembea, M.M.; Sarma, S.V.; Winslow, R.L. Data-driven discovery of a novel sepsis pre-shock state predicts impending septic shock in the icu. Sci. Rep. 2019, 9, 6145. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Shankar, A.; Vojjini, R.; Cheungpasitporn, W.; Sundaragiri, P.R.; DuBrock, H.M.; Sekiguchi, H.; Frantz, R.P.; Cajigas, H.R.; Kane, G.C.; et al. Impact of right ventricular dysfunction on short-term and long-term mortality in sepsis: A meta-analysis of 1,373 patients. Chest 2021, 159, 2254–2263. [Google Scholar] [CrossRef]
- Beesley, S.J.; Sorensen, J.; Walkey, A.J.; Tonna, J.E.; Lanspa, M.J.; Hirshberg, E.; Grissom, C.K.; Horne, B.D.; Burk, R.; Abraham, T.P.; et al. Long-term implications of abnormal left ventricular strain during sepsis. Crit. Care Med. 2021, 49, e444–e453. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Nalos, M.; McLean, A.S. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit. Care 2013, 17, R96. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Huang, S.; Messina, A.; Franchi, F.; Oliveri, F.; Vieillard-Baron, A.; Cecconi, M.; Astuto, M. Systolic dysfunction as evaluated by tissue doppler imaging echocardiography and mortality in septic patients: A systematic review and meta-analysis. J. Crit. Care 2021, 62, 256–264. [Google Scholar] [CrossRef]
- Esteban, A.; Frutos-Vivar, F.; Ferguson, N.D.; Penuelas, O.; Lorente, J.A.; Gordo, F.; Honrubia, T.; Algora, A.; Bustos, A.; Garcia, G.; et al. Sepsis incidence and outcome: Contrasting the intensive care unit with the hospital ward. Crit. Care Med. 2007, 35, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J.; Piel, D.A.; Acton, P.D.; Zhou, R.; Ferrari, V.A.; Karp, J.S.; Deutschman, C.S. Evidence of myocardial hibernation in the septic heart. Crit. Care Med. 2005, 33, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Davin, F.; Guignant, C.; Larue, A.; Cazalis, M.A.; Darbon, R.; Allombert, C.; Mougin, B.; Malcus, C.; Poitevin-Later, F.; et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock 2010, 34, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; O’Dea, K.; Gordon, A. Immune therapy in sepsis: Are we ready to try again? J. Intensive Care Soc. 2018, 19, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Conway-Morris, A.; Wilson, J.; Shankar-Hari, M. Immune activation in sepsis. Crit. Care Clin. 2018, 34, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Hawiger, J. Heartfelt sepsis: Microvascular injury due to genomic storm. Kardiol. Pol. 2018, 76, 1203–1216. [Google Scholar] [CrossRef]
- Rosengart, M.R.; Nathens, A.B.; Arbabi, S.; Neff, M.J.; Garcia, I.; Martin, T.R.; Maier, R.V. Mitogen-activated protein kinases in the intensive care unit: Prognostic potential. Ann. Surg. 2003, 237, 94–100. [Google Scholar] [CrossRef]
- Martin, L.; Schmitz, S.; De Santis, R.; Doemming, S.; Haase, H.; Hoeger, J.; Heinbockel, L.; Brandenburg, K.; Marx, G.; Schuerholz, T. Peptide 19-2.5 inhibits heparan sulfate-triggered inflammation in murine cardiomyocytes stimulated with human sepsis serum. PLoS ONE 2015, 10, e0127584. [Google Scholar] [CrossRef]
- Wang, H.; Ward, M.F.; Sama, A.E. Novel hmgb1-inhibiting therapeutic agents for experimental sepsis. Shock 2009, 32, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Alhamdi, Y.; Abrams, S.T.; Cheng, Z.; Jing, S.; Su, D.; Liu, Z.; Lane, S.; Welters, I.; Wang, G.; Toh, C.H. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit. Care Med. 2015, 43, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, A.F.; Fromm, R.E.; Parker, M.M.; Brenner, M.; Kovacs, J.A.; Wesley, R.A.; Parrillo, J.E. The cardiovascular response of normal humans to the administration of endotoxin. N. Engl. J. Med. 1989, 321, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Wesley, R.A.; Reilly, J.M.; Parillo, J.E. Endotoxemia in human septic shock. Chest 1991, 99, 169–175. [Google Scholar] [CrossRef]
