Clinical Aspects of B Cell Immunodeficiencies: The Past, the Present and the Future
Abstract
:1. Introduction
2. The Past
3. The Present
3.1. PU.1
3.2. Disorders of the Ikaros Family of Transcription Factors
3.2.1. Ikaros Zinc Finger 1 (IKZF1; Ikaros)
3.2.2. Ikaros Zinc Finger 2 (IKZF2; Helios) and Ikaros Zinc Finger 3 (IKZF3; Aiolos)
3.3. Hyper IgM Syndromes
3.3.1. CD40L (and CD40) Deficiency
3.3.2. AID
3.3.3. UNG
3.4. Common Variable Immunodeficiency (CVID) and CVID-like Disorders
3.4.1. CVID
3.4.2. Variants in the TNF Receptor Superfamily Member 13B (TNFRSF13B)
3.4.3. Mutations in NFKB1
3.4.4. Cytotoxic T-Lymphocyte-Associated Protein-4 (CTLA-4) Insufficiency
3.4.5. LPS Responsive Beige-like Anchor Protein (LRBA) Deficiency
3.4.6. Activated Phosphoinositide 3-Kinase Delta Syndrome (APDS)-1/2 (Defect in PIK3CD/PIK3R1)
4. The Future
4.1. The Role of Kappa (Light Chain Gene) Rearrangement Excision Circle (KREC) Quantification, Serum Biomarkers and Gene Sequencing in the Diagnosis of B Cell IEI
4.2. Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)
4.3. Therapeutic IgA
4.4. Gene Therapy
4.5. Gene Editing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Chenoweth, A.; Crescioli, S.; Reichert, J.M. Antibodies to watch in 2022. mAbs 2022, 14, 2014296. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Cunningham-Rundles, C. Primary B-cell immunodeficiencies. Hum. Immunol. 2018, 80, 351–362. [Google Scholar] [CrossRef]
- Bruton, O.C. Agammaglobulinemia. Pediatrics 1952, 9, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Saffran, D.C.; Rawlings, D.J.; Parolini, O.; Allen, R.; Klisak, I.; Sparkes, R.S.; Kubagawa, H.; Mohandas, T.; Quan, S.; et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 1993, 72, 279–290. [Google Scholar] [CrossRef]
- Vetrie, D.; Vořechovský, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.A.; Hammarström, L.; Kinnon, C.; Levinsky, R.J.; Bobrow, M.; et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 1993, 361, 226–233. [Google Scholar] [CrossRef]
- Smith, C.I.; Baskin, B.; Humire-Greiff, P.; Zhou, J.N.; Olsson, P.G.; Maniar, H.S.; Kjellén, P.; Lambris, J.; Christensson, B.; Hammarström, L. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J. Immunol. 1994, 152, 557–565. [Google Scholar] [PubMed]
- Durandy, A.; Kracker, S.; Fischer, A. Chapter 25—Immune Deficiencies Caused by B Cell Defects. In Molecular Biology of B Cells, 2nd ed.; Alt, F.W., Honjo, T., Radbruch, A., Reth, M., Eds.; Academic Press: London, UK, 2015; pp. 463–479. [Google Scholar]
- Horwood, N.; Mahon, T.; McDaid, J.P.; Campbell, J.; Mano, H.; Brennan, F.M.; Webster, D.; Foxwell, B.M. Bruton’s Tyrosine Kinase Is Required for Lipopolysaccharide-induced Tumor Necrosis Factor α Production. J. Exp. Med. 2003, 197, 1603–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferies, C.A.; Doyle, S.; Brunner, C.; Dunne, A.; Brint, E.; Wietek, C.; Walch, E.; Wirth, T.; O’Neill, L. Bruton’s Tyrosine Kinase Is a Toll/Interleukin-1 Receptor Domain-binding Protein That Participates in Nuclear Factor κB Activation by Toll-like Receptor 4. J. Biol. Chem. 2003, 278, 26258–26264. [Google Scholar] [CrossRef] [Green Version]
