Role of mGlu5 in Persistent Forms of Hippocampal Synaptic Plasticity and the Encoding of Spatial Experience
Abstract
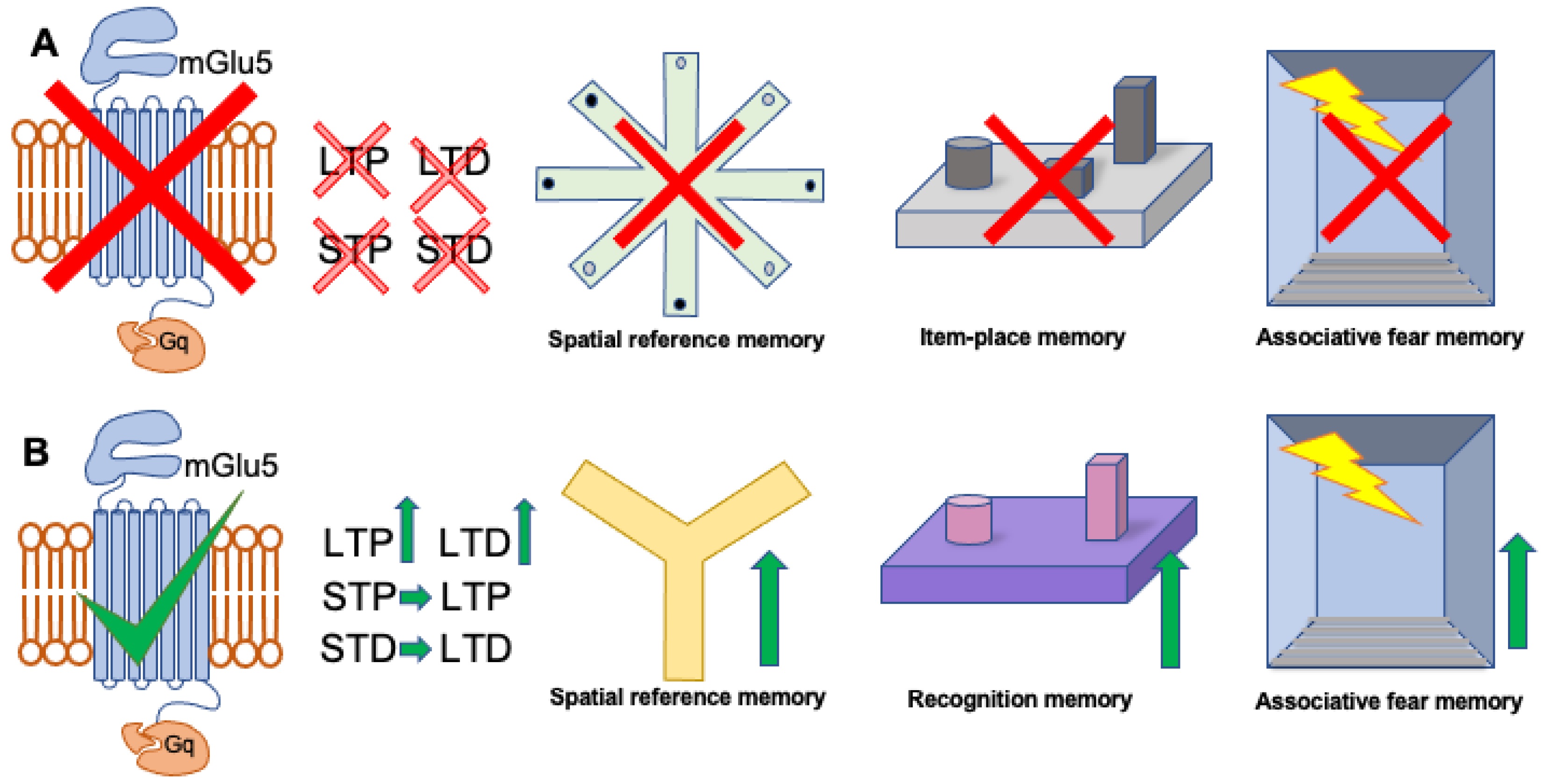
:1. Introduction
2. Contribution of mGlu5 to Persistent Forms of Hippocampal Synaptic Plasticity
2.1. Contribution of mGlu5 to Long-Term Potentiation
2.2. Contribution of mGlu5 Receptors to Long-Term Depression
3. Contribution of mGlu5 Receptors to Forms of Hippocampal Synaptic Plasticity That Are Enabled by Spatial Experience
| Hippocampus-Dependent Learning Task | Species | Outcome | References |
|---|---|---|---|
| Antagonist/NAM | |||
| object-place configuration | rat | LTD and memory inhibited | [5] |
| acquisition of novel environment | rat | impaired place field stability | [17] |
| acquisition of novel audiospatial cues | rat | LTD inhibited | [169] |
| eight-arm radial maze | rat | reference and working memory impaired | [11,16,124] |
| four-arm plus maze | rat | impairment of spontaneous alternation behaviour | [195] |
| Y-Maze spatial alternation task | rat | impairment of retention; no effect if antagonist applied immediately after training | [10] |
| T-Maze | rat | extinction of consolidated context impaired | [196] |
| working and reference memory | rat | impaired performance | [124] |
| inhibitory avoidance learning | rat | impairment in retention | [197] |
| extinction learning | rat | impaired extinction of consolidated information | [196] |
| fear conditioning | rat | impaired expression of contextual fear conditioning | [198] |
| fear conditioning | mouse | attenuation of cue-elicited freezing during fear conditioning | [199] |
| spatial object recognition | mouse | LTD and learning inhibited | [4] |
| environmental enrichment (EE) | mouse | impairment of EE-mediated LTP | [18] |
| Agonist/PAM | |||
| object recognition | rat | enhancement with low concentration of PAM | [200] |
| Y-maze spatial alternation task | rat | improvement in spatial alternation retention | [201] |
| T-maze | rat | enhanced memory abilities | [202] |
| MWM | mouse | enhanced learning and memory performance | [127] |
| MWM | mouse | impaired spatial learning | [203] |
| MWM | mouse | enhanced reversal learning | [204] |
| Barnes maze | mouse | improved performance during reversal training | [202] |
| fear extinction | mouse | enhanced fear extinction learning | |
| mGlu5 KO | |||
| MWM | mouse | impaired spatial learning | [153] |
| fear-conditioning | mouse | impaired processing of contextual information | [153] |
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferraguti, F.; Shigemoto, R. Metabotropic Glutamate Receptors. Cell Tissue Res. 2006, 326, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’Malley, K.; den Pol, A.N.V.; Olney, J.W. Distribution of Metabotropic Glutamate Receptor MGluR5 Immunoreactivity in Rat Brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Hagena, H.; Manahan-Vaughan, D. MGlu5 Acts as a Switch for Opposing Forms of Synaptic Plasticity at Mossy Fiber-CA3 and Commissural Associational-CA3 Synapses. J. Neurosci. 2015, 35, 4999–5006. [Google Scholar] [CrossRef] [Green Version]
- Goh, J.J.; Manahan-Vaughan, D. Endogenous Hippocampal LTD That Is Enabled by Spatial Object Recognition Requires Activation of NMDA Receptors and the Metabotropic Glutamate Receptor, MGlu5. Hippocampus 2012, 23, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Popkirov, S.G.; Manahan-Vaughan, D. Involvement of the Metabotropic Glutamate Receptor MGluR5 in NMDA Receptor-Dependent, Learning-Facilitated Long-Term Depression in CA1 Synapses. Cereb. Cortex 2011, 21, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, A.; Lim, Y.J.; Kumar, K.; Baby, N.; Pang, K.L.K.; Benoy, A.; Behnisch, T.; Sajikumar, S. Group III Metabotropic Glutamate Receptors Gate Long-Term Potentiation and Synaptic Tagging/Capture in Rat Hippocampal Area CA2. elife 2020, 9, e55344. [Google Scholar] [CrossRef]
- Neyman, S.; Braunewell, K.-H.; O’Connell, K.E.; Dev, K.K.; Manahan-Vaughan, D. Inhibition of the Interaction Between Group I Metabotropic Glutamate Receptors and PDZ-Domain Proteins Prevents Hippocampal Long-Term Depression, but Not Long-Term Potentiation. Front. Synaptic Neurosci. 2019, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Lv, X.; Maksymetz, J.; Stansley, B.J.; Ghoshal, A.; Gogliotti, R.G.; Niswender, C.M.; Lindsley, C.W.; Conn, P.J. MGlu5 Positive Allosteric Modulators Facilitate Long-Term Potentiation via Disinhibition Mediated by MGlu5-Endocannabinoid Signaling. ACS Pharm. Transl. Sci. 2019, 2, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Balschun, D.; Manahan-Vaughan, D.; Wagner, T.; Behnisch, T.; Reymann, K.G.; Wetzel, W. A Specific Role for Group I MGluRs in Hippocampal LTP and Hippocampus-Dependent Spatial Learning. Learn. Mem. 1999, 6, 138–152. [Google Scholar] [CrossRef]
- Balschun, D.; Wetzel, W. Inhibition of MGluR5 Blocks Hippocampal LTP in Vivo and Spatial Learning in Rats. Pharmacol. Biochem. Behav. 2002, 73, 375–380. [Google Scholar] [CrossRef]
- Naie, K.; Manahan-Vaughan, D. Regulation by Metabotropic Glutamate Receptor 5 of LTP in the Dentate Gyrus of Freely Moving Rats: Relevance for Learning and Memory Formation. Cereb. Cortex 2004, 14, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Naie, K.; Manahan-Vaughan, D. Investigations of the Protein Synthesis Dependency of MGluR-Induced Long-Term Depression in the Dentate Gyrus of Freely Moving Rats. Neuropharmacology 2005, 49 (Suppl. S1), 35–44. [Google Scholar] [CrossRef]
- Altinbilek, B.; Manahan-Vaughan, D. Antagonism of Group III Metabotropic Glutamate Receptors Results in Impairment of LTD but Not LTP in the Hippocampal CA1 Region, and Prevents Long-Term Spatial Memory. Eur. J. Neurosci. 2007, 26, 1166–1172. [Google Scholar] [CrossRef]
- Altinbilek, B.; Manahan-Vaughan, D. A Specific Role for Group II Metabotropic Glutamate Receptors in Hippocampal Long-Term Depression and Spatial Memory. Neuroscience 2009, 158, 149–158. [Google Scholar] [CrossRef]
- Teleuca, A.E.; Alemà, G.S.; Casolini, P.; Barberis, I.; Ciabattoni, F.; Orlando, R.; Menna, L.D.; Iacovelli, L.; Scioli, M.R.; Nicoletti, F.; et al. Changes in MGlu5 Receptor Signaling Are Associated with Associative Learning and Memory Extinction in Mice. Life 2022, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Bikbaev, A.; Neyman, S.; Ngomba, R.T.; Conn, P.J.; Conn, J.; Nicoletti, F.; Manahan-Vaughan, D. MGluR5 Mediates the Interaction between Late-LTP, Network Activity, and Learning. PLoS ONE 2008, 3, e2155. [Google Scholar] [CrossRef]
- Zhang, S.; Manahan-Vaughan, D. Place Field Stability Requires the Metabotropic Glutamate Receptor, MGlu5. Hippocampus 2014, 24, 1330–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschler, A.; Manahan-Vaughan, D. Metabotropic Glutamate Receptor, MGlu5, Mediates Enhancements of Hippocampal Long-Term Potentiation after Environmental Enrichment in Young and Old Mice. Neuropharmacology 2017, 115, 42–50. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L. The Physiology of Excitatory Amino Acids in the Vertebrate Central Nervous System. Prog. Neurobiol. 1987, 28, 197–276. [Google Scholar] [CrossRef]
- Schoepp, D.D.; Conn, P.J. Metabotropic Glutamate Receptors in Brain Function and Pathology. Trends Pharmacol. Sci. 1993, 14, 13–20. [Google Scholar] [CrossRef]
- Asztély, F.; Gustafsson, B. Ionotropic Glutamate Receptors. Mol. Neurobiol. 1996, 12, 1. [Google Scholar] [CrossRef]
- Hollmann, M.; Heinemann, S. Cloned Glutamate Receptors. Annu. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Nakajima, Y.; Masu, M.; Ueda, Y.; Nakahara, K.; Watanabe, D.; Yamaguchi, S.; Kawabata, S.; Okada, M. Glutamate Receptors: Brain Function and Signal Transduction. Brain Res. Brain Res. Rev. 1998, 26, 230–235. [Google Scholar] [CrossRef]