- Yucel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.H.; Cyganek, L.; Utikal, J.; et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 2935. [Google Scholar] [CrossRef]
- Sattler, K.; El-Battrawy, I.; Cyganek, L.; Lang, S.; Lan, H.; Li, X.; Zhao, Z.; Utikal, J.; Wieland, T.; Borggrefe, M.; et al. Trpv1 activation and internalization is part of the lps-induced inflammation in human ipsc-derived cardiomyocytes. Sci. Rep. 2021, 11, 14689. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Li, Y.; Fan, X.; Yang, Z.; El-Battrawy, I.; Zhou, X.; Akin, I. Lipopolysaccharide modifies sodium current kinetics through ros and pkc signalling in induced pluripotent stem-derived cardiomyocytes from brugada syndrome patient. J. Cardiovasc. Dev. Dis. 2022, 9, 119. [Google Scholar] [CrossRef]
- Fan, X.; Yang, G.; Kowitz, J.; Akin, I.; Zhou, X.; El-Battrawy, I. Takotsubo syndrome: Translational implications and pathomechanisms. Int. J. Mol. Sci. 2022, 23, 1951. [Google Scholar] [CrossRef] [PubMed]
- Natanson, C.; Eichenholz, P.W.; Danner, R.L.; Eichacker, P.Q.; Hoffman, W.D.; Kuo, G.C.; Banks, S.M.; MacVittie, T.J.; Parrillo, J.E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J. Exp. Med. 1989, 169, 823–832. [Google Scholar] [CrossRef]
- Kalbitz, M.; Fattahi, F.; Grailer, J.J.; Jajou, L.; Malan, E.A.; Zetoune, F.S.; Huber-Lang, M.; Russell, M.W.; Ward, P.A. Complement-induced activation of the cardiac nlrp3 inflammasome in sepsis. FASEB J. 2016, 30, 3997–4006. [Google Scholar] [CrossRef]
- Wagner, S.; Schurmann, S.; Hein, S.; Schuttler, J.; Friedrich, O. Septic cardiomyopathy in rat lps-induced endotoxemia: Relative contribution of cellular diastolic ca(2+) removal pathways, myofibrillar biomechanics properties and action of the cardiotonic drug levosimendan. Basic Res. Cardiol. 2015, 110, 507. [Google Scholar] [CrossRef]
- Koentges, C.; Cimolai, M.C.; Pfeil, K.; Wolf, D.; Marchini, T.; Tarkhnishvili, A.; Hoffmann, M.M.; Odening, K.E.; Diehl, P.; von Zur Muhlen, C.; et al. Impaired sirt3 activity mediates cardiac dysfunction in endotoxemia by calpain-dependent disruption of atp synthesis. J. Mol. Cell. Cardiol. 2019, 133, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Fan, C.; Yang, M.; Dong, M.; Bucala, R.; Pei, Z.; Zhang, Y.; Ren, J. Cd74 knockout protects against lps-induced myocardial contractile dysfunction through ampk-skp2-suv39h1-mediated demethylation of bclb. Br. J. Pharm. 2020, 177, 1881–1897. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.C.; Reyes, M.V.; Lakkadi, K.R.; Gowen, B.H.; Hasko, G.; Drosatos, K.; Morrow, J.P. Pkcdelta causes sepsis-induced cardiomyopathy by inducing mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H778–H786. [Google Scholar] [CrossRef] [PubMed]
- Hobai, I.A.; Edgecomb, J.; LaBarge, K.; Colucci, W.S. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock 2015, 43, 3–15. [Google Scholar] [CrossRef]
- DeGrande, S.T.; Little, S.C.; Nixon, D.J.; Wright, P.; Snyder, J.; Dun, W.; Murphy, N.; Kilic, A.; Higgins, R.; Binkley, P.F.; et al. Molecular mechanisms underlying cardiac protein phosphatase 2a regulation in heart. J. Biol. Chem. 2013, 288, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, E.; Fiaccadori, E.; Donadello, K.; Taccone, F.S.; Franchi, F.; Scolletta, S. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. J. Crit. Care 2014, 29, 500–511. [Google Scholar] [CrossRef]
- Zaky, A.; Deem, S.; Bendjelid, K.; Treggiari, M.M. Characterization of cardiac dysfunction in sepsis: An ongoing challenge. Shock 2014, 41, 12–24. [Google Scholar] [CrossRef]
- Vallejo, J.G. Role of toll-like receptors in cardiovascular diseases. Clin. Sci. 2011, 121, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Jiang, X.; Lv, J.; Li, Y.; Ye, H.; Liu, W.; Wang, G.; Zhang, C.; Zheng, N.; et al. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum ca(2+) leakage promote cardiac contractile dysfunction in sepsis. J. Biol. Chem. 2018, 293, 794–807. [Google Scholar] [CrossRef]
