C/EBPδ Suppresses Motility-Associated Gene Signatures and Reduces PDAC Cell Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture Reagents
2.2. Cloning Strategy and Lentiviral Transduction
2.3. RNA Extraction and RNA Sequencing
2.4. Bioinformatic Analyses
2.5. Scratch Migration Assays
2.6. Manual Cell Tracking
2.7. Chemotaxis Assays
2.8. Fluorescence-Activated Cell Sorting (FACS)
2.9. Phalloidin Staining
2.10. Gene Knockdown
3. Results
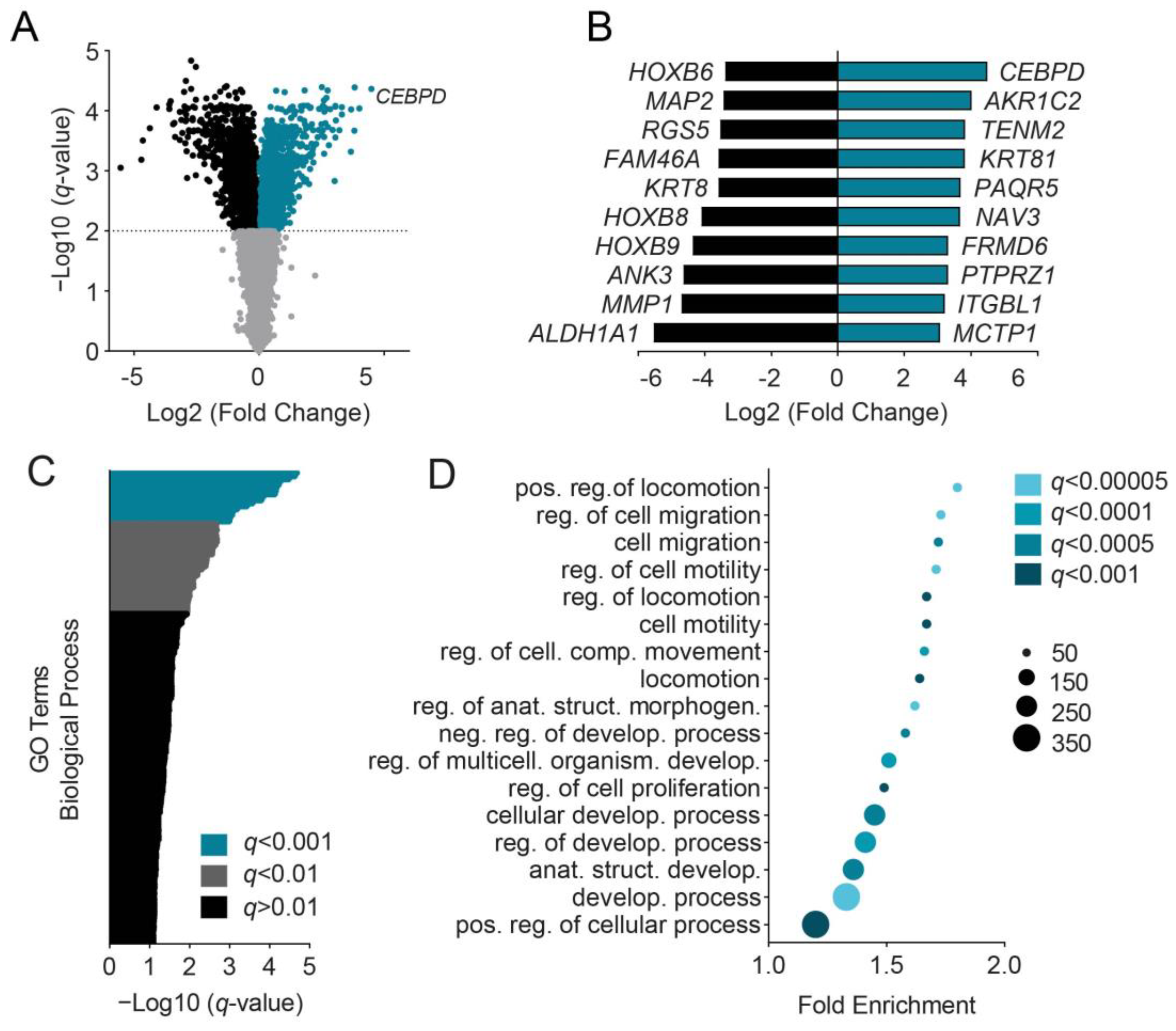
3.1. C/EBPδ Induces Migration-Associated Gene Signatures
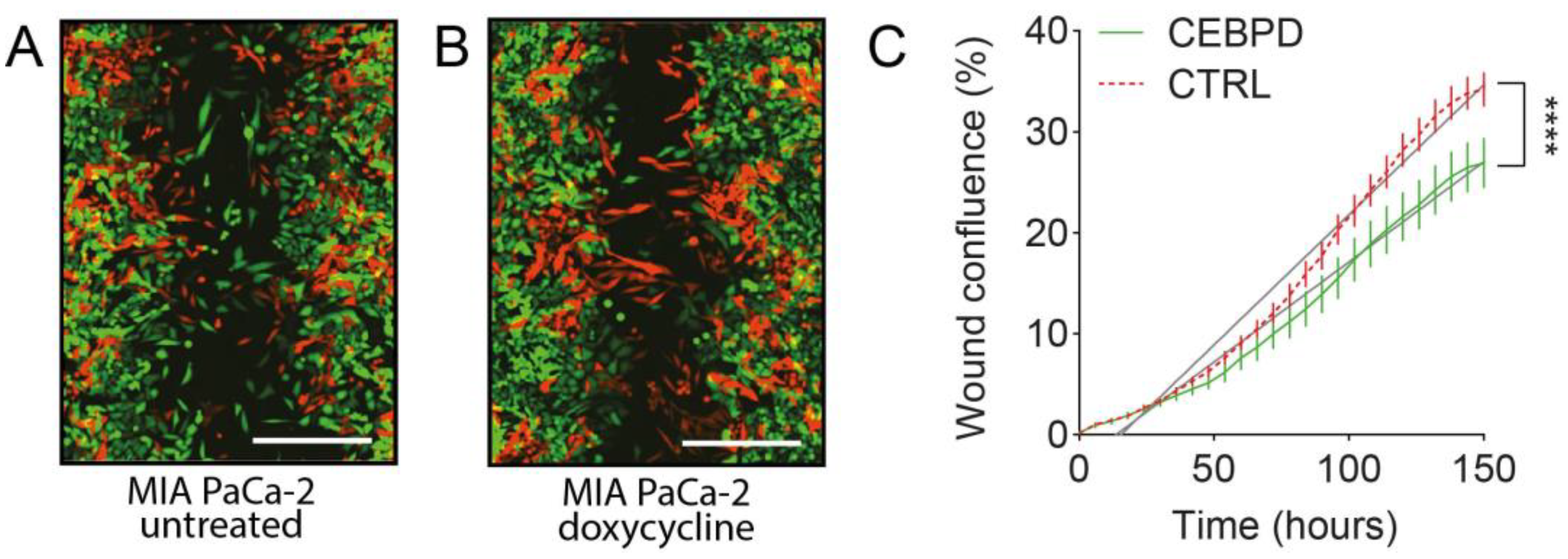
3.2. Induction of C/EBPδ Expression Reduces Migration in PDAC Cells
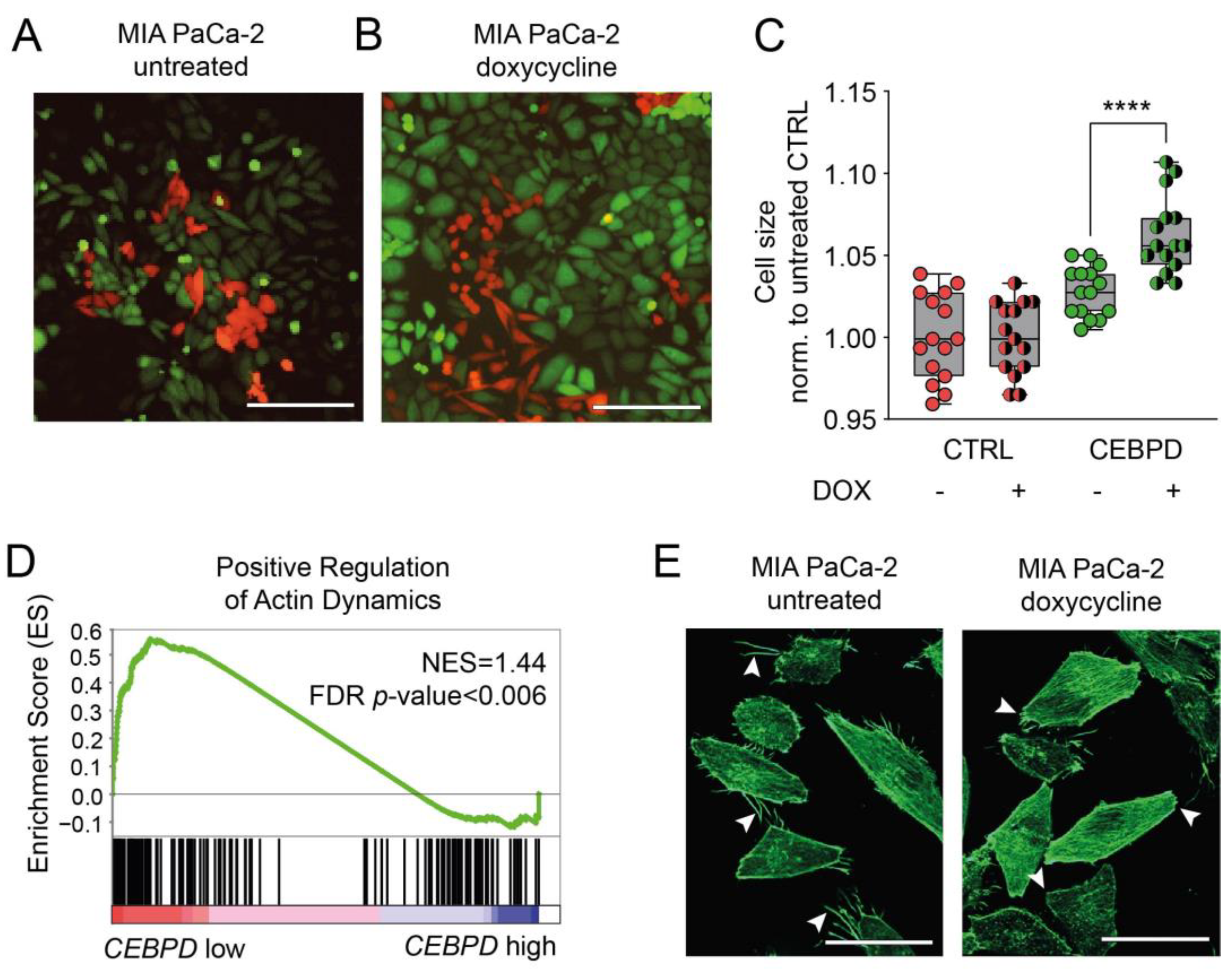
3.3. C/EBPδ Induces a Gene Signature That Negatively Correlates with Actin Dynamics
3.4. C/EBPδ Suppresses EGFR and GSN to Reduce MIA PaCa-2 Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022.
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Das, S. Pancreatic cancer metastasis: Are we being pre-EMTed? Curr. Pharm. Des. 2015, 21, 1249–1255. [Google Scholar] [CrossRef]
- Martin, T.A.; Ye, L.; Sanders, A.; Lane, J.; Jiang, W. Cancer Invasion and Metastasis: Molecular and Cellular Perspective; Jandial, R., Ed.; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Thomas, S.; Lee, J.; Beatty, G.L. Beatty, Paracrine and cell autonomous signalling in pancreatic cancer progression and metastasis. EBioMedicine 2020, 53, 102662. [Google Scholar] [CrossRef]
- Paz, H.; Pathak, N.; Yang, J. Invading one step at a time: The role of invadopodia in tumor metastasis. Oncogene 2014, 33, 4193–4202. [Google Scholar] [CrossRef]
