Effects of Human Deciduous Dental Pulp-Derived Mesenchymal Stem Cell-Derived Conditioned Medium on the Metabolism of HUVECs, Osteoblasts, and BMSCs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Preparation of SHED-CM
2.3. Analysis of Cell Proliferation Using the Bromodeoxyuridine Immunoassay
2.4. Endothelial Tube Formation Assay
2.5. Effects of SHED-CM on the Proliferation of Osteoblasts and hBMSCs
2.6. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.7. Western Blot Analysis of ALP Protein Expression
2.8. Statistical Analyses
3. Results
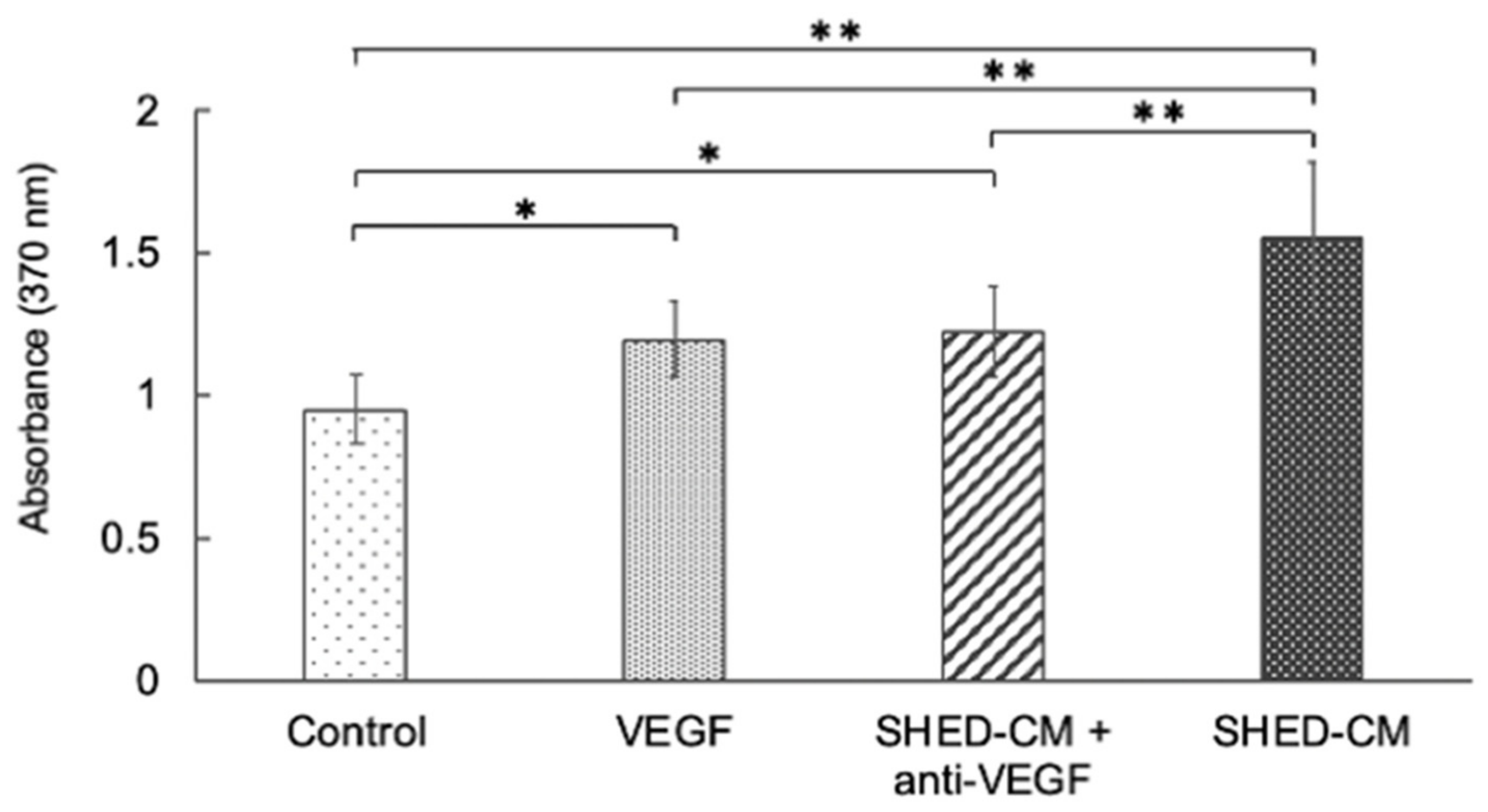
3.1. Effects of VEGF-A Blockade on the Proliferation Potential of SHED-CM in HUVECs
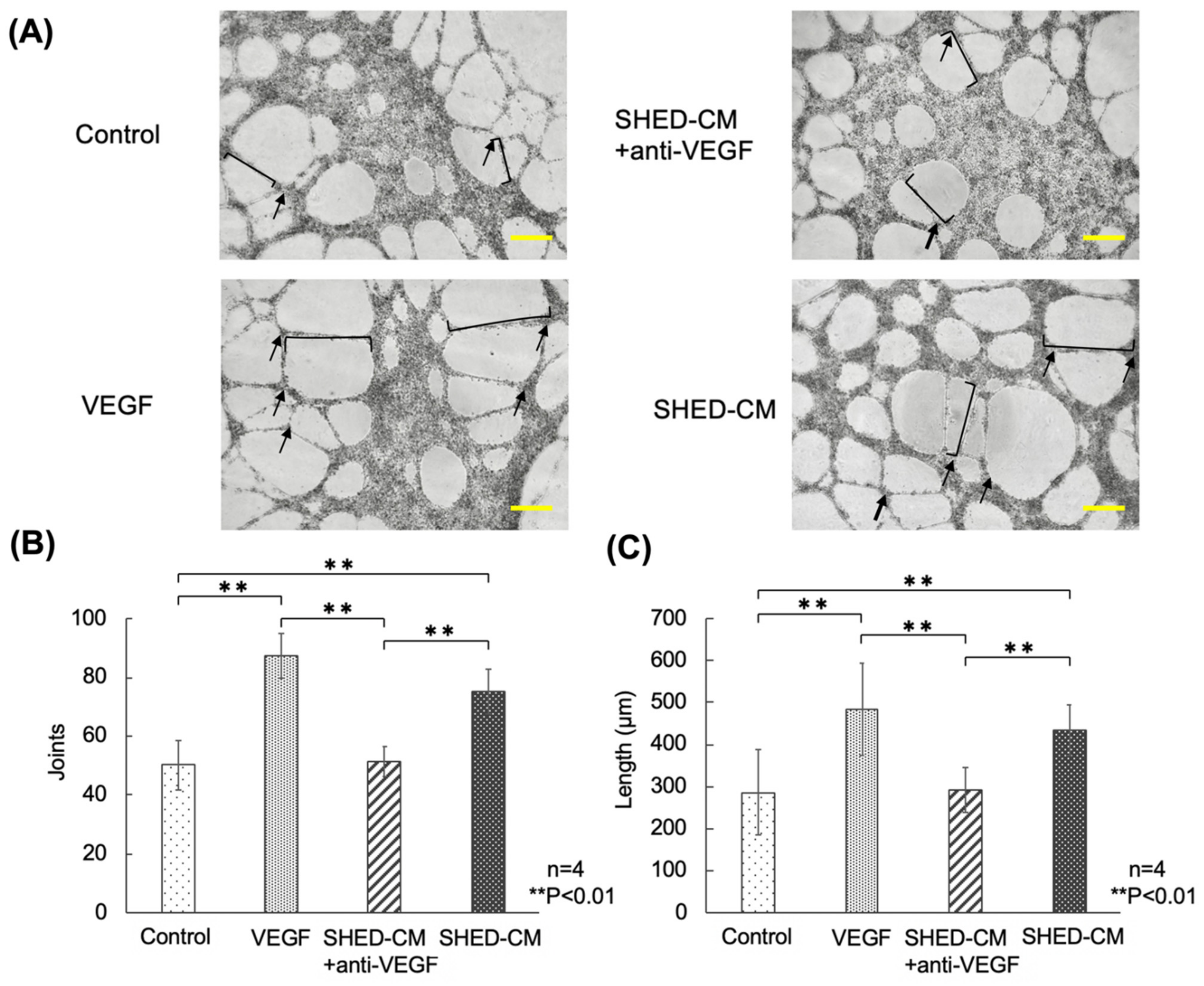
3.2. Effects of VEGF-A Blockade on the Angiogenic Potential of SHED-CM in HUVECs
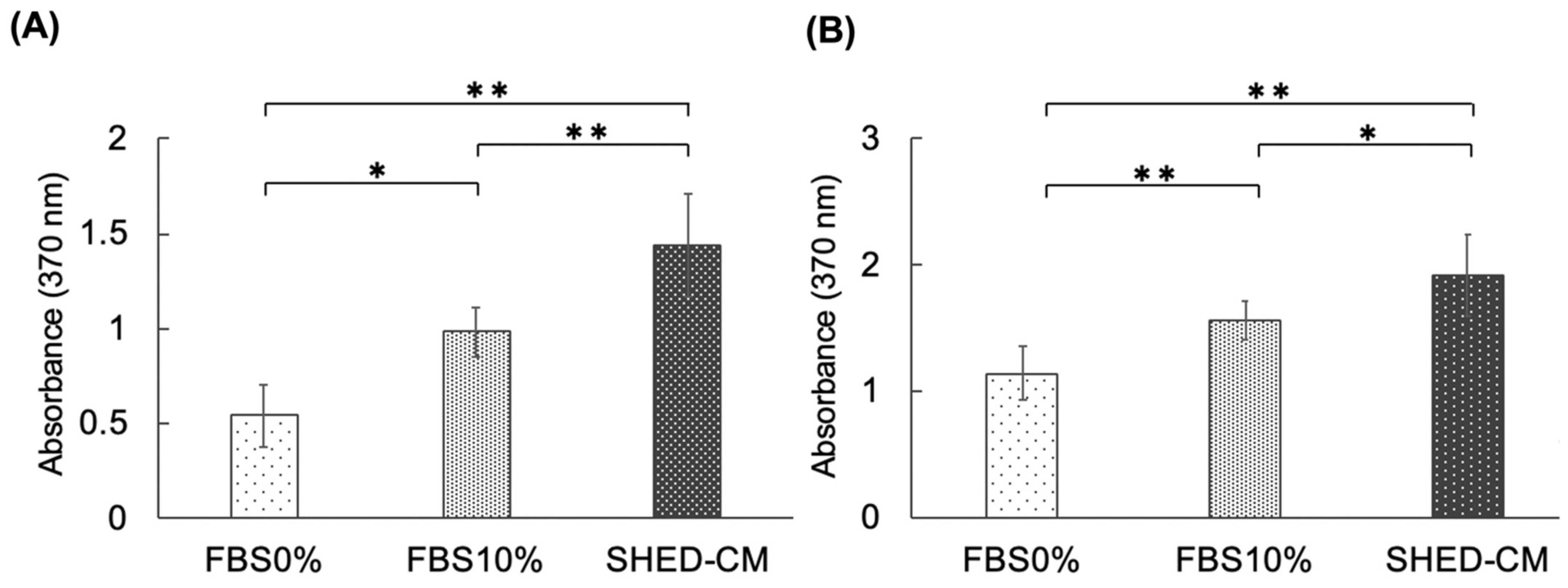
3.3. Effect of SHED-CM on the Proliferative Ability of hBMSCs and MC3T3-E1 Cells
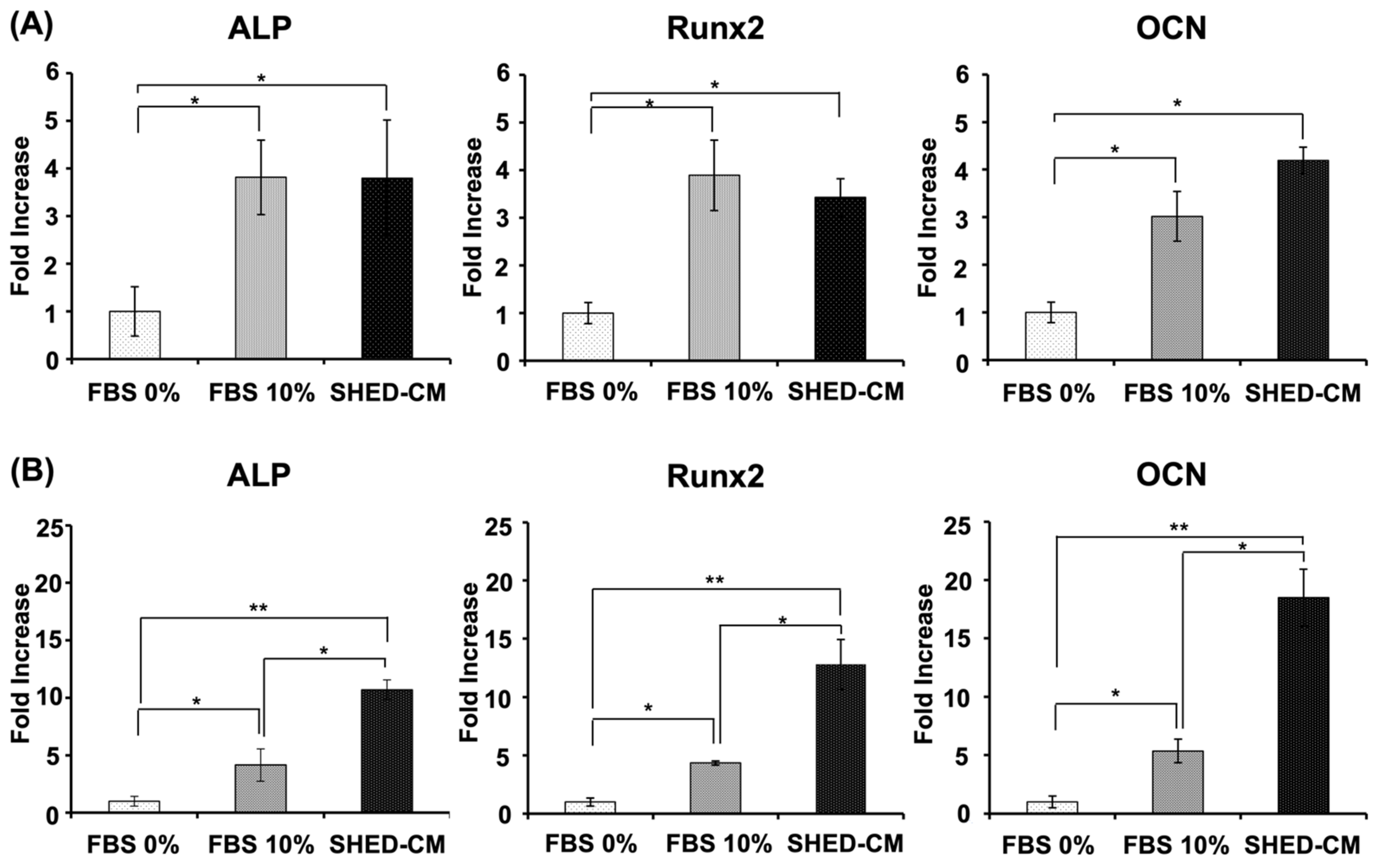
3.4. Effects of SHED-CM on hBMSC and MC3T3-E1 Gene Expression
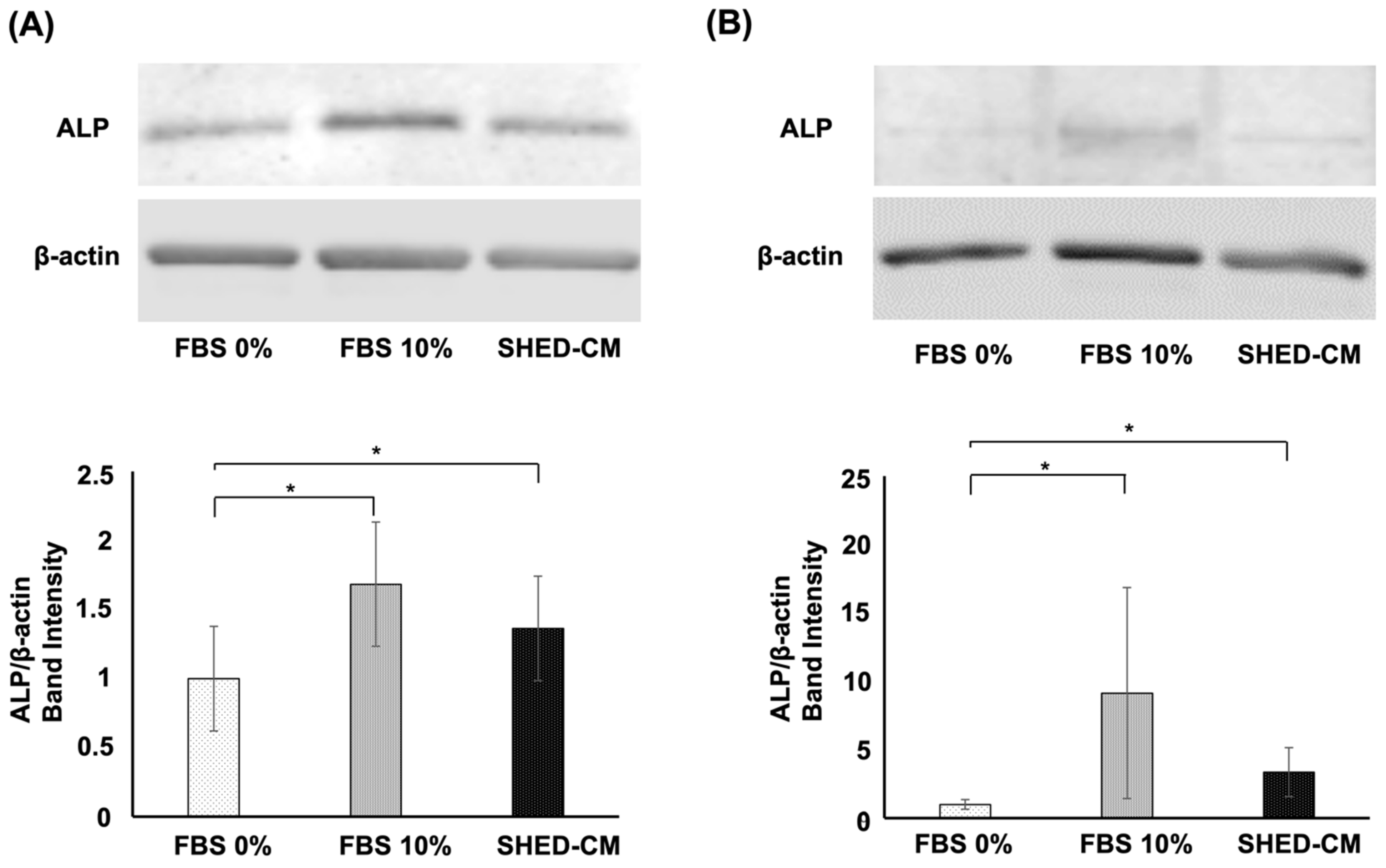
3.5. Effects of SHED-CM on the Protein Expression of ALP in the hBMSCs and MC3T3-E1 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S. Periodontal tissue regeneration by signaling molecule(s): What role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontology 2000 2011, 56, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, B.; Pierantozzi, E.; Sorrentino, V. Mesenchymal stem cells: From the perivascular environment to clinical applications. Histol. Histopathol. 