- Conn, P.J.; Pin, J.P. Pharmacology and Functions of Metabotropic Glutamate Receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef] [Green Version]
- Minakami, R.; Katsuki, F.; Sugiyama, H. A Variant of Metabotropic Glutamate Receptor Subtype 5: An Evolutionally Conserved Insertion with No Termination Codon. Biochem. Biophys. Res. Commun. 1993, 194, 622–627. [Google Scholar] [CrossRef]
- Joly, C.; Gomeza, J.; Brabet, I.; Curry, K.; Bockaert, J.; Pin, J.P. Molecular, Functional, and Pharmacological Characterization of the Metabotropic Glutamate Receptor Type 5 Splice Variants: Comparison with MGluR1. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 3970–3981. [Google Scholar] [CrossRef]
- Mukherjee, S.; Manahan-Vaughan, D. Role of Metabotropic Glutamate Receptors in Persistent Forms of Hippocampal Plasticity and Learning. Neuropharmacology 2012, 66, 65–81. [Google Scholar] [CrossRef]
- Harris, E.W.; Cotman, C.W. Long-Term Potentiation of Guinea Pig Mossy Fiber Responses Is Not Blocked by N-Methyl D-Aspartate Antagonists. Neurosci. Lett. 1986, 70, 132–137. [Google Scholar] [CrossRef]
- Kamiya, H.; Shinozaki, H.; Yamamoto, C. Activation of Metabotropic Glutamate Receptor Type 2/3 Suppresses Transmission at Rat Hippocampal Mossy Fibre Synapses. J. Physiol. 1996, 493 Pt 2, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Macek, T.A.; Winder, D.G.; Gereau, R.W.; Ladd, C.O.; Conn, P.J. Differential Involvement of Group II and Group III MGluRs as Autoreceptors at Lateral and Medial Perforant Path Synapses. J. Neurophysiol. 1996, 76, 3798–3806. [Google Scholar] [CrossRef]
- Min, M.Y.; Rusakov, D.A.; Kullmann, D.M. Activation of AMPA, Kainate, and Metabotropic Receptors at Hippocampal Mossy Fiber Synapses: Role of Glutamate Diffusion. Neuron 1998, 21, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.-B.; Castillo, P.E. Role of Glutamate Autoreceptors at Hippocampal Mossy Fiber Synapses. Neuron 2008, 60, 1082–1094. [Google Scholar] [CrossRef] [Green Version]
- Manahan-Vaughan, D. Group 1 and 2 Metabotropic Glutamate Receptors Play Differential Roles in Hippocampal Long-Term Depression and Long-Term Potentiation in Freely Moving Rats. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 3303–3311. [Google Scholar] [CrossRef] [Green Version]
- Manahan-Vaughan, D.; Reymann, K.G. 1S,3R-ACPD Dose-Dependently Induces a Slow-Onset Potentiation in the Rat Hippocampal CA1 Region in Vivo. Neuropharmacology 1995, 34, 1103–1105. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D. Group III Metabotropic Glutamate Receptors Modulate Long-Term Depression in the Hippocampal CA1 Region of Two Rat Strains in Vivo. Neuropharmacology 2000, 39, 1952–1958. [Google Scholar] [CrossRef]
- Klausnitzer, J.; Kulla, A.; Manahan-Vaughan, D. Role of the Group III Metabotropic Glutamate Receptor in LTP, Depotentiation and LTD in Dentate Gyrus of Freely Moving Rats. Neuropharmacology 2004, 46, 160–170. [Google Scholar] [CrossRef]
- Naie, K.; Gundimi, S.; Siegmund, H.; Heinemann, U.; Manahan-Vaughan, D. Group III Metabotropic Glutamate Receptor-Mediated, Chemically Induced Long-Term Depression Differentially Affects Cell Viability in the Hippocampus. Eur. J. Pharmacol. 2006, 535, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Baude, A.; Nusser, Z.; Roberts, J.D.; Mulvihill, E.; McIlhinney, R.A.; Somogyi, P. The Metabotropic Glutamate Receptor (MGluR1 Alpha) Is Concentrated at Perisynaptic Membrane of Neuronal Subpopulations as Detected by Immunogold Reaction. Neuron 1993, 11, 771–787. [Google Scholar] [CrossRef]
- Lujan, R.; Roberts, J.D.; Shigemoto, R.; Ohishi, H.; Somogyi, P. Differential Plasma Membrane Distribution of Metabotropic Glutamate Receptors MGluR1 Alpha, MGluR2 and MGluR5, Relative to Neurotransmitter Release Sites. J. Chem. Neuroanat. 1997, 13, 219–241. [Google Scholar] [CrossRef]
- Lujan, R.; Nusser, Z.; Roberts, J.D.; Shigemoto, R.; Somogyi, P. Perisynaptic Location of Metabotropic Glutamate Receptors MGluR1 and MGluR5 on Dendrites and Dendritic Spines in the Rat Hippocampus. Eur. J. Neurosci. 1996, 8, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, R.; Kinoshita, A.; Wada, E.; Nomura, S.; Ohishi, H.; Takada, M.; Flor, P.J.; Neki, A.; Abe, T.; Nakanishi, S.; et al. Differential Presynaptic Localization of Metabotropic Glutamate Receptor Subtypes in the Rat Hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 7503–7522. [Google Scholar] [CrossRef] [Green Version]
- Bach, P.; Isaac, M.; Slassi, A. Metabotropic Glutamate Receptor 5 Modulators and Their Potential Therapeutic Applications. Expert Opin. Ther. Pat. 2007, 17, 371–384. [Google Scholar] [CrossRef]
- Ure, J.; Baudry, M.; Perassolo, M. Metabotropic Glutamate Receptors and Epilepsy. J. Neurol. Sci. 2006, 247, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moldrich, R.X.; Chapman, A.G.; Sarro, G.D.; Meldrum, B.S. Glutamate Metabotropic Receptors as Targets for Drug Therapy in Epilepsy. Eur. J. Pharmacol. 2003, 476, 3–16. [Google Scholar] [CrossRef]
- Cicco, G.D.; Marzano, E.; Iacovelli, L.; Celli, R.; van Luijtelaar, G.; Nicoletti, F.; Ngomba, R.T.; Wall, M.J. Group I Metabotropic Glutamate Receptor-Mediated Long Term Depression Is Disrupted in the Hippocampus of WAG/Rij Rats Modelling Absence Epilepsy. Neuropharmacology 2021, 196, 108686. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.J.; Lindsley, C.W.; Jones, C.K. Activation of Metabotropic Glutamate Receptors as a Novel Approach for the Treatment of Schizophrenia. Trends Pharmacol. Sci. 2009, 30, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [Green Version]
- Dubovyk, V.; Manahan-Vaughan, D. Time-Dependent Alterations in the Expression of NMDA Receptor Subunits along the Dorsoventral Hippocampal Axis in an Animal Model of Nascent Psychosis. ACS Chem. Neurosci. 2018, 9, 2241–2251. [Google Scholar] [CrossRef]
- Vardigan, J.D.; Huszar, S.L.; McNaughton, C.H.; Hutson, P.H.; Uslaner, J.M. MK-801 Produces a Deficit in Sucrose Preference That Is Reversed by Clozapine, d-Serine, and the Metabotropic Glutamate 5 Receptor Positive Allosteric Modulator CDPPB: Relevance to Negative Symptoms Associated with Schizophrenia? Pharmacol. Biochem. Behav. 2010, 95, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, C.R.; Veselinović, T.; Rajkumar, R.; Mauler, J.; Orth, L.; Ruch, A.; Ramkiran, S.; Heekeren, K.; Kawohl, W.; Wyss, C.; et al. MGluR5 Receptor Availability Is Associated with Lower Levels of Negative Symptoms and Better Cognition in Male Patients with Chronic Schizophrenia. Hum. Brain Mapp. 2020, 41, 2762–2781. [Google Scholar] [CrossRef]
- Matosin, N.; Fernandez-Enright, F.; Fung, S.J.; Lum, J.S.; Engel, M.; Andrews, J.L.; Huang, X.-F.; Weickert, C.S.; Newell, K.A. Alterations of MGluR5 and Its Endogenous Regulators Norbin, Tamalin and Preso1 in Schizophrenia: Towards a Model of MGluR5 Dysregulation. Acta Neuropathol. 2015, 130, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic Glutamate Receptors: From the Workbench to the Bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dhull, D.K.; Mishra, P.S. Therapeutic Potential of MGluR5 Targeting in Alzheimer’s Disease. Front. Neurosci. 2015, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruiz, R.; Albasanz, J.L.; Martín, M.; Moreno-Martínez, A.E.; Fukazawa, Y.; Luján, R. The Density of Group I MGlu5 Receptors Is Reduced along the Neuronal Surface of Hippocampal Cells in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 5867. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, P.; Zhang, Y.; Yang, Y.; Zhu, M.; Qin, S.; Xu, J.-T.; Duan, D.; Wu, Y.; Wang, D.; et al. Inhibition of the ISR Abrogates MGluR5-Dependent Long-Term Depression and Spatial Memory Deficits in a Rat Model of Alzheimer’s Disease. Transl. Psychiat. 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrahman, K.S.; Hamilton, A.; Albaker, A.; Ferguson, S.S.G. MGluR5 Contribution to Neuropathology in Alzheimer Mice Is Disease Stage-Dependent. ACS Pharmacol. Transl. Sci. 2020, 3, 334–344. [Google Scholar] [CrossRef]