- Bousfiha, A.; Jeddane, L.; Picard, C.; Al-Herz, W.; Ailal, F.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 2020, 40, 66–81. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020, 40, 24–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. The Ever-Increasing Array of Novel Inborn Errors of Immunity: An Interim Update by the IUIS Committee. J. Clin. Immunol. 2021, 41, 666–679. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Le Coz, C.; Nguyen, D.N.; Su, C.; Nolan, B.E.; Albrecht, A.V.; Xhani, S.; Sun, D.; Demaree, B.; Pillarisetti, P.; Khanna, C.; et al. Constrained chromatin accessibility in PU.1-mutated agammaglobulinemia patients. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Read, K.A.; Jones, D.M.; Freud, A.G.; Oestreich, K.J. Established and emergent roles for Ikaros transcription factors in lymphoid cell development and function. Immunol. Rev. 2020, 300, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Santos, C.J.; Kuehn, H.S.; Rosenzweig, S.D. IKAROS Family Zinc Finger 1–Associated Diseases in Primary Immunodeficiency Patients. Immunol. Allergy Clin. N. Am. 2020, 40, 461–470. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Niemela, J.E.; Stoddard, J.; Mannurita, S.C.; Shahin, T.; Goel, S.; Hintermeyer, M.; Heredia, R.J.; Garofalo, M.; Lucas, L.; et al. Germline IKAROS dimerization haploinsufficiency causes hematologic cytopenias and malignancies. Blood 2021, 137, 349–363. [Google Scholar] [CrossRef]
- Hoshino, A.; Boutboul, D.; Zhang, Y.; Kuehn, H.S.; Hadjadj, J.; Özdemir, N.; Celkan, T.; Walz, C.; Picard, C.; Lenoir, C.; et al. Gain-of-function IKZF1 variants in humans cause immune dysregulation associated with abnormal T/B cell late differentiation. Sci. Immunol. 2022, 7, eabi7160. [Google Scholar] [CrossRef]
- Hetemäki, I.; Kaustio, M.; Kinnunen, M.; Heikkilä, N.; Keskitalo, S.; Nowlan, K.; Miettinen, S.; Sarkkinen, J.; Glumoff, V.; Andersson, N.; et al. Loss-of-function mutation in IKZF2 leads to immunodeficiency with dysregulated germinal center reactions and reduction of MAIT cells. Sci. Immunol. 2021, 6, eabe3454. [Google Scholar] [CrossRef]
- Shahin, T.; Mayr, D.; Shoeb, M.R.; Kuehn, H.S.; Hoeger, B.; Giuliani, S.; Gawriyski, L.M.; Petronczki, Y.; Hadjadj, J.; Bal, S.K.; et al. Identification of germline monoallelic mutations in IKZF2 in patients with immune dysregulation. Blood Adv. 2022, 6, 2444–2451. [Google Scholar] [CrossRef]
- Yamashita, M.; Kuehn, H.S.; Okuyama, K.; Okada, S.; Inoue, Y.; Mitsuiki, N.; Imai, K.; Takagi, M.; Kanegane, H.; Takeuchi, M.; et al. A variant in human AIOLOS impairs adaptive immunity by interfering with IKAROS. Nat. Immunol. 2021, 22, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, H.S.; Chang, J.; Yamashita, M.; Niemela, J.E.; Zou, C.; Okuyama, K.; Harada, J.; Stoddard, J.L.; Nunes-Santos, C.J.; Boast, B.; et al. T and B cell abnormalities, pneumocystis pneumonia, and chronic lymphocytic leukemia associated with an AIOLOS defect in patients. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Fekrvand, S.; Shahkarami, S.; Azizi, G.; Moazzami, B.; Abolhassani, H.; Aghamohammadi, A. The hyper IgM syndromes: Epidemiology, pathogenesis, clinical manifestations, diagnosis and management. Clin. Immunol. 2018, 198, 19–30. [Google Scholar] [CrossRef]