2018, 33, 1235–1246. [Google Scholar] [CrossRef]
- Kichenbrand, C.; Velot, E.; Menu, P.; Moby, V. Dental pulp stem cell-derived conditioned medium: An attractive alternative for regenerative therapy. Tissue Eng. Part B Rev. 2019, 25, 78–88. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- De Mendonça Costa, A.; Bueno, D.F.; Martins, M.T.; Kerkis, I.; Kerkis, A.; Fanganiello, R.D.; Cerruti, H.; Alonso, N.; Passos-Bueno, M.R. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J. Craniofac. Surg. 2008, 19, 204–210. [Google Scholar] [CrossRef]
- Gandia, C.; Armiñan, A.; García-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Riccio, M.; Maraldi, T.; Pisciotta, A.; La Sala, G.B.; Ferrari, A.; Bruzzesi, G.; Motta, A.; Migliaresi, C.; De Pol, A. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng. Part A 2012, 18, 1006–1013. [Google Scholar] [CrossRef]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef]
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [Green Version]
- Hung, S.C.; Pochampally, R.R.; Chen, S.C.; Hsu, S.C.; Prockop, D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef]
- Katagiri, W.; Osugi, M.; Kawai, T.; Ueda, M. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int. J. Oral Maxillofac. Implants 2013, 28, 1009–1016. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef] [Green Version]
- Oskowitz, A.; McFerrin, H.; Gutschow, M.; Carter, M.L.; Pochampally, R. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res. 2011, 6, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Hiraki, T.; Kunimatsu, R.; Nakajima, K.; Abe, T.; Yamada, S.; Rikitake, K.; Tanimoto, K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020, 26, 381–390. [Google Scholar] [CrossRef]
- Rikitake, K.; Kunimatsu, R.; Yoshimi, Y.; Nakajima, K.; Hiraki, T.; Aisyah Rizky Putranti, N.; Tsuka, Y.; Abe, T.; Ando, K.; Hayashi, Y.; et al. Effect of CD146+ SHED on bone regeneration in a mouse calvaria defect model. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef]
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015, 33, 35–42. [Google Scholar] [CrossRef]
- Van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Lee, S.C.; Kim, S.J. A novel cell-free strategy for promoting mouse liver regeneration: Utilization of a conditioned medium from adipose-derived stem cells. Hepatol. Int. 2015, 9, 310–320. [Google Scholar] [CrossRef]