- Nath, K.A.; Belcher, J.D.; Nath, M.C.; Grande, J.P.; Croatt, A.J.; Ackerman, A.W.; Katusic, Z.S.; Vercellotti, G.M. Role of tlr4 signaling in the nephrotoxicity of heme and heme proteins. Am. J. Physiol. Ren. Physiol. 2018, 314, F906–F914. [Google Scholar] [CrossRef]
- Fattahi, F.; Frydrych, L.M.; Bian, G.; Kalbitz, M.; Herron, T.J.; Malan, E.A.; Delano, M.J.; Ward, P.A. Role of complement c5a and histones in septic cardiomyopathy. Mol. Immunol. 2018, 102, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Raeburn, C.D.; Calkins, C.M.; Zimmerman, M.A.; Song, Y.; Ao, L.; Banerjee, A.; Harken, A.H.; Meng, X. Icam-1 and vcam-1 mediate endotoxemic myocardial dysfunction independent of neutrophil accumulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R477–R486. [Google Scholar] [CrossRef] [PubMed]
- Rastaldo, R.; Pagliaro, P.; Cappello, S.; Penna, C.; Mancardi, D.; Westerhof, N.; Losano, G. Nitric oxide and cardiac function. Life Sci. 2007, 81, 779–793. [Google Scholar] [CrossRef]
- Kakihana, Y.; Ito, T.; Nakahara, M.; Yamaguchi, K.; Yasuda, T. Sepsis-induced myocardial dysfunction: Pathophysiology and management. J. Intensive Care 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Spiller, F.; Oliveira Formiga, R.; Fernandes da Silva Coimbra, J.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide 2019, 89, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Khadour, F.H.; Panas, D.; Ferdinandy, P.; Schulze, C.; Csont, T.; Lalu, M.M.; Wildhirt, S.M.; Schulz, R. Enhanced no and superoxide generation in dysfunctional hearts from endotoxemic rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1108–H1115. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Kirov, M.Y.; Evgenov, O.V.; Evgenov, N.V.; Egorina, E.M.; Sovershaev, M.A.; Sveinbjørnsson, B.; Nedashkovsky, E.V.; Bjertnaes, L.J. Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Crit. Care Med. 2001, 29, 1860–1867. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Cunnion, R.E.; Zimmerberg, J. Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to catecholamines in septic rats. Am. J. Physiol. 1993, 264, H660–H663. [Google Scholar] [CrossRef]
- Rudiger, A.; Dyson, A.; Felsmann, K.; Carré, J.E.; Taylor, V.; Hughes, S.; Clatworthy, I.; Protti, A.; Pellerin, D.; Lemm, J.; et al. Early functional and transcriptomic changes in the myocardium predict outcome in a long-term rat model of sepsis. Clin. Sci. 2013, 124, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Yu, M.M.; Shou, S.T.; Chai, Y.F. Sepsis-induced cardiomyopathy: Mechanisms and treatments. Front. Immunol. 2017, 8, 1021. [Google Scholar] [CrossRef]
- Rehman, A.; Baloch, N.U.; Morrow, J.P.; Pacher, P.; Haskó, G. Targeting of g-protein coupled receptors in sepsis. Pharmacol. Ther. 2020, 211, 107529. [Google Scholar] [CrossRef]
- Celes, M.R.; Malvestio, L.M.; Suadicani, S.O.; Prado, C.M.; Figueiredo, M.J.; Campos, E.C.; Freitas, A.C.; Spray, D.C.; Tanowitz, H.B.; da Silva, J.S.; et al. Disruption of calcium homeostasis in cardiomyocytes underlies cardiac structural and functional changes in severe sepsis. PLoS ONE 2013, 8, e68809. [Google Scholar] [CrossRef]
- Macarthur, H.; Westfall, T.C.; Riley, D.P.; Misko, T.P.; Salvemini, D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc. Natl. Acad. Sci. USA 2000, 97, 9753–9758. [Google Scholar]
- Kalbitz, M.; Fattahi, F.; Herron, T.J.; Grailer, J.J.; Jajou, L.; Lu, H.; Huber-Lang, M.; Zetoune, F.S.; Sarma, J.V.; Day, S.M.; et al. Complement destabilizes cardiomyocyte function in vivo after polymicrobial sepsis and in vitro. J. Immunol. 2016, 197, 2353–2361. [Google Scholar] [CrossRef]
- Nuding, S.; Schröder, J.; Presek, P.; Wienke, A.; Müller-Werdan, U.; Ebelt, H.; Werdan, K. Reducing elevated heart rates in patients with multiple organ dysfunction syndrome with the if (funny channel current) inhibitor ivabradine. Shock 2018, 49, 402–411. [Google Scholar] [CrossRef]