- Mecca, A.P.; McDonald, J.W.; Michalak, H.R.; Godek, T.A.; Harris, J.E.; Pugh, E.A.; Kemp, E.C.; Chen, M.-K.; Salardini, A.; Nabulsi, N.B.; et al. PET Imaging of MGluR5 in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020, 12, 15. [Google Scholar] [CrossRef]
- Abd-Elrahman, K.S.; Albaker, A.; de Souza, J.M.; Ribeiro, F.M.; Schlossmacher, M.G.; Tiberi, M.; Hamilton, A.; Ferguson, S.S.G. Aβ Oligomers Induce Pathophysiological MGluR5 Signaling in Alzheimer’s Disease Model Mice in a Sex-Selective Manner. Sci. Signal 2020, 13, eabd2494. [Google Scholar] [CrossRef]
- Abd-Elrahman, K.S.; Ferguson, S.S.G. Noncanonical Metabotropic Glutamate Receptor 5 Signaling in Alzheimer’s Disease. Annu. Rev. Pharmacol. 2021, 62, 235–254. [Google Scholar] [CrossRef]
- Sebastianutto, I.; Goyet, E.; Andreoli, L.; Font-Ingles, J.; Moreno-Delgado, D.; Bouquier, N.; Jahannault-Talignani, C.; Moutin, E.; Menna, L.D.; Maslava, N.; et al. D1-MGlu5 Heteromers Mediate Noncanonical Dopamine Signaling in Parkinson’s Disease. J. Clin. Investig. 2020, 130, 1168–1184. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Fan, J.-K.; Gu, L.; Yang, H.-M.; Zhan, S.-Q.; Zhang, H. Metabotropic Glutamate Receptor 5 Inhibits α-Synuclein-Induced Microglia Inflammation to Protect from Neurotoxicity in Parkinson’s Disease. J. Neuroinflamm. 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Turle-Lorenzo, N.; Breysse, N.; Baunez, C.; Amalric, M. Functional Interaction between MGlu 5 and NMDA Receptors in a Rat Model of Parkinson’s Disease. Psychopharmacology 2005, 179, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.; Fu, P.; Zhang, Z.; Lin, K.; Ko, J.K.-S.; Yung, K.K.-L. Roles of Glutamate Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4391. [Google Scholar] [CrossRef] [Green Version]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The MGluR Theory of Fragile X Mental Retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bassell, G.J.; Warren, S.T. Fragile X Syndrome: Loss of Local MRNA Regulation Alters Synaptic Development and Function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Waung, M.W.; Huber, K.M. Protein Translation in Synaptic Plasticity: MGluR-LTD, Fragile X. Curr. Opin. Neurobiol. 2009, 19, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dölen, G.; Carpenter, R.L.; Ocain, T.D.; Bear, M.F. Mechanism-Based Approaches to Treating Fragile X. Pharmacol. Ther. 2010, 127, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.D.; Bear, M.F. Toward Fulfilling the Promise of Molecular Medicine in Fragile X Syndrome. Annu. Rev. Med. 2011, 62, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Michalon, A.; Sidorov, M.; Ballard, T.M.; Ozmen, L.; Spooren, W.; Wettstein, J.G.; Jaeschke, G.; Bear, M.F.; Lindemann, L. Chronic Pharmacological MGlu5 Inhibition Corrects Fragile X in Adult Mice. Neuron 2012, 74, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Stoppel, D.C.; McCamphill, P.K.; Senter, R.K.; Heynen, A.J.; Bear, M.F. MGluR5 Negative Modulators for Fragile X: Treatment Resistance and Persistence. Front. Psychiatry 2021, 12, 718953. [Google Scholar] [CrossRef]
- Mody, M.; Petibon, Y.; Han, P.; Kuruppu, D.; Ma, C.; Yokell, D.; Neelamegam, R.; Normandin, M.D.; Fakhri, G.E.; Brownell, A.-L. In Vivo Imaging of MGlu5 Receptor Expression in Humans with Fragile X Syndrome towards Development of a Potential Biomarker. Sci. Rep. 2021, 11, 15897. [Google Scholar] [CrossRef] [PubMed]
- Brašić, J.R.; Goodman, J.A.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Martin, S.D.; Slifer, K.; Sedlak, T.; Mathur, A.K.; et al. Fragile X Mental Retardation Protein and Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome: A Pilot Study. Brain Sci. 2022, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, H.; Chang, Q. MeCP2 Phosphorylation Is Required for Modulating Synaptic Scaling through MGluR5. J. Neurosci. 2012, 32, 12841–12847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogliotti, R.G.; Senter, R.K.; Rook, J.M.; Ghoshal, A.; Zamorano, R.; Malosh, C.; Stauffer, S.R.; Bridges, T.M.; Bartolome, J.M.; Daniels, J.S.; et al. MGlu5 Positive Allosteric Modulation Normalizes Synaptic Plasticity Defects and Motor Phenotypes in a Mouse Model of Rett Syndrome. Hum. Mol. Genet 2016, 25, 1990–2004. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Wu, H.; Coronado, A.A.; de Laittre, E.; Osterweil, E.K.; Zhang, Y.; Bear, M.F. Negative Allosteric Modulation of MGluR5 Partially Corrects Pathophysiology in a Mouse Model of Rett Syndrome. J. Neurosci. 2016, 36, 11946–11958. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, S.; Mironov, S.L. CA1 Neurons Acquire Rett Syndrome Phenotype After Brief Activation of Glutamatergic Receptors: Specific Role of MGluR1/5. Front. Cell Neurosci. 2018, 12, 363. [Google Scholar] [CrossRef]
- Ding, S.; Fellin, T.; Zhu, Y.; Lee, S.-Y.; Auberson, Y.P.; Meaney, D.F.; Coulter, D.A.; Carmignoto, G.; Haydon, P.G. Enhanced Astrocytic Ca2+ Signals Contribute to Neuronal Excitotoxicity after Status Epilepticus. J. Neurosci. 2007, 27, 10674–10684. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, K.C.; Lacor, P.N.; Pitt, J.; Klein, W.L. Aβ Oligomer-Induced Synapse Degeneration in Alzheimer’s Disease. Cell Mol. Neurobiol. 2011, 31, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic Glutamate Receptor 5 Is a Coreceptor for Alzheimer Aβ Oligomer Bound to Cellular Prion Protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.-W.; Nicoll, A.J.; Zhang, D.; Mably, A.J.; O’Malley, T.; Purro, S.A.; Terry, C.; Collinge, J.; Walsh, D.M.; Rowan, M.J. MGlu5 Receptors and Cellular Prion Protein Mediate Amyloid-β-Facilitated Synaptic Long-Term Depression in Vivo. Nat. Commun. 2014, 5, 3374. [Google Scholar] [CrossRef] [Green Version]
- Cleva, R.M.; Olive, M.F. Positive Allosteric Modulators of Type 5 Metabotropic Glutamate Receptors (MGluR5) and Their Therapeutic Potential for the Treatment of CNS Disorders. Molecules 2011, 16, 2097–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levenga, J.; Hayashi, S.; de Vrij, F.M.S.; Koekkoek, S.K.; van der Linde, H.C.; Nieuwenhuizen, I.; Song, C.; Buijsen, R.A.M.; Pop, A.S.; GomezMancilla, B.; et al. AFQ056, a New MGluR5 Antagonist for Treatment of Fragile X Syndrome. Neurobiol. Dis. 2011, 42, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Grauer, S.; Kelley, C.; Navarra, R.; Graf, R.; Zhang, G.; Atkinson, P.J.; Popiolek, M.; Wantuch, C.; Khawaja, X.; et al. ADX47273 [S-(4-Fluoro-Phenyl)-{3-[3-(4-Fluoro-Phenyl)-[1,2,4]-Oxadiazol-5-Yl]-Piperidin-1-Yl}-Methanone]: A Novel Metabotropic Glutamate Receptor 5-Selective Positive Allosteric Modulator with Preclinical Antipsychotic-Like and Procognitive Activities. J. Pharmacol. Exp. Ther. 2008, 327, 827–839. [Google Scholar] [CrossRef]
- Musazzi, L. Targeting Metabotropic Glutamate Receptors for Rapid-Acting Antidepressant Drug Discovery. Expert Opin. Drug Dis. 2020, 16, 147–157. [Google Scholar] [CrossRef]
- Scharf, S.H.; Jaeschke, G.; Wettstein, J.G.; Lindemann, L. Metabotropic Glutamate Receptor 5 as Drug Target for Fragile X Syndrome. Curr. Opin. Pharmacol. 2015, 20, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Stansley, B.J.; Conn, P.J. The Therapeutic Potential of Metabotropic Glutamate Receptor Modulation for Schizophrenia. Curr. Opin. Pharmacol. 2018, 38, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.; Manahan-Vaughan, D. Hippocampal Long-Term Depression: Master or Minion in Declarative Memory Processes? Trends Neurosci. 2007, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.; Manahan-Vaughan, D. Hippocampal Long-Term Depression and Long-Term Potentiation Encode Different Aspects of Novelty Acquisition. Proc. Natl. Acad. Sci. USA 2004, 101, 8192–8197. [Google Scholar] [CrossRef] [Green Version]