- Leiva, L.E.; Junprasert, J.; Hollenbaugh, D.; Sorensen, R.U. Central Nervous System Toxoplasmosis with an Increased Proportion of Circulating γδ T Cells in a Patient with Hyper-IgM Syndrome. J. Clin. Immunol. 1998, 18, 283–290. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Chung, J.-H.; Shin, J.-H.; Hwang, T.-J.; Kim, K.-S.; Lee, J.-H.; Nam, J.-H.; Park, C.-S.; Juhng, S.-W.; Choi, C.; et al. Lymphonodular Cryptococcosis Diagnosed by Fine Needle Aspiration Cytology in Hyper-IgM Syndrome: A case report. Acta Cytol. 2001, 45, 241–244. [Google Scholar] [CrossRef] [PubMed]
- de la Morena, M.T.; Leonard, D.; Torgerson, T.R.; Cabral-Marques, O.; Slatter, M.; Aghamohammadi, A.; Chandra, S.; Murguia-Favela, L.; Bonilla, F.A.; Kanariou, M.; et al. Long-term outcomes of 176 patients with X-linked hyper-IgM syndrome treated with or without hematopoietic cell transplantation. J. Allergy Clin. Immunol. 2016, 139, 1282–1292. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhou, K.; Yu, D.; Cai, X.; Hua, Y.; Zhou, H.; Wang, C. A delayed diagnosis of X-linked hyper IgM syndrome complicated with toxoplasmic encephalitis in a child: A case report and literature review. Medicine 2017, 96, e8989. [Google Scholar] [CrossRef]
- Subauste, C.S.; Wessendarp, M.; Sorensen, R.U.; Leiva, L.E. CD40-CD40 ligand interaction is central to cell-mediated immun-ity against Toxoplasma gondii: Patients with hyper IgM syndrome have a defective type 1 immune response that can be re-stored by soluble CD40 ligand trimer. J. Immunol. 1999, 162, 6690–6700. [Google Scholar]
- Tsuge, I.; Matsuoka, H.; Nakagawa, A.; Kamachi, Y.; Aso, K.; Negoro, T.; Ito, M.; Torii, S.; Watanabe, K. Necrotizing toxoplasmic encephalitis in a child with the X-linked hyper-IgM syndrome. Eur. J. Pediatr. 1998, 157, 735–737. [Google Scholar] [CrossRef]
- Romani, L.; Williamson, P.R.; Di Cesare, S.; Di Matteo, G.; De Luca, M.; Carsetti, R.; Figà-Talamanca, L.; Cancrini, C.; Rossi, P.; Finocchi, A. Cryptococcal Meningitis and Post-Infectious Inflammatory Response Syndrome in a Patient With X-Linked Hyper IgM Syndrome: A Case Report and Review of the Literature. Front. Immunol. 2021, 12, 708837. [Google Scholar] [CrossRef]
- Malheiro, L.; Lazzara, D.; Xerinda, S.; Pinheiro, M.D.; Sarmento, A. Cryptococcal meningoencephalitis in a patient with hyper immunoglobulin M (IgM) syndrome: A case report. BMC Res. Notes 2014, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pacharn, P.; Phongsamart, W.; Boonyawat, B.; Jirapongsananuruk, O.; Visitsunthorn, N.; Chokephaibulkit, K. Disseminated cryptococcosis in two boys with novel mutation of CD40 Ligand-Associated X-linked hyper-IgM syndrome. Asian Pac. J. Allergy Immunol. 2021, 39, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.P.; de la Morena, M.T. X-Linked Hyper IgM Syndrome; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Lopez-Granados, E.; Temmerman, S.T.; Wu, L.; Reynolds, J.C.; Follmann, D.; Liu, S.; Nelson, D.L.; Rauch, F.; Jain, A. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 5056–5061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanford, J.P.; Favour, C.B.; Tribeman, M.S. Absence of Serum Gamma Globulins in an Adult. N. Engl. J. Med. 1954, 250, 1027–1029. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.A.; the USIDNET Consortium; Maggadottir, S.M.; Pantell, M.S.; Lugar, P.; Rundles, C.C.; Sullivan, K.E. Two Sides of the Same Coin: Pediatric-Onset and Adult-Onset Common Variable Immune Deficiency. J. Clin. Immunol. 2017, 37, 592–602. [Google Scholar] [CrossRef]