- Matsushita, Y.; Ishigami, M.; Matsubara, K.; Kondo, M.; Wakayama, H.; Goto, H.; Ueda, M.; Yamamoto, A. Multifaceted therapeutic benefits of factors derived from stem cells from human exfoliated deciduous teeth for acute liver failure in rats. J. Tissue Eng. Regen. Med. 2017, 11, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Shibata, R.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Tanigawa, T.; Ueda, M.; Murohara, T.; Yamamoto, A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci. Rep. 2015, 5, 16295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part A 2013, 19, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mita, T.; Furukawa-Hibi, Y.; Takeuchi, H.; Hattori, H.; Yamada, K.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 293, 189–197. [Google Scholar] [CrossRef]
- Sugimura-Wakayama, Y.; Katagiri, W.; Osugi, M.; Kawai, T.; Ogata, K.; Sakaguchi, K.; Hibi, H. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev. 2015, 24, 2687–2699. [Google Scholar] [CrossRef]
- Izumoto-Akita, T.; Tsunekawa, S.; Yamamoto, A.; Uenishi, E.; Ishikawa, K.; Ogata, H.; Iida, A.; Ikeniwa, M.; Hosokawa, K.; Niwa, Y.; et al. Secreted factors from dental pulp stem cells improve glucose intolerance in streptozotocin-induced diabetic mice by increasing pancreatic β-cell function. BMJ Open Diabetes Res. Care 2015, 3, e000128. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, J.; Takahashi, N.; Matsumoto, T.; Yoshioka, Y.