- Dal-Secco, D.; DalBó, S.; Lautherbach, N.E.S.; Gava, F.N.; Celes, M.R.N.; Benedet, P.O.; Souza, A.H.; Akinaga, J.; Lima, V.; Silva, K.P.; et al. Cardiac hyporesponsiveness in severe sepsis is associated with nitric oxide-dependent activation of g protein receptor kinase. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H149–H163. [Google Scholar] [CrossRef]
- Nakano, T.; Onoue, K.; Nakada, Y.; Nakagawa, H.; Kumazawa, T.; Ueda, T.; Nishida, T.; Soeda, T.; Okayama, S.; Watanabe, M.; et al. Alteration of β-adrenoceptor signaling in left ventricle of acute phase takotsubo syndrome: A human study. Sci. Rep. 2018, 8, 12731. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The endothelium in sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Liu, S.F.; Newton, R.; Evans, T.W.; Barnes, P.J. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin. Sci. 1996, 90, 301–306. [Google Scholar] [CrossRef]
- Zidar, N.; Dolenc-Strazar, Z.; Jeruc, J.; Jerse, M.; Balazic, J.; Gartner, U.; Jermol, U.; Zupanc, T.; Stajer, D. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the normal human heart and in myocardial infarction. Cardiovasc. Pathol. 2007, 16, 300–304. [Google Scholar] [CrossRef]
- Reines, H.D.; Halushka, P.V.; Cook, J.A.; Wise, W.C.; Rambo, W. Plasma thromboxane concentrations are raised in patients dying with septic shock. Lancet 1982, 2, 174–175. [Google Scholar] [CrossRef]
- Stewart, D.J.; Cernacek, P.; Costello, K.B.; Rouleau, J.L. Elevated endothelin-1 in heart failure and loss of normal response to postural change. Circulation 1992, 85, 510–517. [Google Scholar] [CrossRef]
- Freeman, B.D.; Machado, F.S.; Tanowitz, H.B.; Desruisseaux, M.S. Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 2014, 118, 110–119. [Google Scholar] [CrossRef]
- Yang, L.L.; Gros, R.; Kabir, M.G.; Sadi, A.; Gotlieb, A.I.; Husain, M.; Stewart, D.J. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation 2004, 109, 255–261. [Google Scholar] [CrossRef]
- Carrara, M.; Ferrario, M.; Bollen Pinto, B.; Herpain, A. The autonomic nervous system in septic shock and its role as a future therapeutic target: A narrative review. Ann. Intensive Care 2021, 11, 80. [Google Scholar] [CrossRef]
- Fillmore, N.; Mori, J.; Lopaschuk, G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharm. 2014, 171, 2080–2090. [Google Scholar] [CrossRef]
- Tessier, J.P.; Thurner, B.; Jüngling, E.; Lückhoff, A.; Fischer, Y. Impairment of glucose metabolism in hearts from rats treated with endotoxin. Cardiovasc. Res. 2003, 60, 119–130. [Google Scholar] [CrossRef]
- Memon, R.A.; Fuller, J.; Moser, A.H.; Smith, P.J.; Feingold, K.R.; Grunfeld, C. In vivo regulation of acyl-coa synthetase mrna and activity by endotoxin and cytokines. Am. J. Physiol. 1998, 275, E64–E72. [Google Scholar] [CrossRef]
- Memon, R.A.; Bass, N.M.; Moser, A.H.; Fuller, J.; Appel, R.; Grunfeld, C.; Feingold, K.R. Down-regulation of liver and heart specific fatty acid binding proteins by endotoxin and cytokines in vivo. Biochim. Biophys. Acta 1999, 1440, 118–126. [Google Scholar] [CrossRef]
- Drosatos, K.; Khan, R.S.; Trent, C.M.; Jiang, H.; Son, N.H.; Blaner, W.S.; Homma, S.; Schulze, P.C.; Goldberg, I.J. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ. Heart Fail. 2013, 6, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Stanzani, G.; Duchen, M.R.; Singer, M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 759–773. [Google Scholar] [CrossRef]
- Brealey, D.; Karyampudi, S.; Jacques, T.S.; Novelli, M.; Stidwill, R.; Taylor, V.; Smolenski, R.T.; Singer, M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R491–R497. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Nicholson, D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006, 6, 813–822. [Google Scholar] [CrossRef]