- Chiang, S.P.H.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef]
- Sznurkowska, M.K.; Aceto, N. The gate to metastasis: Key players in cancer cell intravasation. Febs J. 2022, 289, 4336–4354. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Huang, C.; Li, N.; Li, Z.; Chang, A.; Chen, Y.; Zhao, T.; Li, Y.; Wang, X.; Zhang, W.; Wang, Z.; et al. Tumour-derived Interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nat. Commun. 2017, 8, 14035. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wu, W.-J.; Wang, W.-J.; Huang, H.-Y.; Li, W.-M.; Yeh, B.-W.; Wu, T.-F.; Shiue, Y.-L.; Sheu, J.J.-C.; Wang, J.-M.; et al. CEBPD amplification and overexpression in urothelial carcinoma: A driver of tumor metastasis indicating adverse prognosis. Oncotarget 2015, 6, 31069–31084. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Duitman, J.; Aberson, H.L.; Chen, K.; Dijk, F.; Roelofs, J.; Dings, M.P.G.; Hooijer, G.K.J.; Hernanda, P.Y.; Pan, Q.; et al. CCAAT/Enhancer-Binding Protein Delta (C/EBPδ): A Previously Unrecognized Tumor Suppressor that Limits the Oncogenic Potential of Pancreatic Ductal Adenocarcinoma Cells. Cancers 2020, 12, 2546. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Bartsch, U.; Stocking, C.; Fehse, B. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol. Ther. 2008, 16, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2014, 31, 166–169. [Google Scholar] [CrossRef]
- R2: Genomics Analysis and Visualization Platform. Available online: http://r2.amc.nl (accessed on 1 June 2022).
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 2009, 10, 48. [Google Scholar] [CrossRef]