- El-Helou, S.M.; Biegner, A.-K.; Bode, S.; Ehl, S.R.; Heeg, M.; Maccari, M.E.; Ritterbusch, H.; Speckmann, C.; Rusch, S.; Scheible, R.; et al. The German National Registry of Primary Immunodeficiencies (2012–2017). Front. Immunol. 2019, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Ameratunga, R.; Woon, S.-T.; Gillis, D.; Koopmans, W.; Steele, R. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin. Exp. Immunol. 2013, 174, 203–211. [Google Scholar] [CrossRef]
- Seidel, M.G.; Kindle, G.; Gathmann, B.; Quinti, I.; Buckland, M.; van Montfrans, J.; Scheible, R.; Rusch, S.; Gasteiger, L.M.; Grimbacher, B.; et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J. Allergy Clin. Immunol. Pract. 2019, 7, 1763–1770. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Barlan, I.; Chapel, H.; Costa-Carvalho, B.T.; Cunningham-Rundles, C.; de la Morena, M.T.; Espinosa-Rosales, F.J.; Hammarström, L.; Nonoyama, S.; Quinti, I.; et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J. Allergy Clin. Immunol. Pract. 2015, 4, 38–59. [Google Scholar] [CrossRef] [Green Version]
- Schauer, U.; Stemberg, F.; Rieger, C.H.L.; Büttner, W.; Borte, M.; Schubert, S.; Möllers, H.; Riedel, F.; Herz, U.; Renz, H.; et al. Levels of Antibodies Specific to Tetanus Toxoid, Haemophilus influenzae Type b, and Pneumococcal Capsular Polysaccharide in Healthy Children and Adults. Clin. Vaccine Immunol. 2003, 10, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Publication WHO. Pneumococcal vaccines WHO position paper—2012—Recommendations. Vaccine 2012, 30, 4717–4718. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.K.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M.; et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130, S1–S24. [Google Scholar] [CrossRef] [PubMed]
- LaFon, D.C.; Nahm, M.H. Measuring immune responses to pneumococcal vaccines. J. Immunol. Methods 2018, 461, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-E.; Cunningham-Rundles, C. Non-infectious Complications of Common Variable Immunodeficiency: Updated Clinical Spectrum, Sequelae, and Insights to Pathogenesis. Front. Immunol. 2020, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- Odnoletkova, I.; Kindle, G.; Quinti, I.; Grimbacher, B.; Knerr, V.; Gathmann, B.; Ehl, S.; Mahlaoui, N.; Van Wilder, P.; Bogaerts, K.; et al. The burden of common variable immunodeficiency disorders: A retrospective analysis of the European Society for Immunodeficiency (ESID) registry data. Orphanet J. Rare Dis. 2018, 13, 201. [Google Scholar] [CrossRef] [Green Version]
- Resnick, E.S.; Moshier, E.L.; Godbold, J.H.; Cunningham-Rundles, C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012, 119, 1650–1657. [Google Scholar] [CrossRef]
- Notarangelo, L.D.; Fischer, A.; Geha, R.S.; Casanova, J.-L.; Chapel, H.; Conley, M.E.; Cunningham-Rundles, C.; Etzioni, A.; Hammartröm, L.; Nonoyama, S.; et al. Primary immunodeficiencies: 2009 update. J. Allergy Clin. Immunol. 2009, 124, 1161–1178. [Google Scholar] [CrossRef] [Green Version]
- Romberg, N.; Chamberlain, N.; Saadoun, D.; Gentile, M.; Kinnunen, T.; Ng, Y.S.; Virdee, M.; Menard, L.; Cantaert, T.; Morbach, H.; et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J. Clin. Investig. 2013, 123, 4283–4293. [Google Scholar] [CrossRef] [Green Version]