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Ishigro, N.; Ueda, M.; Furukawa, K.; et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone 2016, 83, 210–219. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsushita, Y.; Sakai, K.; Kano, F.; Kondo, M.; Noda, M.; Hashimoto, N.; Imagama, S.; Ishiguro, N.; Suzumura, A.; et al. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J. Neurosci. 2015, 35, 2452–2464. [Google Scholar] [CrossRef] [Green Version]
- Hirata, M.; Ishigami, M.; Matsushita, Y.; Ito, T.; Hattori, H.; Hibi, H.; Goto, H.; Ueda, M.; Yamamoto, A. Multifaceted therapeutic benefits of factors derived from dental pulp stem cells for mouse liver fibrosis. Stem Cells Transl. Med. 2016, 5, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Wakayama, H.; Hashimoto, N.; Matsushita, Y.; Matsubara, K.; Yamamoto, N.; Hasegawa, Y.; Ueda, M.; Yamamoto, A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy 2015, 17, 1119–1129. [Google Scholar] [CrossRef]
- Kikuchi, H. Differential influences of bFGF and VEGF on the expression of vascular cell adhesion molecule-1 on human umbilical vein endothelial cells. Nihon Rinsho Meneki Gakkai Kaishi 2000, 23, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Alkharobi, H.; Alhodhodi, A.; Hawsawi, Y.; Alkafaji, H.; Devine, D.; El-Gendy, R.; Beattie, J. IGFBP-2 and -3 co-ordinately regulate IGF1 induced matrix