- Eden, E.; Lipson, D.; Yogev, S.; Yakhini, Z. Discovering motifs in ranked lists of DNA sequences. PLoS Comput. Biol. 2007, 3, e39. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Kwon, O.-S.; Cha, H.-J.; Sung, B.J. Stochastic and Heterogeneous Cancer Cell Migration: Experiment and Theory. Sci. Rep. 2019, 9, 16297. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ficca, M.L.; Meyer, R.G.; Kaiser, H.; Brack, A.R.; Kandolf, R.; Küpper, J.-H. Comparative analysis of inducible expression systems in transient transfection studies. Anal. Biochem. 2004, 334, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Manz, X.D.; Albers, H.J.; Symersky, P.; Aman, J.; Van Der Meer, A.D.; Bogaard, H.J.; Szulcek, R. In Vitro Microfluidic Disease Model to Study Whole Blood-Endothelial Interactions and Blood Clot Dynamics in Real-Time. JoVE 2020, 159, e61068. [Google Scholar] [CrossRef] [PubMed]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, 85, e51300. [Google Scholar] [CrossRef]
- Wiejak, J.; Tsimbouri, P.M.; Herzyk, P.; Dalby, M.J.; Hamilton, G.; Yarwood, S.J. Genomic analysis of the role of transcription factor C/EBPδ in the regulation of cell behaviour on nanometric grooves. Biomaterials 2013, 34, 1967–1979. [Google Scholar] [CrossRef][Green Version]
- Du, W.; Phinney, N.Z.; Huang, H.; Wang, Z.; Westcott, J.; Toombs, J.E.; Zhang, Y.; Beg, M.S.; Wilkie, T.M.; Lorens, J.B.; et al. AXL Is a Key Factor for Cell Plasticity and Promotes Metastasis in Pancreatic Cancer. Mol. Cancer Res. 2021, 19, 1412–1421. [Google Scholar] [CrossRef]
- Leconet, W.; Larbouret, C.; Chardès, T.; Thomas, G.; Neiveyans, M.; Busson, M.; Jarlier, M.; Radosevic-Robin, N.; Pugnière, M.; Bernex, F.; et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene 2014, 33, 5405–5414. [Google Scholar] [CrossRef]