- Fliegauf, M.; Bryant, V.L.; Frede, N.; Slade, C.; Woon, S.-T.; Lehnert, K.; Winzer, S.; Bulashevska, A.; Scerri, T.; Leung, E.; et al. Haploinsufficiency of the NF-κB1 Subunit p50 in Common Variable Immunodeficiency. Am. J. Hum. Genet. 2015, 97, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Lorenzini, T.; Fliegauf, M.; Klammer, N.; Frede, N.; Proietti, M.; Bulashevska, A.; Camacho-Ordonez, N.; Varjosalo, M.; Kinnunen, M.; de Vries, E.; et al. Characterization of the clinical and immunologic phenotype and management of 157 individuals with 56 distinct heterozygous NFKB1 mutations. J. Allergy Clin. Immunol. 2020, 146, 901–911. [Google Scholar] [CrossRef]
- Li, J.; Lei, W.-T.; Zhang, P.; Rapaport, F.; Seeleuthner, Y.; Lyu, B.; Asano, T.; Rosain, J.; Hammadi, B.; Zhang, Y.; et al. Biochemically deleterious human NFKB1 variants underlie an autosomal dominant form of common variable immunodeficiency. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehn, H.S.; Ouyang, W.; Lo, B.; Deenick, E.K.; Niemela, J.E.; Avery, D.T.; Schickel, J.-N.; Tran, D.Q.; Stoddard, J.; Zhang, Y.; et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014, 345, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, D.; Bode, C.; Kenefeck, R.; Hou, T.Z.; Wing, J.B.; Kennedy, A.; Bulashevska, A.; Petersen, B.-S.; Schäffer, A.A.; Grüning, B.; et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014, 20, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Gabrysch, A.; Olbrich, P.; Patiño, V.; Warnatz, K.; Wolff, D.; Hoshino, A.; Kobayashi, M.; Imai, K.; Takagi, M.; et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4–insufficient subjects. J. Allergy Clin. Immunol. 2018, 142, 1932–1946. [Google Scholar] [CrossRef] [Green Version]
- Ayrignac, X.; Goulabchand, R.; Jeziorski, E.; Rullier, P.; Carra-Dallière, C.; Lozano, C.; Portales, P.; Vincent, T.; Viallard, J.F.; de Champfleur, N.M.; et al. Two neurologic facets of CTLA4-related haploinsufficiency. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e751. [Google Scholar] [CrossRef]
- Egg, D.; Rump, I.C.; Mitsuiki, N.; Rojas-Restrepo, J.; Maccari, M.-E.; Schwab, C.; Gabrysch, A.; Warnatz, K.; Goldacker, S.; Patiño, V.; et al. Therapeutic options for CTLA-4 insufficiency. J. Allergy Clin. Immunol. 2021, 149, 736–746. [Google Scholar] [CrossRef]
- Lanz, A.-L.; Riester, M.; Peters, P.; Schwerd, T.; Lurz, E.; Hajji, M.S.; Rohlfs, M.; Ley-Zaporozhan, J.; Walz, C.; Kotlarz, D.; et al. Abatacept for treatment-refractory pediatric CTLA4-haploinsufficiency. Clin. Immunol. 2021, 229, 108779. [Google Scholar] [CrossRef]
- Gámez-Díaz, L.; August, D.; Stepensky, P.; Revel-Vilk, S.; Seidel, M.G.; Noriko, M.; Morio, T.; Worth, A.J.; Blessing, J.; Van de Veerdonk, F.; et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J. Allergy Clin. Immunol. 2016, 137, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagdas, D.; Halaçlı, S.O.; Tan, D.; Lo, B.; Çetinkaya, P.G.; Esenboğa, S.; Karaatmaca, B.; Matthews, H.; Balcı-Hayta, B.; Arıkoğlu, T.; et al. A Spectrum of Clinical Findings from ALPS to CVID: Several Novel LRBA Defects. J. Clin. Immunol. 2019, 39, 726–738. [Google Scholar] [CrossRef]