- Stacho, M.; Manahan-Vaughan, D. The Intriguing Contribution of Hippocampal Long-Term Depression to Spatial Learning and Long-Term Memory. Front. Behav. Neurosci. 2022, 16, 806356. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D.; Braunewell, K.-H. Novelty Acquisition Is Associated with Induction of Hippocampal Long-Term Depression. Proc. Natl. Acad. Sci. USA 1999, 96, 8739–8744. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning Induces Long-Term Potentiation in the Hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manahan-Vaughan, D. Item-Place Encoding Through Hippocampal Long-Term Depression. In Handbook of Object Novelty Recognition; Ennaceur, A., de Souza Silva, M., Eds.; Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 273–289. ISBN 9780128120125. [Google Scholar]
- Manahan-Vaughan, D. Learning-Related Hippocampal Long-Term Potentiation and Long-Term Depression; Byrne, H., John, Eds.; Academic Press: Oxford, UK, 2017; pp. 585–609. ISBN 978-0-12-805291-4. [Google Scholar]
- Abe, T.; Sugihara, H.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular Characterization of a Novel Metabotropic Glutamate Receptor MGluR5 Coupled to Inositol Phosphate/Ca2+ Signal Transduction. J. Biol. Chem. 1992, 267, 13361–13368. [Google Scholar] [CrossRef]
- Pin, J.P.; Duvoisin, R. The Metabotropic Glutamate Receptors: Structure and Functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef]
- Matter, N.; Ritz, M.F.; Freyermuth, S.; Rogue, P.; Malviya, A.N. Stimulation of Nuclear Protein Kinase C Leads to Phosphorylation of Nuclear Inositol 1,4,5-Trisphosphate Receptor and Accelerated Calcium Release by Inositol 1,4,5-Trisphosphate from Isolated Rat Liver Nuclei. J. Biol. Chem. 1993, 268, 732–736. [Google Scholar] [CrossRef]
- Bartlett, P.J.; Metzger, W.; Gaspers, L.D.; Thomas, A.P. Differential Regulation of Multiple Steps in Inositol 1,4,5-Trisphosphate Signaling by Protein Kinase C Shapes Hormone-Stimulated Ca2+ Oscillations. J. Biol. Chem. 2015, 290, 18519–18533. [Google Scholar] [CrossRef] [Green Version]
- Irvine, R.F. 20 Years of Ins(1,4,5)P3, and 40 Years Before. Nat. Rev. Mol. Cell Bio. 2003, 4, 586–590. [Google Scholar] [CrossRef]
- Thatcher, J.D. The Inositol Trisphosphate (IP3) Signal Transduction Pathway. Sci. Signal. 2010, 3, tr3. [Google Scholar] [CrossRef]
- Wang, J.H.; Feng, D.P. Postsynaptic Protein Kinase C Essential to Induction and Maintenance of Long-Term Potentiation in the Hippocampal CA1 Region. Proc. Natl. Acad. Sci. USA 1992, 89, 2576–2580. [Google Scholar] [CrossRef] [Green Version]
- Barnes, C.A.; Mizumori, S.J.Y.; Lovinger, D.M.; Sheu, F.-S.; Murakami, K.; Chan, S.Y.; Linden, D.J.; Nelson, R.B.; Routtenberg, A. Selective Decline in Protein F1 Phosphorylation in Hippocampus of Senescent Rats. Neurobiol. Aging 1988, 9, 393–398. [Google Scholar] [CrossRef]
- Lovinger, D.M.; Colley, P.A.; Akers, R.F.; Nelson, R.B.; Routtenberg, A. Direct Relation of Long-Term Synaptic Potentiation to Phosphorylation of Membrane Protein F1, a Substrate for Membrane Protein Kinase C. Brain Res. 1986, 399, 205–211. [Google Scholar] [CrossRef]
- Boehm, J.; Kang, M.-G.; Johnson, R.C.; Esteban, J.; Huganir, R.L.; Malinow, R. Synaptic Incorporation of AMPA Receptors during LTP Is Controlled by a PKC Phosphorylation Site on GluR1. Neuron 2006, 51, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.J.; Carpenter, D.O. A Comparison of the Roles of Protein Kinase C in Long-Term Potentiation in Rat Hippocampal Areas CA1 and CA3. Cell Mol. Neurobiol. 2005, 25, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and Pathology of Calcium Signaling in the Brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, R.F.; Lovinger, D.M.; Colley, P.A.; Linden, D.J.; Routtenberg, A. Translocation of Protein Kinase C Activity May Mediate Hippocampal Long-Term Potentiation. Science 1986, 231, 587–589. [Google Scholar] [CrossRef]
- Klann, E.; Chen, S.J.; Sweatt, J.D. Mechanism of Protein Kinase C Activation during the Induction and Maintenance of Long-Term Potentiation Probed Using a Selective Peptide Substrate. Proc. Natl. Acad. Sci. USA 1993, 90, 8337–8341. [Google Scholar] [CrossRef] [Green Version]
- Malenka, R.C.; Madison, D.V.; Nicoll, R.A. Potentiation of Synaptic Transmission in the Hippocampus by Phorbol Esters. Nature 1986, 321, 175–177. [Google Scholar] [CrossRef]
- Sacktor, T.C.; Osten, P.; Valsamis, H.; Jiang, X.; Naik, M.U.; Sublette, E. Persistent Activation of the Zeta Isoform of Protein Kinase C in the Maintenance of Long-Term Potentiation. Proc. Natl. Acad. Sci. USA 1993, 90, 8342–8346. [Google Scholar] [CrossRef] [Green Version]
- Lovinger, D.M.; Akers, R.F.; Nelson, R.B.; Barnes, C.A.; McNaughton, B.L.; Routtenberg, A. A Selective Increase in Phosphorylation of Protein F1, a Protein Kinase C Substrate, Directly Related to Three Day Growth of Long Term Synaptic Enhancement. Brain Res. 1985, 343, 137–143. [Google Scholar] [CrossRef]
- Ballesteros, J.J.; Buschler, A.; Köhr, G.; Manahan-Vaughan, D. Afferent Input Selects NMDA Receptor Subtype to Determine the Persistency of Hippocampal LTP in Freely Behaving Mice. Front. Synaptic Neurosci. 2016, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Manahan-Vaughan, D.; Reiser, M.; Pin, J.-P.; Wilsch, V.; Bockaert, J.; Reymann, K.G.; Riedel, G. Physiological and Pharmacological Profile Oftrans-Azetidine-2,4-Dicarboxylic Acid: Metabotropic Glutamate Receptor Agonism and Effects on Long-Term Potentiation. Neuroscience 1996, 72, 999–1008. [Google Scholar] [CrossRef]
- Aksoy-Aksel, A.; Manahan-Vaughan, D. Synaptic Strength at the Temporoammonic Input to the Hippocampal CA1 Region in Vivo Is Regulated by NMDA Receptors, Metabotropic Glutamate Receptors and Voltage-Gated Calcium Channels. Neuroscience 2015, 309, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.C.; Mason, S.E. Effects of the NMDA Receptor/Channel Antagonists CPP and MK801 on Hippocampal Field Potentials and Long-Term Potentiation in Anesthetized Rats. Brain Res. 1988, 462, 40–46. [Google Scholar] [CrossRef]
- Morris, R.G. Synaptic Plasticity and Learning: Selective Impairment of Learning Rats and Blockade of Long-Term Potentiation in Vivo by the N-Methyl-D-Aspartate Receptor Antagonist AP5. J. Neurosci. Off. J. Soc. Neurosci. 1989, 9, 3040–3057. [Google Scholar] [CrossRef]
- Morris, R.G.; Anderson, E.; Lynch, G.S.; Baudry, M. Selective Impairment of Learning and Blockade of Long-Term Potentiation by an N-Methyl-D-Aspartate Receptor Antagonist, AP5. Nature 1986, 319, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Gottschling, C.; Faissner, A.; Manahan-Vaughan, D. Intrinsic Cellular and Molecular Properties of in Vivo Hippocampal Synaptic Plasticity Are Altered in the Absence of Key Synaptic Matrix Molecules. Hippocampus 2017, 27, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Buschler, A.; Goh, J.J.; Manahan-Vaughan, D. Frequency Dependency of NMDA Receptor-Dependent Synaptic Plasticity in the Hippocampal CA1 Region of Freely Behaving Mice. Hippocampus 2012, 22, 2238–2248. [Google Scholar] [CrossRef]
- Aarse, J.; Herlitze, S.; Manahan-Vaughan, D. The Requirement of BDNF for Hippocampal Synaptic Plasticity Is Experience-Dependent. Hippocampus 2015, 26, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.; Bliss, T.V.P.; Dutrieux, G.; Laroche, S.; Errington, M.L. Induction and Duration of Long-Term Potentiation in the Hippocampus of the Freely Moving Mouse. J. Neurosci. Meth. 1997, 75, 75–80. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D.; Braunewell, K.-H.; Reymann, K.G. Subtype-Specific Involvement of Metabotropic Glutamate Receptors in Two Forms of Long-Term Potentiation in the Dentate Gyrus of Freely Moving Rats. Neuroscience 1998, 86, 709–721. [Google Scholar] [CrossRef]