- Stock, A.-M.; Hahn, S.; Troost, G.; Niggemann, B.; Zänker, K.S.; Entschladen, F. Induction of pancreatic cancer cell migration by an autocrine epidermal growth factor receptor activation. Exp. Cell. Res. 2014, 326, 307–314. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Chen, Y.; Zhou, M.; Zhang, H.; Chen, R.; Shi, F.; Wang, C.; Rui, Z. Transcriptional silencing of ETS-1 abrogates epithelial-mesenchymal transition resulting in reduced motility of pancreatic cancer cells. Oncol. Rep. 2015, 33, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, K.; Furihata, M.; Naganuma, S.; Dabanaka, K.; Hanazaki, K.; Saibara, T. Podocalyxin-like protein, linked to poor prognosis of pancreatic cancers, promotes cell invasion by binding to gelsolin. Cancer Sci. 2016, 107, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Ashcroft, F.J.; Patel, S.; Saraga, G.; Vimalachandran, D.; Prime, W.; Campbell, F.; Dodson, A.; Jenkins, R.; Lemoine, N.R.; et al. Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility. Gut 2007, 56, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Masugi, Y.; Yamazaki, K.; Emoto, K.; Effendi, K.; Tsujikawa, H.; Kitago, M.; Itano, O.; Kitagawa, Y.; Sakamoto, M. Upregulation of integrin β4 promotes epithelial-mesenchymal transition and is a novel prognostic marker in pancreatic ductal adenocarcinoma. Lab. Invest. 2015, 95, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Otey, C.A.; Rachlin, A.; Moza, M.; Arneman, D.; Carpen, O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int. Rev. Cytol. 2005, 246, 31–58. [Google Scholar] [CrossRef]