- Redenbaugh, V.; Coulter, T. Disorders Related to PI3Kδ Hyperactivation: Characterizing the Clinical and Immunological Features of Activated PI3-Kinase Delta Syndromes. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.K.; Webster, S.; Dalm, V.A.S.H.; Šedivá, A.; Van Hagen, P.M.; Holland, S.; Rosenzweig, S.D.; Christoph, B.; Sloth, B.; Cabanski, M.; et al. Effective “activated PI3Kδ syndrome”–targeted therapy with the PI3Kδ inhibitor leniolisib. Blood 2017, 130, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Lougaris, V.; Soresina, A.; Baronio, M.; Montin, D.; Martino, S.; Signa, S.; Volpi, S.; Zecca, M.; Marinoni, M.; Baselli, L.A.; et al. Long-term follow-up of 168 patients with X-linked agammaglobulinemia reveals increased morbidity and mortality. J. Allergy Clin. Immunol. 2020, 146, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mayer, L. Pathogenesis and treatment of gastrointestinal disease in antibody deficiency syndromes. J. Allergy Clin. Immunol. 2009, 124, 658–664. [Google Scholar] [CrossRef] [Green Version]
- van Zelm, M.C.; van der Burg, M.; Langerak, A.W.; van Dongen, J.J.M. PID Comes Full Circle: Applications of V(D)J Recombination Excision Circles in Research, Diagnostics and Newborn Screening of Primary Immunodeficiency Disorders. Front. Immunol. 2011, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Castagnoli, R.; Delmonte, O.M.; Calzoni, E.; Notarangelo, L.D. Hematopoietic Stem Cell Transplantation in Primary Immunodeficiency Diseases: Current Status and Future Perspectives. Front. Pediatr. 2019, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, N.; Imai, K.; Kanegane, H.; Sato, H.; Yamada, M.; Kondoh, K.; Okada, S.; Kobayashi, M.; Agematsu, K.; Takada, H.; et al. Quantification of κ-deleting recombination excision circles in Guthrie cards for the identification of early B-cell maturation defects. J. Allergy Clin. Immunol. 2011, 128, 223–225.e222. [Google Scholar] [CrossRef]
- Nourizadeh, M.; Shakerian, L.; Borte, S.; Fazlollahi, M.R.; Badalzadeh, M.; Houshmand, M.; Alizadeh, Z.; Dalili, H.; Rashidi-Nezhad, A.; Kazemnejad, A.; et al. Newborn screening using TREC/KREC assay for severe T and B cell lymphopenia in Iran. Scand. J. Immunol. 2018, 88, e12699. [Google Scholar] [CrossRef] [Green Version]
- Trück, J.; Prader, S.; Natalucci, G.; Hagmann, C.; Brotschi, B.; Kelly, J.; Bassler, D.; Steindl, K.; Rauch, A.; Baumgartner, M.R.; et al. Swiss newborn screening for severe T and B cell deficiency with a combined TREC/KREC assay—Management recommendations. Swiss Med. Wkly 2020, 150, w20254. [Google Scholar] [CrossRef]
- Lodi, L.; Ricci, S.; Romano, F.; Ghiori, F.; Canessa, C.; Lippi, F.; Bianchi, L.; Azzari, C. Newborn screening for PIDs using both TREC and KREC identifies late occurrence of B cells. Pediatr. Allergy Immunol. 2017, 28, 498–500. [Google Scholar] [CrossRef]
- Fouriki, A.; Schnider, C.; Theodoropoulou, K.; Pachlopnik, J.; Hofer, M.; Candotti, F. [Newborn screening for severe T and B lymphocyte deficiencies in Switzerland]. Rev. Med. Suisse 2021, 17, 68–76. [Google Scholar] [PubMed]
- Guevara-Hoyer, K.; Ochoa-Grullón, J.; Fernández-Arquero, M.; Cárdenas, M.; De Diego, R.P.; Sánchez-Ramón, S. Serum Free Immunoglobulins Light Chains: A Common Feature of Common Variable Immunodeficiency? Front. Immunol. 2020, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hedayat, M.; Aghamohammadi, A.; Rezaei, N. Soluble CD26 and CD30 levels in patients with common var-iable immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2013, 23, 120–124. [Google Scholar] [PubMed]