- Derrick, B.E.; Weinberger, S.B.; Martinez, J.L. Opioid Receptors Are Involved in an NMDA Receptor-Independent Mechanism of LTP Induction at Hippocampal Mossy Fiber-CA3 Synapses. Brain Res. Bull. 1991, 27, 219–223. [Google Scholar] [CrossRef]
- Neyman, S.; Manahan-Vaughan, D. Metabotropic Glutamate Receptor 1 (MGluR1) and 5 (MGluR5) Regulate Late Phases of LTP and LTD in the Hippocampal CA1 Region in Vitro. Eur. J. Neurosci. 2008, 27, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Manahan-Vaughan, D.; Braunewell, K.-H. The Metabotropic Glutamate Receptor, MGluR5, Is a Key Determinant of Good and Bad Spatial Learning Performance and Hippocampal Synaptic Plasticity. Cereb. Cortex 2005, 15, 1703–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.S.; Raymond, C.R.; Abraham, W.C. Priming of Long-Term Potentiation Induced by Activation of Metabotropic Glutamate Receptors Coupled to Phospholipase C. Hippocampus 1998, 8, 160–170. [Google Scholar] [CrossRef]
- Raymond, C.R.; Thompson, V.L.; Tate, W.P.; Abraham, W.C. Metabotropic Glutamate Receptors Trigger Homosynaptic Protein Synthesis to Prolong Long-Term Potentiation. J. Neurosci. 2000, 20, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Ayala, J.E.; Chen, Y.; Banko, J.L.; Sheffler, D.J.; Williams, R.; Telk, A.N.; Watson, N.L.; Xiang, Z.; Zhang, Y.; Jones, P.J.; et al. MGluR5 Positive Allosteric Modulators Facilitate Both Hippocampal LTP and LTD and Enhance Spatial Learning. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009, 34, 2057–2071. [Google Scholar] [CrossRef] [Green Version]
- Rook, J.M.; Xiang, Z.; Lv, X.; Ghoshal, A.; Dickerson, J.W.; Bridges, T.M.; Johnson, K.A.; Foster, D.J.; Gregory, K.J.; Vinson, P.N.; et al. Biased MGlu5-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating MGlu5 Modulation of NMDAR Currents. Neuron 2015, 86, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Noetzel, M.J.; Gregory, K.J.; Vinson, P.N.; Manka, J.T.; Stauffer, S.R.; Lindsley, C.W.; Niswender, C.M.; Xiang, Z.; Conn, P.J. A Novel Metabotropic Glutamate Receptor 5 Positive Allosteric Modulator Acts at a Unique Site and Confers Stimulus Bias to MGlu5 Signaling. Mol. Pharmacol. 2013, 83, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Bikbaev, A.; Manahan-Vaughan, D. Metabotropic Glutamate Receptor, MGlu5, Regulates Hippocampal Synaptic Plasticity and Is Required for Tetanisation-Triggered Changes in Theta and Gamma Oscillations. Neuropharmacology 2016, 115, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Krug, M.; Lössner, B.; Ott, T. Anisomycin Blocks the Late Phase of Long-Term Potentiation in the Dentate Gyrus of Freely Moving Rats. Brain Res. Bull. 1984, 13, 39–42. [Google Scholar] [CrossRef]
- Otani, S.; Abraham, W.C. Inhibition of Protein Synthesis in the Dentate Gyrus, but Not the Entorhinal Cortex, Blocks Maintenance of Long-Term Potentiation in Rats. Neurosci. Lett. 1989, 106, 175–180. [Google Scholar] [CrossRef]
- Matthies, H.; Frey, U.; Reymann, K.; Krug, M.; Jork, R.; Schröeder, H. Different Mechanisms and Multiple Stages of LTP. Adv. Exp. Med. Biol. 1990, 268, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.; Huang, Y.Y.; Kandel, E.R. Effects of CAMP Simulate a Late Stage of LTP in Hippocampal CA1 Neurons. Science 1993, 260, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.W.; Ganong, A.H.; Cotman, C.W. Long-Term Potentiation in the Hippocampus Involves Activation of N-Methyl-D-Aspartate Receptors. Brain Res. 1984, 323, 132–137. [Google Scholar] [CrossRef]
- Coan, E.J.; Collingridge, G.L. Characterization of an N-Methyl-d-Aspartate Receptor Component of Synaptic Transmission in Rat Hippocampal Slices. Neuroscience 1987, 22, 1–8. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Blake, J.F.; Brown, M.W.; Bashir, Z.I.; Ryan, E. Involvement of Excitatory Amino Acid Receptors in Long-Term Potentiation in the Schaffer Collateral-Commissural Pathway of Rat Hippocampal Slices. Can. J. Physiol. Pharmacol. 1991, 69, 1084–1090. [Google Scholar] [CrossRef]
- Bear, M.F.; Malenka, R.C. Synaptic Plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, Y.; Henderson, J.; Taverna, F.; Romano, C.; Abramow-Newerly, W.; Wojtowicz, J.M.; Roder, J. Selective Abolition of the NMDA Component of Long-Term Potentiation in Mice Lacking MGluR5. Learn. Mem. 1998, 5, 331–343. [Google Scholar] [CrossRef]
- Awad, H.; Hubert, G.W.; Smith, Y.; Levey, A.I.; Conn, P.J. Activation of Metabotropic Glutamate Receptor 5 Has Direct Excitatory Effects and Potentiates NMDA Receptor Currents in Neurons of the Subthalamic Nucleus. J. Neurosci. 2000, 20, 7871–7879. [Google Scholar] [CrossRef] [Green Version]
- Benquet, P.; Gee, C.E.; Gerber, U. Two Distinct Signaling Pathways Upregulate NMDA Receptor Responses via Two Distinct Metabotropic Glutamate Receptor Subtypes. J. Neurosci. 2002, 22, 9679–9686. [Google Scholar] [CrossRef] [Green Version]
- Doherty, A.J.; Palmer, M.J.; Henley, J.M.; Collingridge, G.L.; Jane, D.E. (RS)-2-Chloro-5-Hydroxyphenylglycine (CHPG) Activates MGlu5, but Not MGlu1, Receptors Expressed in CHO Cells and Potentiates NMDA Responses in the Hippocampus. Neuropharmacology 1997, 36, 265–267. [Google Scholar] [CrossRef]
- Mannaioni, G.; Marino, M.J.; Valenti, O.; Traynelis, S.F.; Conn, P.J. Metabotropic Glutamate Receptors 1 and 5 Differentially Regulate CA1 Pyramidal Cell Function. J. Neurosci. 2001, 21, 5925–5934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attucci, S.; Carlà, V.; Mannaioni, G.; Moroni, F. Activation of Type 5 Metabotropic Glutamate Receptors Enhances NMDA Responses in Mice Cortical Wedges. Br. J. Pharmacol. 2001, 132, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyoshi, K.; Masu, M.; Ishii, T.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular Cloning and Characterization of the Rat NMDA Receptor. Nature 1991, 354, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and Regional Expression in the Rat Brain and Functional Properties of Four NMDA Receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Sheng, M.; Cummings, J.; Roldan, L.A.; Jan, Y.N.; Jan, L.Y. Changing Subunit Composition of Heteromeric NMDA Receptors during Development of Rat Cortex. Nature 1994, 368, 144–147. [Google Scholar] [CrossRef]
- Sans, N.; Petralia, R.S.; Wang, Y.X.; Blahos, J.; Hell, J.W.; Wenthold, R.J. A Developmental Change in NMDA Receptor-Associated Proteins at Hippocampal Synapses. J. Neurosci. 2000, 20, 1260–1271. [Google Scholar] [CrossRef] [Green Version]
- Matta, J.A.; Ashby, M.C.; Sanz-Clemente, A.; Roche, K.W.; Isaac, J.T.R. MGluR5 and NMDA Receptors Drive the Experience- and Activity-Dependent NMDA Receptor NR2B to NR2A Subunit Switch. Neuron 2011, 70, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wong, T.P.; Pozza, M.F.; Lingenhoehl, K.; Wang, Y.; Sheng, M.; Auberson, Y.P.; Wang, Y.T. Role of NMDA Receptor Subtypes in Governing the Direction of Hippocampal Synaptic Plasticity. Science 2004, 304, 1021–1024. [Google Scholar] [CrossRef] [Green Version]
- Massey, P.V.; Johnson, B.E.; Moult, P.R.; Auberson, Y.P.; Brown, M.W.; Molnar, E.; Collingridge, G.L.; Bashir, Z.I. Differential Roles of NR2A and NR2B-Containing NMDA Receptors in Cortical Long-Term Potentiation and Long-Term Depression. J. Neurosci. 2004, 24, 7821–7828. [Google Scholar] [CrossRef] [Green Version]
- O’Riordan, K.J.; Hu, N.-W.; Rowan, M.J. Physiological Activation of MGlu5 Receptors Supports the Ion Channel Function of NMDA Receptors in Hippocampal LTD Induction in Vivo. Sci. Rep. 2018, 8, 4391. [Google Scholar] [CrossRef]