- Bennett, G.; Sadlier, D.; Doran, P.P.; MacMathuna, P.; Murray, D.W. A functional and transcriptomic analysis of NET1 bioactivity in gastric cancer. BMC Cancer 2011, 11, 50. [Google Scholar] [CrossRef]
- Huang, S. Non-genetic heterogeneity of cells in development: More than just noise. Development 2009, 136, 3853. [Google Scholar] [CrossRef]
- Monberg, M.E.; Geiger, H.; Lee, J.J.; Sharma, R.; Semaan, A.; Bernard, V.; Wong, J.; Wang, F.; Liang, S.; Swartzlander, D.B.; et al. Occult polyclonality of preclinical pancreatic cancer models drives in vitro evolution. Nat. Commun. 2022, 13, 3652. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Sahoo, S.; Brien, R.; Jung, S.; Humphries, B.; Lee, W.; Cheng, Y.-H.; Zhang, Z.; Luker, K.E.; Wicha, M.S.; et al. Single-cell RNA-sequencing of migratory breast cancer cells: Discovering genes associated with cancer metastasis. Analyst 2019, 144, 7296–7309. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, Z. Prognostic and Clinicopathological Significance of E-Cadherin in Pancreatic Cancer Patients: A Meta-Analysis. Front. Oncol. 2021, 11, 627116. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Beetham, H.; Black, M.; Priya, R.; Telford, B.J.; Guest, J.; Wiggins, G.A.R.; Godwin, T.D.; Yap, A.S.; Guilford, P.J. E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition. BMC Cancer 2014, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; He, M.; Jiang, X.; Liu, H.; Xie, T.; Qin, Z.; Huang, Q.; Liao, S.; Lin, C.; He, J.; et al. Single-Cell RNA Sequencing Reveals the Migration of Osteoclasts in Giant Cell Tumor of Bone. Front. Oncol. 2021, 11, 715552. [Google Scholar] [CrossRef]