- Callery, E.; Morais, C.L.M.; Paraskevaidi, M.; Brusic, V.; Vijayadurai, P.; Anantharachagan, A.; Martin, F.L.; Rowbottom, A.W. New approach to investigate Common Variable Immunodeficiency patients using spectrochemical analysis of blood. Sci. Rep. 2019, 9, 7239. [Google Scholar] [CrossRef]
- Maglione, P.J.; Ko, H.M.; Tokuyama, M.; Gyimesi, G.; Soof, C.; Li, M.; Sanchez, E.; Chen, H.; Radigan, L.; Berenson, J.; et al. Serum B-Cell Maturation Antigen (BCMA) Levels Differentiate Primary Antibody Deficiencies. J. Allergy Clin. Immunol. Pract. 2019, 8, 283–291 e281. [Google Scholar] [CrossRef]
- Slade, C.A.; Bosco, J.J.; Giang, T.B.; Kruse, E.; Stirling, R.G.; Cameron, P.U.; Hore-Lacy, F.; Sutherland, M.F.; Barnes, S.L.; Holdsworth, S.; et al. Delayed Diagnosis and Complications of Predominantly Antibody Deficiencies in a Cohort of Australian Adults. Front. Immunol. 2018, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Maffucci, P.; Filion, C.A.; Boisson, B.; Itan, Y.; Shang, L.; Casanova, J.-L.; Cunningham-Rundles, C. Genetic Diagnosis Using Whole Exome Sequencing in Common Variable Immunodeficiency. Front. Immunol. 2016, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Abolhassani, H.; Hammarström, L.; Cunningham-Rundles, C. Current genetic landscape in common variable immune deficiency. Blood 2020, 135, 656–667. [Google Scholar] [CrossRef]
- Segundo, G.R.S.; Condino-Neto, A. Treatment of patients with immunodeficiency: Medication, gene therapy, and transplantation. J. Pediatr. 2020, 97 (Suppl. 1), S17–S23. [Google Scholar] [CrossRef]
- Heimall, J. Genetic Testing to Diagnose Primary Immunodeficiency Disorders and to Identify Targeted Therapy. Immunol. Allergy Clin. N. Am. 2018, 39, 129–140. [Google Scholar] [CrossRef]
- Sun, D.; Heimall, J.R.; Greenhawt, M.J.; Bunin, N.J.; Shaker, M.S.; Romberg, N. Cost Utility of Lifelong Immunoglobulin Replacement Therapy vs Hematopoietic Stem Cell Transplant to Treat Agammaglobulinemia. JAMA Pediatr. 2022, 176, 176–184. [Google Scholar] [CrossRef]
- Albin, S.; Cunningham-Rundles, C. An update on the use of immunoglobulin for the treatment of immunodeficiency disorders. Immunotherapy 2014, 6, 1113–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, U.; Miescher, S.; Vonarburg, C. Immunoglobulin replacement therapy in antibody deficiency syndromes: Are we really doing enough? Clin. Exp. Immunol. 2014, 178 (Suppl. 1), 83–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shillitoe, B.M.J.; Ponsford, M.; Slatter, M.A.; Evans, J.; Struik, S.; Cosgrove, M.; Doull, I.; Jolles, S.; Gennery, A.R. Haematopoietic Stem Cell Transplant for Norovirus-Induced Intestinal Failure in X-linked Agammaglobulinemia. J. Clin. Immunol. 2021, 41, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Bucciol, G.; Tousseyn, T.; Jansen, K.; Casteels, I.; Tangye, S.G.; Breuer, J.; Brown, J.R.; Wollants, E.; Van Ranst, M.; Moens, L.; et al. Hematopoietic Stem Cell Transplantation Cures Chronic Aichi Virus Infection in a Patient with X-linked Agammaglobulinemia. J. Clin. Immunol. 2021, 41, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Wehr, C.; Gennery, A.R.; Lindemans, C.; Schulz, A.; Hoenig, M.; Marks, R.; Recher, M.; Gruhn, B.; Holbro, A.; Heijnen, I.; et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J. Allergy Clin. Immunol. 2015, 135, 988–997 e986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Sterlin, D.; Gorochov, G. When Therapeutic IgA Antibodies Might Come of Age. Pharmacology 2020, 106, 9–19. [Google Scholar] [CrossRef]