- Crouser, E.D. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 2004, 4, 729–741. [Google Scholar] [CrossRef]
- Dikalov, S. Cross talk between mitochondria and nadph oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef]
- Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015, 78, 100–106. [Google Scholar] [CrossRef]
- Turdi, S.; Han, X.; Huff, A.F.; Roe, N.D.; Hu, N.; Gao, F.; Ren, J. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: Role of autophagy. Free Radic. Biol. Med. 2012, 53, 1327–1338. [Google Scholar] [CrossRef]
- Torraco, A.; Carrozzo, R.; Piemonte, F.; Pastore, A.; Tozzi, G.; Verrigni, D.; Assenza, M.; Orecchioni, A.; D’Egidio, A.; Marraffa, E.; et al. Effects of levosimendan on mitochondrial function in patients with septic shock: A randomized trial. Biochimie 2014, 102, 166–173. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Al Khiami, B.; Efimov, I.R.; Evans, S.; Vader, J.; Jain, A.; Brownstein, B.H.; Hotchkiss, R.S.; Mann, D.L. Widespread down-regulation of cardiac mitochondrial and sarcomeric genes in patients with sepsis. Crit. Care Med. 2017, 45, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevilla Berrios, R.A.; O’Horo, J.C.; Velagapudi, V.; Pulido, J.N. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: A systematic review and meta-analysis. J. Crit. Care 2014, 29, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Paonessa, J.R.; Brennan, T.; Pimentel, M.; Steinhaus, D.; Feng, M.; Celi, L.A. Hyperdynamic left ventricular ejection fraction in the intensive care unit. Crit. Care 2015, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Tritapepe, L.; Lorini, F.L.; Arcadipane, A.; Vieillard-Baron, A.; Cecconi, M. Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2018, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Corredor, C.; Arcadipane, A.; Landesberg, G.; Vieillard-Baron, A.; Cecconi, M.; Fletcher, N. Tissue doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: A systematic review and meta-analysis. Br. J. Anaesth. 2017, 119, 583–594. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.J.; Kim, M.; Ryoo, S.M.; Kim, W.Y. Association between right ventricle dysfunction and poor outcome in patients with septic shock. Heart 2020, 106, 1665–1671. [Google Scholar] [CrossRef]
- Lanspa, M.J.; Cirulis, M.M.; Wiley, B.M.; Olsen, T.D.; Wilson, E.L.; Beesley, S.J.; Brown, S.M.; Hirshberg, E.L.; Grissom, C.K. Right ventricular dysfunction in early sepsis and septic shock. Chest 2021, 159, 1055–1063. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Kumar, M.; Pandompatam, G.; Sakhuja, A.; Kashyap, R.; Kashani, K.; Gajic, O.; Geske, J.B.; Jentzer, J.C. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: An 8-year historical cohort study. Ann. Intensive Care 2017, 7, 94. [Google Scholar] [CrossRef]
- Dugar, S.P.; Vallabhajosyula, S. Right ventricle in sepsis: Clinical and research priority. Heart 2020, 106, 1629–1630. [Google Scholar] [CrossRef]
- Werdan, K.; Oelke, A.; Hettwer, S.; Nuding, S.; Bubel, S.; Hoke, R.; Russ, M.; Lautenschlager, C.; Mueller-Werdan, U.; Ebelt, H. Septic cardiomyopathy: Hemodynamic quantification, occurrence, and prognostic implications. Clin. Res. Cardiol. 2011, 100, 661–668. [Google Scholar] [CrossRef]
- Chen, W.Y.; Zhang, Z.H.; Tao, L.L.; Xu, Q.; Wei, X.; Chen, M.S. Afterload-related cardiac performance identifies cardiac impairment and associates with outcome in patients with septic shock: A retrospective cohort study. J. Intensive Care 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.C.; Zhang, L.R.; Liu, L.X.; Sun, L.X.; Hu, Z.J. Afterload-related cardiac performance predicts prognosis in critical ill patients with sepsis: A prospective observational pilot study. Medicine 2021, 100, e27235. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, J.; Hettwer, S.; Schuermann, M.; Bagger, S.; Gerhardt, F.; Mundt, S.; Muschik, S.; Zimmermann, J.; Bubel, S.; Amoury, M.; et al. Severity of cardiac impairment in the early stage of community-acquired sepsis determines worse prognosis. Clin. Res. Cardiol. 2013, 102, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Lee, T.H.; Bang, C.H.; Kim, J.H.; Hong, S.J. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine 2018, 97, e0263. [Google Scholar] [CrossRef]