mineralisation of differentiating human dental pulp cells. Stem Cell Res. 2016, 17, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Ma, Z.; Zhang, X.; Wang, W.; Yang, Z.; Zhang, M.; Wu, G.; Lu, W.; Deng, Z.; Jin, Y. Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J. Endod. 2009, 35, 1546–1553. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Ren, K. Exosomes in perspective: A potential surrogate for stem cell therapy. Odontology 2019, 107, 271–284. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem cell-derived exosomes—An emerging tool for myocardial regeneration. World J. Stem Cells 2018, 10, 106–115. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Picou, F.; Court, F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013, 61, 1795–1806. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Sequence ( 5′ → 3′) | |
|---|---|---|
| GAPDH | Forward | CCA CTC CTC CAC CTT TGA |
| Reverse | CAC CAC CCT GTT GCT GTA | |
| Runx2 | Forward | CCA GAT GGG ACT GTG GTT TAC TG |
| Reverse | TTC CGG AGC TCA GCA GAA TAA | |
| ALP | Forward | ATG GTG GAC TGC TCA CAA C |
| Reverse | GAC GTA GTT CTG CTC GTG GA | |
| OCN | Forward | GCA GAG TCC AGG AAA GGG TG |
| Reverse | GTC AGC AAC TCG TCA CAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunimatsu, R.; Hiraki, T.; Rikitake, K.; Nakajima, K.; Putranti, N.A.R.; Abe, T.; Ando, K.; Nakatani, A.; Sakata, S.; Tanimoto, K. Effects of Human Deciduous Dental Pulp-Derived Mesenchymal Stem Cell-Derived Conditioned Medium on the Metabolism of HUVECs, Osteoblasts, and BMSCs. Cells 2022, 11, 3222. https://doi.org/10.3390/cells11203222
Kunimatsu R, Hiraki T, Rikitake K, Nakajima K, Putranti NAR, Abe T, Ando K, Nakatani A, Sakata S, Tanimoto K. Effects of Human Deciduous Dental Pulp-Derived Mesenchymal Stem Cell-Derived Conditioned Medium on the Metabolism of HUVECs, Osteoblasts, and BMSCs. Cells. 2022; 11(20):3222. https://doi.org/10.3390/cells11203222
Chicago/Turabian StyleKunimatsu, Ryo, Tomoka Hiraki, Kodai Rikitake, Kengo Nakajima, Nurul Aisyah Rizky Putranti, Takaharu Abe, Kazuyo Ando, Ayaka Nakatani, Shuzo Sakata, and Kotaro Tanimoto. 2022. "Effects of Human Deciduous Dental Pulp-Derived Mesenchymal Stem Cell-Derived Conditioned Medium on the Metabolism of HUVECs, Osteoblasts, and BMSCs" Cells 11, no. 20: 3222. https://doi.org/10.3390/cells11203222
APA StyleKunimatsu, R., Hiraki, T., Rikitake, K., Nakajima, K., Putranti, N. A. R., Abe, T., Ando, K., Nakatani, A., Sakata, S., & Tanimoto, K. (2022). Effects of Human Deciduous Dental Pulp-Derived Mesenchymal Stem Cell-Derived Conditioned Medium on the Metabolism of HUVECs, Osteoblasts, and BMSCs. Cells, 11(20), 3222. https://doi.org/10.3390/cells11203222