- Lu, Y.M.; Jia, Z.; Janus, C.; Henderson, J.T.; Gerlai, R.; Wojtowicz, J.M.; Roder, J.C. Mice Lacking Metabotropic Glutamate Receptor 5 Show Impaired Learning and Reduced CA1 Long-Term Potentiation (LTP) but Normal CA3 LTP. J. Neurosci. 1997, 17, 5196–5205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, U.; Krug, M.; Reymann, K.G.; Matthies, H. Anisomycin, an Inhibitor of Protein Synthesis, Blocks Late Phases of LTP Phenomena in the Hippocampal CA1 Region in Vitro. Brain Res. 1988, 452, 57–65. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D.; Kulla, A.; Frey, J.U. Requirement of Translation but Not Transcription for the Maintenance of Long-Term Depression in the CA1 Region of Freely Moving Rats. J. Neurosci. 2000, 20, 8572–8576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagena, H.; Manahan-Vaughan, D. Differentiation in the Protein Synthesis-Dependency of Persistent Synaptic Plasticity in Mossy Fiber and Associational/Commissural CA3 Synapses in Vivo. Front. Integr. Neurosci. 2013, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Cavigelli, M.; Dolfi, F.; Claret, F.X.; Karin, M. Induction of C-Fos Expression through JNK-Mediated TCF/Elk-1 Phosphorylation. EMBO J. 1995, 14, 5957–5964. [Google Scholar] [CrossRef]
- Jong, Y.-J.I.; Kumar, V.; O’Malley, K.L. Intracellular Metabotropic Glutamate Receptor 5 (MGluR5) Activates Signaling Cascades Distinct from Cell Surface Counterparts. J. Biol. Chem. 2009, 284, 35827–35838. [Google Scholar] [CrossRef] [Green Version]
- Brakeman, P.R.; Lanahan, A.A.; O’Brien, R.; Roche, K.; Barnes, C.A.; Huganir, R.L.; Worley, P.F. Homer: A Protein That Selectively Binds Metabotropic Glutamate Receptors. Nature 1997, 386, 284–288. [Google Scholar] [CrossRef]
- Tu, J.C.; Xiao, B.; Yuan, J.P.; Lanahan, A.A.; Leoffert, K.; Li, M.; Linden, D.J.; Worley, P.F. Homer Binds a Novel Proline-Rich Motif and Links Group 1 Metabotropic Glutamate Receptors with IP3 Receptors. Neuron 1998, 21, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Sala, C.; Piëch, V.; Wilson, N.R.; Passafaro, M.; Liu, G.; Sheng, M. Regulation of Dendritic Spine Morphology and Synaptic Function by Shank and Homer. Neuron 2001, 31, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Tu, J.C.; Xiao, B.; Naisbitt, S.; Yuan, J.P.; Petralia, R.S.; Brakeman, P.; Doan, A.; Aakalu, V.K.; Lanahan, A.A.; Sheng, M.; et al. Coupling of MGluR/Homer and PSD-95 Complexes by the Shank Family of Postsynaptic Density Proteins. Neuron 1999, 23, 583–592. [Google Scholar] [CrossRef]
- Naisbitt, S.; Kim, E.; Tu, J.C.; Xiao, B.; Sala, C.; Valtschanoff, J.; Weinberg, R.J.; Worley, P.F.; Sheng, M. Shank, a Novel Family of Postsynaptic Density Proteins That Binds to the NMDA Receptor/PSD-95/GKAP Complex and Cortactin. Neuron 1999, 23, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Bridi, M.; Schoch, H.; Florian, C.; Poplawski, S.G.; Banerjee, A.; Hawk, J.D.; Porcari, G.S.; Lejards, C.; Hahn, C.-G.; Giese, K.-P.; et al. Transcriptional Corepressor SIN3A Regulates Hippocampal Synaptic Plasticity via Homer1/MGluR5 Signaling. JCI Insight 2020, 5, e92385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugi, T.; Oyama, T.; Muto, T.; Nakanishi, S.; Morikawa, K.; Jingami, H. Crystal Structures of Autoinhibitory PDZ Domain of Tamalin: Implications for Metabotropic Glutamate Receptor Trafficking Regulation. EMBO J. 2007, 26, 2192–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitano, J.; Kimura, K.; Yamazaki, Y.; Soda, T.; Shigemoto, R.; Nakajima, Y.; Nakanishi, S. Tamalin, a PDZ Domain-Containing Protein, Links a Protein Complex Formation of Group 1 Metabotropic Glutamate Receptors and the Guanine Nucleotide Exchange Factor Cytohesins. J. Neurosci. 2002, 22, 1280–1289. [Google Scholar] [CrossRef] [Green Version]
- Bortolotto, Z.A.; Collett, V.J.; Conquet, F.; Jia, Z.; van der Putten, H.; Collingridge, G.L. The Regulation of Hippocampal LTP by the Molecular Switch, a Form of Metaplasticity, Requires MGlu5 Receptors. Neuropharmacology 2005, 49 (Suppl. S1), 13–25. [Google Scholar] [CrossRef]
- Naie, K.; Tsanov, M.; Manahan-Vaughan, D. Group I Metabotropic Glutamate Receptors Enable Two Distinct Forms of Long-Term Depression in the Rat Dentate Gyrus in Vivo. Eur. J. Neurosci. 2007, 25, 3264–3275. [Google Scholar] [CrossRef]
- Dietz, B.; Manahan-Vaughan, D. Hippocampal Long-Term Depression Is Facilitated by the Acquisition and Updating of Memory of Spatial Auditory Content and Requires MGlu5 Activation. Neuropharmacology 2016, 115, 30–41. [Google Scholar] [CrossRef]
- Francesconi, W.; Cammalleri, M.; Sanna, P.P. The Metabotropic Glutamate Receptor 5 Is Necessary for Late-Phase Long-Term Potentiation in the Hippocampal CA1 Region. Brain Res. 2004, 1022, 12–18. [Google Scholar] [CrossRef]
- Balu, D.T.; Li, Y.; Takagi, S.; Presti, K.T.; Ramikie, T.S.; Rook, J.M.; Jones, C.K.; Lindsley, C.W.; Conn, P.J.; Bolshakov, V.Y.; et al. An MGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia. Neuropsychopharmacol 2016, 41, 2052–2061. [Google Scholar] [CrossRef]
- Huber, K.M.; Roder, J.C.; Bear, M.F. Chemical Induction of MGluR5- and Protein Synthesis-Dependent Long-Term Depression in Hippocampal Area CA1. J. Neurophysiol. 2001, 86, 321–325. [Google Scholar] [CrossRef]
- Harney, S.C.; Rowan, M.; Anwyl, R. Long-Term Depression of NMDA Receptor-Mediated Synaptic Transmission Is Dependent on Activation of Metabotropic Glutamate Receptors and Is Altered to Long-Term Potentiation by Low Intracellular Calcium Buffering. J. Neurosci. 2006, 26, 1128–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparini, F.; Lingenhöhl, K.; Stoehr, N.; Flor, P.J.; Heinrich, M.; Vranesic, I.; Biollaz, M.; Allgeier, H.; Heckendorn, R.; Urwyler, S.; et al. 2-Methyl-6-(Phenylethynyl)-Pyridine (MPEP), a Potent, Selective and Systemically Active MGlu5 Receptor Antagonist. Neuropharmacology 1999, 38, 1493–1503. [Google Scholar] [CrossRef]
- Faas, G.C.; Adwanikar, H.; Gereau, R.W.; Saggau, P. Modulation of Presynaptic Calcium Transients by Metabotropic Glutamate Receptor Activation: A Differential Role in Acute Depression of Synaptic Transmission and Long-Term Depression. J. Neurosci. 2002, 22, 6885–6890. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; You, J.-L.; Wu, M.-Y.; Hsu, K.-S. Rap1-Induced P38 Mitogen-Activated Protein Kinase Activation Facilitates AMPA Receptor Trafficking via the GDI·Rab5 Complex Potential Role in (S)-3,5-Dihydroxyphenylglycine-Induced Long Term Depression. J. Biol. Chem. 2004, 279, 12286–12292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-C.; Hsu, K.-S. Sustained Activation of Metabotropic Glutamate Receptor 5 and Protein Tyrosine Phosphatases Mediate the Expression of (S)-3,5-Dihydroxyphenylglycine-Induced Long-Term Depression in the Hippocampal CA1 Region. J. Neurochem. 2006, 96, 179–194. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A Synaptic Model of Memory: Long-Term Potentiation in the Hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Braunewell, K.-H.; Manahan-Vaughan, D. Long-Term Depression: A Cellular Basis for Learning? Rev. Neurosci. 2001, 12, 121–140. [Google Scholar] [CrossRef]
- Hagena, H.; Manahan-Vaughan, D. Learning-Facilitated Synaptic Plasticity at CA3 Mossy Fiber and Commissural-Associational Synapses Reveals Different Roles in Information Processing. Cereb. Cortex 2011, 21, 2442–2449. [Google Scholar] [CrossRef] [Green Version]
- Mercerón-Martínez, D.; Almaguer-Melian, W.; Bergado, J.A. Basolateral Amygdala Stimulation plus Water Maze Training Restore Dentate Gyrus LTP and Improve Spatial Learning and Memory. Behav. Brain Res. 2022, 417, 113589. [Google Scholar] [CrossRef]
- Choi, J.-H.; Park, P.; Baek, G.-C.; Sim, S.-E.; Kang, S.J.; Lee, Y.; Ahn, S.-H.; Lim, C.-S.; Lee, Y.-S.; Collingridge, G.L.; et al. Effects of PI3Kβ Overexpression in the Hippocampus on Synaptic Plasticity and Spatial Learning. Mol. Brain 2014, 7, 78. [Google Scholar] [CrossRef]