- Lader, A.S.; Lee, J.J.; Cicchetti, G.; Kwiatkowski, D.J. Mechanisms of gelsolin-dependent and -independent EGF-stimulated cell motility in a human lung epithelial cell line. Exp. Cell Res. 2005, 307, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Murphy-Ullrich, J.E.; Wells, A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J Cell Biol 1996. 134, 689–698. [CrossRef]
- Nag, S.; Larsson, M.; Robinson, R.C.; Burtnick, L.D. Gelsolin: The tail of a molecular gymnast. Cytoskeleton 2013, 70, 360–384. [Google Scholar] [CrossRef]
- Sun, H.Q.; Yamamoto, M.; Mejillano, M.; Yin, H.L. Gelsolin, a multifunctional actin regulatory protein. J. Biol. Chem. 1999, 274, 33179–33182. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Aiello, N.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Condeelis, Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Fishilevich, S.; Nudel, R.; Rappaport, N.; Hadar, R.; Plaschkes, I.; Stein, T.I.; Rosen, N.; Kohn, A.; Twik, M.; Safran, M.; et al. GeneHancer: Genome-wide integration of enhancers and target genes in GeneCards. Database 2017, 2017, bax028. [Google Scholar] [CrossRef] [PubMed]
- Osada, S.; Yamamoto, H.; Nishihara, T.; Imagawa, M. DNA Binding Specificity of the CCAAT/Enhancer-binding Protein Transcription Factor Family (∗). J. Biol. Chem. 1996, 271, 3891–3896. [Google Scholar] [CrossRef] [PubMed]
- Gibieža, P.; Petrikaitė, V. The regulation of actin dynamics during cell division and malignancy. Am. J. Cancer Res. 2021, 11, 4050–4069. [Google Scholar]


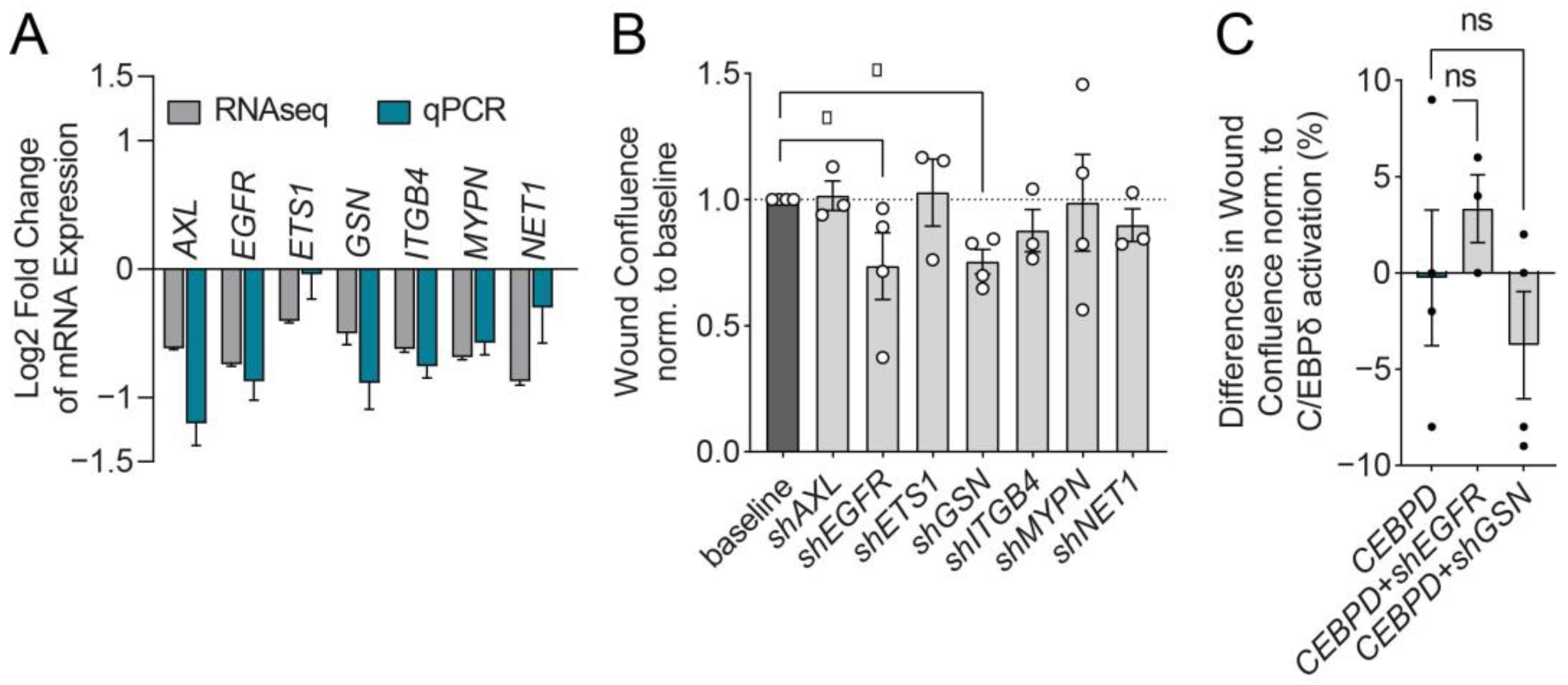
| Gene Name | Accession No. | Log2 Fold Change | p-Value |
|---|---|---|---|