- Landesberg, G.; Jaffe, A.S.; Gilon, D.; Levin, P.D.; Goodman, S.; Abu-Baih, A.; Beeri, R.; Weissman, C.; Sprung, C.L.; Landesberg, A. Troponin elevation in severe sepsis and septic shock: The role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit. Care Med. 2014, 42, 790–800. [Google Scholar] [CrossRef]
- Pandompatam, G.; Kashani, K.; Vallabhajosyula, S. The role of natriuretic peptides in the management, outcomes and prognosis of sepsis and septic shock. Rev. Bras. Ter. Intensiva 2019, 31, 368–378. [Google Scholar] [CrossRef]
- Bai, Y.L.; Hu, B.L.; Wen, H.C.; Zhang, Y.L.; Zhu, J.J. Prognostic value of plasma brain natriuretic peptide value for patientswith sepsis: A meta-analysis. J. Crit. Care 2018, 48, 145–152. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Wang, Z.; Murad, M.H.; Vallabhajosyula, S.; Sundaragiri, P.R.; Kashani, K.; Miller, W.L.; Jaffe, A.S.; Vallabhajosyula, S. Natriuretic peptides to predict short-term mortality in patients with sepsis: A systematic review and meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 50–64. [Google Scholar] [CrossRef]
- Beltran-Garcia, J.; Osca-Verdegal, R.; Nacher-Sendra, E.; Cardona-Monzonis, A.; Sanchis-Gomar, F.; Carbonell, N.; Pallardo, F.V.; Lavie, C.J.; Garcia-Gimenez, J.L. Role of non-coding rnas as biomarkers of deleterious cardiovascular effects in sepsis. Prog. Cardiovasc. Dis. 2021, 68, 70–77. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Dong, R.; Zhang, D.; Qin, S. A retrospective observational study of the association between plasma levels of interleukin 8 in 42 patients with sepsis-induced myocardial dysfunction at a single center between 2017 and 2020. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e933065. [Google Scholar] [CrossRef]
- Chen, M.; Kong, C.; Zheng, Z.; Li, Y. Identification of biomarkers associated with septic cardiomyopathy based on bioinformatics analyses. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2020, 27, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Pro, C.I.; Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar]
- Investigators, A.; Group, A.C.T.; Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Refs | Phenotypes | Defining Criteria |
|---|---|---|
| Diagnostic criteria for sepsis-associated cardiac dysfunction unrelated to ischemia *. Beesley et al., 2018 [5] Martin at al., 2019 [6] |
| NA |
| LVEF < 40–50%, ↓ LVFAC | |
| LVEF < 40–50%, ↓ LVFAC, e’ velocity, MPI, Afterload-related cardiac performance, Ventricular arterial decoupling | |
| Cardiovascular phenotypes of septic cardiomyopathy classification performance Geri et al., 2019 [7] |
| – |
| LVEF < 40%, LVFAC < 33%, aortic VTI < 14 cm | |
| Aortic VTI > 20 cm, LVFAC > 58%, heart rate < 106 bpm | |
| RV/LV EDA > 0.8, sBP < 100 mmHg, dBP < 51 mmHg | |
| Aortic VTI < 16 cm, E wave < 67 cm/s, ΔDSVC > 39% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, F.; Liberale, L.; Preda, A.; Schindler, T.H.; Montecucco, F. Septic Cardiomyopathy: From Pathophysiology to the Clinical Setting. Cells 2022, 11, 2833. https://doi.org/10.3390/cells11182833
Carbone F, Liberale L, Preda A, Schindler TH, Montecucco F. Septic Cardiomyopathy: From Pathophysiology to the Clinical Setting. Cells. 2022; 11(18):2833. https://doi.org/10.3390/cells11182833
Chicago/Turabian StyleCarbone, Federico, Luca Liberale, Alberto Preda, Thomas Hellmut Schindler, and Fabrizio Montecucco. 2022. "Septic Cardiomyopathy: From Pathophysiology to the Clinical Setting" Cells 11, no. 18: 2833. https://doi.org/10.3390/cells11182833