- Davis, C.D.; Jones, F.L.; Derrick, B.E. Novel Environments Enhance the Induction and Maintenance of Long-Term Potentiation in the Dentate Gyrus. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6497–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Cullen, W.K.; Anwyl, R.; Rowan, M.J. Dopamine-Dependent Facilitation of LTP Induction in Hippocampal CA1 by Exposure to Spatial Novelty. Nat. Neurosci. 2003, 6, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mercado, D.; Dieguez, D.; Barea-Rodriguez, E.J. Brief Novelty Exposure Facilitates Dentate Gyrus LTP in Aged Rats. Hippocampus 2008, 18, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, A.; Manahan-Vaughan, D. The Hippocampal CA1 Region and Dentate Gyrus Differentiate between Environmental and Spatial Feature Encoding through Long-Term Depression. Cereb. Cortex 2008, 18, 968–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashby, D.M.; Floresco, S.B.; Phillips, A.G.; McGirr, A.; Seamans, J.K.; Wang, Y.T. LTD Is Involved in the Formation and Maintenance of Rat Hippocampal CA1 Place-Cell Fields. Nat. Commun. 2021, 12, 100. [Google Scholar] [CrossRef]
- Sasaki-Hamada, S.; Ayumu, F.; Satoh, S.; Iwai, T.; Oka, J.-I. GLP-2 Restores Impairments in Spatial Working Memory and Hippocampal LTD via the MEK/ERK Pathway in Juvenile-Onset Diabetes Rats. Behav. Brain Res. 2021, 406, 113235. [Google Scholar] [CrossRef]
- Chang, C.-P.; Lee, C.-T.; Hou, W.-H.; Lin, M.-S.; Lai, H.-L.; Chien, C.-L.; Chang, C.; Cheng, P.-L.; Lien, C.-C.; Chern, Y. Type VI Adenylyl Cyclase Negatively Regulates GluN2B-Mediated LTD and Spatial Reversal Learning. Sci. Rep. 2016, 6, 22529. [Google Scholar] [CrossRef]
- André, M.A.E.; Manahan-Vaughan, D. Spatial Olfactory Learning Facilitates Long-Term Depression in the Hippocampus. Hippocampus 2013, 23, 963–968. [Google Scholar] [CrossRef]
- Hagena, H.; Manahan-Vaughan, D. MGLU Receptors. Recept 2017, 31, 79–101. [Google Scholar] [CrossRef]
- Tan, S.Z.K.; Ganella, D.E.; Dick, A.L.W.; Duncan, J.R.; Ong-Palsson, E.; Bathgate, R.A.D.; Kim, J.H.; Lawrence, A.J. Spatial Learning Requires MGlu5 Signalling in the Dorsal Hippocampus. Neurochem. Res. 2015, 40, 1303–1310. [Google Scholar] [CrossRef]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate Receptor Function in Learning and Memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef]
- Simonyi, A.; Schachtman, T.R.; Christoffersen, G.R.J. The Role of Metabotropic Glutamate Receptor 5 in Learning and Memory Processes. Drug News Perspect. 2005, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, H.; Stefani, M.R.; Adams, B.W.; Tamagan, G.D.; Moghaddam, B. Functional Interaction Between NMDA and MGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004, 29, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- André, M.A.E.; Güntürkün, O.; Manahan-Vaughan, D. The Metabotropic Glutamate Receptor, MGlu5, Is Required for Extinction Learning That Occurs in the Absence of a Context Change. Hippocampus 2014, 25, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Simonyi, A.; Serfozo, P.; Shelat, P.B.; Dopheide, M.M.; Coulibaly, A.P.; Schachtman, T.R. Differential Roles of Hippocampal Metabotropic Glutamate Receptors 1 and 5 in Inhibitory Avoidance Learning. Neurobiol. Learn. Mem. 2007, 88, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Gravius, A.; Barberi, C.; Schäfer, D.; Schmidt, W.J.; Danysz, W. The Role of Group I Metabotropic Glutamate Receptors in Acquisition and Expression of Contextual and Auditory Fear Conditioning in Rats—A Comparison. Neuropharmacology 2006, 51, 1146–1155. [Google Scholar] [CrossRef]
- Handford, C.E.; Tan, S.; Lawrence, A.J.; Kim, J.H. The Effect of the MGlu5 Negative Allosteric Modulator MTEP and NMDA Receptor Partial Agonist D-Cycloserine on Pavlovian Conditioned Fear. Int. J. Neuropsychopharmacol. 2014, 17, 1521–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uslaner, J.M.; Parmentier-Batteur, S.; Flick, R.B.; Surles, N.O.; Lam, J.S.H.; McNaughton, C.H.; Jacobson, M.A.; Hutson, P.H. Dose-Dependent Effect of CDPPB, the MGluR5 Positive Allosteric Modulator, on Recognition Memory Is Associated with GluR1 and CREB Phosphorylation in the Prefrontal Cortex and Hippocampus. Neuropharmacology 2009, 57, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Balschun, D.; Zuschratter, W.; Wetzel, W. Allosteric Enhancement of Metabotropic Glutamate Receptor 5 Function Promotes Spatial Memory. Neuroscience 2006, 142, 691–702. [Google Scholar] [CrossRef]
- Fowler, S.W.; Walker, J.M.; Klakotskaia, D.; Will, M.J.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Effects of a Metabotropic Glutamate Receptor 5 Positive Allosteric Modulator, CDPPB, on Spatial Learning Task Performance in Rodents. Neurobiol. Learn. Mem. 2013, 99, 25–31. [Google Scholar] [CrossRef]
- Steckler, T.; Oliveira, A.F.M.; Dyck, C.V.; Craenendonck, H.V.; Mateus, A.M.A.; Langlois, X.; Lesage, A.S.J.; Prickaerts, J. Metabotropic Glutamate Receptor 1 Blockade Impairs Acquisition and Retention in a Spatial Water Maze Task. Behav. Brain Res. 2005, 164, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, Y.; Kraniotis, S.; He, Q.; Marshall, J.J.; Nomura, T.; Stauffer, S.R.; Lindsley, C.W.; Conn, P.J.; Contractor, A. Potentiating MGluR5 Function with a Positive Allosteric Modulator Enhances Adaptive Learning. Learn. Mem. 2013, 20, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Pang, T.Y.C.; Hannan, A.J. Enhancement of Cognitive Function in Models of Brain Disease through Environmental Enrichment and Physical Activity. Neuropharmacology 2013, 64, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Holloway, J.; Holtkamp, C.C.M.; Larsson, A.; Hartman, L.C.; Pearce, R.; Scherman, B.; Johansson, S.; Thomas, P.W.; Wareing, L.A.; et al. Effects of Multi-sensory Stimulation for People with Dementia. J. Adv. Nurs. 2003, 43, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschler, A.; Manahan-Vaughan, D. Brief Environmental Enrichment Elicits Metaplasticity of Hippocampal Synaptic Potentiation in Vivo. Front. Behav. Neurosci. 2012, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, G.P.; Olin, A.; O’Riordan, K.; Hullinger, R.; Burger, C. Environmental Enrichment Improves Hippocampal Function in Aged Rats by Enhancing Learning and Memory, LTP, and MGluR5-Homer1c Activity. Neurobiol. Aging 2018, 63, 1–11. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D.; Ngomba, R.T.; Storto, M.; Kulla, A.; Catania, M.V.; Chiechio, S.; Rampello, L.; Passarelli, F.; Capece, A.; Reymann, K.G.; et al. An Increased Expression of the MGlu5 Receptor Protein Following LTP Induction at the Perforant Path-Dentate Gyrus Synapse in Freely Moving Rats. Neuropharmacology 2003, 44, 17–25. [Google Scholar] [CrossRef]
- Riedel, G.; Casabona, G.; Platt, B.; Macphail, E.M.; Nicoletti, F. Fear Conditioning-Induced Time- and Subregion-Specific Increase in Expression of MGlu5 Receptor Protein in Rat Hippocampus. Neuropharmacology 2000, 39, 1943–1951. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D. Regulation of Hippocampal Information Encoding by Metabotopic Glutamate Receptors. Neuroforum 2018, 24, A121–A126. [Google Scholar] [CrossRef]
- Kemp, A.; Manahan-Vaughan, D. Passive Spatial Perception Facilitates the Expression of Persistent Hippocampal Long-Term Depression. Cereb. Cortex 2011, 22, 1614–1621. [Google Scholar] [CrossRef]
- Beckmann, D.; Feldmann, M.; Shchyglo, O.; Manahan-Vaughan, D. Hippocampal Synaptic Plasticity, Spatial Memory, and Neurotransmitter Receptor Expression Are Profoundly Altered by Gradual Loss of Hearing Ability. Cereb. Cortex 2020, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Manahan-Vaughan, D. Spatial Olfactory Learning Contributes to Place Field Formation in the Hippocampus. Cereb. Cortex 2015, 25, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsanov, M.; Manahan-Vaughan, D. Visual Cortex Plasticity Evokes Excitatory Alterations in the Hippocampus. Front. Integr. Neurosci. 2009, 3, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, J.; Dostrovsky, J. The Hippocampus as a Spatial Map. Preliminary Evidence from Unit Activity in the Freely-Moving Rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Knierim, J.; Kudrimoti, H.; McNaughton, B. Place Cells, Head Direction Cells, and the Learning of Landmark Stability. J. Neurosci. 1995, 15, 1648–1659. [Google Scholar] [CrossRef] [Green Version]