| AXL Receptor Tyrosine Kinase (AXL) | NM_001699 | −0.62 | 7.5 × 10−6 |
| Epidermal Growth Factor Receptor (EGFR) | NM_005228 | −0.74 | 2.72 × 10−7 |
| ETS Proto-Oncogene 1 (ETS1) | NM_005238 | −0.4 | 5.16 × 10−5 |
| Gelsolin (GSN) | NM_000177 | −0.5 | 7.25 × 10−4 |
| Integrin Subunit beta 4 (ITGB4) | NM_000213 | −0.62 | 6.53 × 10−6 |
| Myopalladin (MYPN) | NM_032578 | −0.68 | 8.67 × 10−5 |
| Neuroepithelial Cell Transforming 1 (NET1) | NM_005863 | −0.51 | 1.41 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartl, L.; Maarschalkerweerd, P.A.F.; Butler, J.M.; Manz, X.D.; Thijssen, V.L.J.L.; Bijlsma, M.F.; Duitman, J.; Spek, C.A. C/EBPδ Suppresses Motility-Associated Gene Signatures and Reduces PDAC Cell Migration. Cells 2022, 11, 3334. https://doi.org/10.3390/cells11213334
Hartl L, Maarschalkerweerd PAF, Butler JM, Manz XD, Thijssen VLJL, Bijlsma MF, Duitman J, Spek CA. C/EBPδ Suppresses Motility-Associated Gene Signatures and Reduces PDAC Cell Migration. Cells. 2022; 11(21):3334. https://doi.org/10.3390/cells11213334
Chicago/Turabian StyleHartl, Leonie, Pien A. F. Maarschalkerweerd, Joe M. Butler, Xue D. Manz, Victor L. J. L. Thijssen, Maarten F. Bijlsma, JanWillem Duitman, and C. Arnold Spek. 2022. "C/EBPδ Suppresses Motility-Associated Gene Signatures and Reduces PDAC Cell Migration" Cells 11, no. 21: 3334. https://doi.org/10.3390/cells11213334
APA StyleHartl, L., Maarschalkerweerd, P. A. F., Butler, J. M., Manz, X. D., Thijssen, V. L. J. L., Bijlsma, M. F., Duitman, J., & Spek, C. A. (2022). C/EBPδ Suppresses Motility-Associated Gene Signatures and Reduces PDAC Cell Migration. Cells, 11(21), 3334. https://doi.org/10.3390/cells11213334