- Axmacher, N.; Mormann, F.; Fernández, G.; Elger, C.E.; Fell, J. Memory Formation by Neuronal Synchronization. Brain Res. Rev. 2006, 52, 170–182. [Google Scholar] [CrossRef]
- Düzel, E.; Penny, W.D.; Burgess, N. Brain Oscillations and Memory. Curr. Opin. Neurobiol. 2010, 20, 143–149. [Google Scholar] [CrossRef]
- Nyhus, E.; Curran, T. Functional Role of Gamma and Theta Oscillations in Episodic Memory. Neurosci. Biobehav. Rev. 2010, 34, 1023–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikbaev, A.; Manahan-Vaughan, D. Hippocampal Network Activity Is Transiently Altered by Induction of Long-Term Potentiation in the Dentate Gyrus of Freely Behaving Rats. Front. Behav. Neurosci. 2007, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Bikbaev, A.; Manahan-Vaughan, D. Relationship of Hippocampal Theta and Gamma Oscillations to Potentiation of Synaptic Transmission. Front. Neurosci. 2008, 2, 56–63. [Google Scholar] [CrossRef]
- Bouton, M.E. Context and Behavioral Processes in Extinction. Learn. Mem. 2004, 11, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Maren, S. Hippocampal Involvement in Contextual Modulation of Fear Extinction. Hippocampus 2007, 17, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Reiserer, R.S.; Harrison, F.E.; Syverud, D.C.; McDonald, M.P. Impaired Spatial Learning in the APPSwe + PSEN1ΔE9 Bigenic Mouse Model of Alzheimer’s Disease. Genes Brain Behav. 2007, 6, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Fowler, S.W.; Miller, D.K.; Sun, A.Y.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Simonyi, A.; Schachtman, T.R. Spatial Learning and Memory Impairment and Increased Locomotion in a Transgenic Amyloid Precursor Protein Mouse Model of Alzheimer’s Disease. Behav. Brain Res. 2011, 222, 169–175. [Google Scholar] [CrossRef]
- Minkeviciene, R.; Banerjee, P.; Tanila, H. Memantine Improves Spatial Learning in a Transgenic Mouse Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2004, 311, 677–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, P.; Levenson, J.M.; Battaglia, F.; Atkinson, R.; Teague, R.; Antalffy, B.; Armstrong, D.; Arancio, O.; Sweatt, J.D.; Zoghbi, H.Y. Learning and Memory and Synaptic Plasticity Are Impaired in a Mouse Model of Rett Syndrome. J. Neurosci. 2006, 26, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Kee, S.E.; Mou, X.; Zoghbi, H.Y.; Ji, D. Impaired Spatial Memory Codes in a Mouse Model of Rett Syndrome. Elife 2018, 7, e31451. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.A.; Pozzo-Miller, L. The Role of MeCP2 in Learning and Memory. Learn. Mem. 2019, 26, 343–350. [Google Scholar] [CrossRef]
- Yan, Q.J.; Asafo-Adjei, P.K.; Arnold, H.M.; Brown, R.E.; Bauchwitz, R.P. A Phenotypic and Molecular Characterization of the Fmr1-tm1Cgr Fragile X Mouse. Genes Brain Behav. 2004, 3, 337–359. [Google Scholar] [CrossRef]
- Guo, W.; Murthy, A.C.; Zhang, L.; Johnson, E.B.; Schaller, E.G.; Allan, A.M.; Zhao, X. Inhibition of GSK3β Improves Hippocampus-Dependent Learning and Rescues Neurogenesis in a Mouse Model of Fragile X Syndrome. Hum. Mol. Genet 2012, 21, 681–691. [Google Scholar] [CrossRef]
- Ehrlichman, R.S.; Luminais, S.N.; White, S.L.; Rudnick, N.D.; Ma, N.; Dow, H.C.; Kreibich, A.S.; Abel, T.; Brodkin, E.S.; Hahn, C.-G.; et al. Neuregulin 1 Transgenic Mice Display Reduced Mismatch Negativity, Contextual Fear Conditioning and Social Interactions. Brain Res. 2009, 1294, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Kasai, A.; Mizuno, M.; Fengyi, L.; Shintani, N.; Maeda, S.; Yokoyama, M.; Ozaki, M.; Nawa, H. Phenotypic Characterization of Transgenic Mice Overexpressing Neuregulin-1. PLoS ONE 2010, 5, e14185. [Google Scholar] [CrossRef] [PubMed]
- Deakin, I.H.; Nissen, W.; Law, A.J.; Lane, T.; Kanso, R.; Schwab, M.H.; Nave, K.-A.; Lamsa, K.P.; Paulsen, O.; Bannerman, D.M.; et al. Transgenic Overexpression of the Type I Isoform of Neuregulin 1 Affects Working Memory and Hippocampal Oscillations but Not Long-Term Potentiation. Cereb. Cortex 2012, 22, 1520–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifton, N.E.; Morisot, N.; Girardon, S.; Millan, M.J.; Loiseau, F. Enhancement of Social Novelty Discrimination by Positive Allosteric Modulators at Metabotropic Glutamate 5 Receptors: Adolescent Administration Prevents Adult-Onset Deficits Induced by Neonatal Treatment with Phencyclidine. Psychopharmacology 2013, 225, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. On the Integration of Space, Time, and Memory. Neuron 2017, 95, 1007–1018. [Google Scholar] [CrossRef]
- Horner, A.J.; Doeller, C.F. Plasticity of Hippocampal Memories in Humans. Curr. Opin. Neurobiol. 2017, 43, 102–109. [Google Scholar] [CrossRef]
- Maksymetz, J.; Moran, S.P.; Conn, P.J. Targeting Metabotropic Glutamate Receptors for Novel Treatments of Schizophrenia. Mol. Brain 2017, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Montana, M.C.; Gereau, R.W. Metabotropic Glutamate Receptors as Targets for Analgesia: Antagonism, Activation, and Allosteric Modulation. Curr. Pharm. Biotechno. 2011, 12, 1681–1688. [Google Scholar] [CrossRef]
| Preparation | Species | Outcome | Hippocampal Region | References |
|---|---|---|---|---|
| mGlu5 antagonist/NAM | ||||
| in vivo | rat | LTP inhibited, STP impaired | DG | [10,11,124] |
| in vivo | rat | L-LTP inhibited | DG | [16,130] |
| in vivo | rat | No effect on LTD | DG | [168] |
| in vivo | rat | LTP inhibited | MF–CA3 | [3] |
| in vivo | rat | No effect on LTP | AC–CA3 | [3] |
| in vivo | rat | No effect on LTD | MF–CA3 | [3] |
| in vivo | rat | LTD inhibited | AC–CA3 | [3] |
| in vivo | rat | LTP inhibited | CA1 | [124] |
| in vivo | rat | LTP enhanced (after prolonged antagonism) | CA1 | [16] |
| in vivo | rat | LTD inhibited | CA1 | [5,33,169] |
| in vitro | rat | STP inhibited | CA1 | [123] |
| in vitro | rat | L-LTP inhibited | CA1 | [123] |
| in vitro | rat | LTD induction inhibited | CA1 | [123] |
| in vitro | rat | L-LTD inhibited | CA1 | [123] |
| in vitro | mouse | LTP inhibited | CA1 | [170] |
| in vivo | mouse | STD, LTD inhibited | CA1 | [4,11] |
| in vivo | mouse (3–4 months old) | LTP inhibited | CA1 | [18] |
| in vivo | mouse (10–14 months old) | No effect | CA1 | [18] |
| mGlu5 agonist/PAM | ||||
| in vivo | rat | LTP enhanced | DG | [130] |
| in vitro, hippocampal slice preparation | rat | LTP enhanced | CA1 | [8,127] |
| in vitro, hippocampal slice preparation | rat | LTD enhanced | CA1 | [127] |
| in vitro, hippocampal slice preparation | mouse | LTP enhanced | CA1 | [8] |
| in vitro, hippocampal slice preparation | mouse | LTP rescued | CA1 | [171] |
| mGlu5 KO | ||||
| in vitro, hippocampal slice preparation | mouse | LTP inhibited | CA1, DG | [153] |
| in vitro, hippocampal slice preparation | mouse | No effect | MF–CA3 | [153] |
| in vitro, hippocampal slice preparation | mouse | DHPG-LTD inhibited | CA1 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagena, H.; Manahan-Vaughan, D. Role of mGlu5 in Persistent Forms of Hippocampal Synaptic Plasticity and the Encoding of Spatial Experience. Cells 2022, 11, 3352. https://doi.org/10.3390/cells11213352
Hagena H, Manahan-Vaughan D. Role of mGlu5 in Persistent Forms of Hippocampal Synaptic Plasticity and the Encoding of Spatial Experience. Cells. 2022; 11(21):3352. https://doi.org/10.3390/cells11213352
Chicago/Turabian StyleHagena, Hardy, and Denise Manahan-Vaughan. 2022. "Role of mGlu5 in Persistent Forms of Hippocampal Synaptic Plasticity and the Encoding of Spatial Experience" Cells 11, no. 21: 3352. https://doi.org/10.3390/cells11213352