- Eibl, M.M.; Wolf, H.M.; Fürnkranz, H.; Rosenkranz, A. Prevention of Necrotizing Enterocolitis in Low-Birth-Weight Infants by IgA–IgG Feeding. N. Engl. J. Med. 1988, 319, 1–7. [Google Scholar] [CrossRef]
- Tjellström, B.; Stenhammar, L.; Eriksson, S.; Magnusson, K.-E. Oral immunoglobulin A supplement in treatment of Clostridium difficile enteritis. Lancet 1993, 341, 701–702. [Google Scholar] [CrossRef]
- Giraudi, V.; Riganti, C.; Torales, M.R.; Sédola, H.; Gaddi, E. Upper respiratory infections in children: Response to endonasal administration of IGA. Int. J. Pediatr. Otorhinolaryngol. 1997, 39, 103–110. [Google Scholar] [CrossRef]
- Ma, J.K.-C.; Hikmat, B.Y.; Wycoff, K.; Vine, N.D.; Chargelegue, D.; Yu, L.; Hein, M.B.; Lehner, T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 1998, 4, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, L.; Cone, R.A.; Whaley, K.J. Using Monoclonal Antibodies to Prevent Mucosal Transmission of Epidemic Infectious Diseases. Emerg. Infect. Dis. 1999, 5, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Corthésy, B. Recombinant Secretory Immunoglobulin A in Passive Immunotherapy: Linking Immunology and Biotechnology. Curr. Pharm. Biotechnol. 2003, 4, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Longet, S.; Miled, S.; Lötscher, M.; Miescher, S.M.; Zuercher, A.W.; Corthésy, B. Human Plasma-derived Polymeric IgA and IgM Antibodies Associate with Secretory Component to Yield Biologically Active Secretory-like Antibodies. J. Biol. Chem. 2013, 288, 4085–4094. [Google Scholar] [CrossRef] [Green Version]
- Yoo, E.M.; Chintalacharuvu, K.R.; Morrison, S.L. Recombinant IgA Antibodies. Mucosal Immune Def. Immunoglobulin A 2007, 390–415. [Google Scholar] [CrossRef]
- Westerhof, L.B.; Wilbers, R.H.P.; Van Raaij, D.R.; Van Wijk, C.Z.; Goverse, A.; Bakker, J.; Schots, A. Transient Expression of Secretory IgA In Planta is Optimal Using a Multi-Gene Vector and may be Further Enhanced by Improving Joining Chain Incorporation. Front. Plant Sci. 2015, 6, 1200. [Google Scholar] [CrossRef] [Green Version]
- Gohil, K. Pharmaceutical approval update. P T 2014, 39, 746–772. [Google Scholar] [PubMed]
- Mukherjee, S.; Thrasher, A.J. Gene therapy for PIDs: Progress, pitfalls and prospects. Gene 2013, 525, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Fox, T.A.; Booth, C. Gene therapy for primary immunodeficiencies. Br. J. Haematol. 2020, 193, 1044–1059. [Google Scholar] [CrossRef]
- Booth, C.; Romano, R.; Roncarolo, M.G.; Thrasher, A.J. Gene therapy for primary immunodeficiency. Hum. Mol. Genet. 2019, 28, R15–R23. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Thrasher, A.J.; Cavazza, A. Gene Editing for the Treatment of Primary Immunodeficiency Diseases. Hum. Gene Ther. 2021, 32, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Thrasher, A.J.; Zhang, F. Gene therapy and genome editing for primary immunodeficiency diseases. Genes Dis. 2020, 7, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.H.; Villegas, I.; Long, J.; Santos, J.; Keir, A.; Abele, A.; Kuo, C.Y.; Kohn, D.B. Optimizing Integration and Expression of Transgenic Bruton’s Tyrosine Kinase for CRISPR-Cas9-Mediated Gene Editing of X-Linked Agammaglobulinemia. CRISPR J. 2021, 4, 191–206. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef]
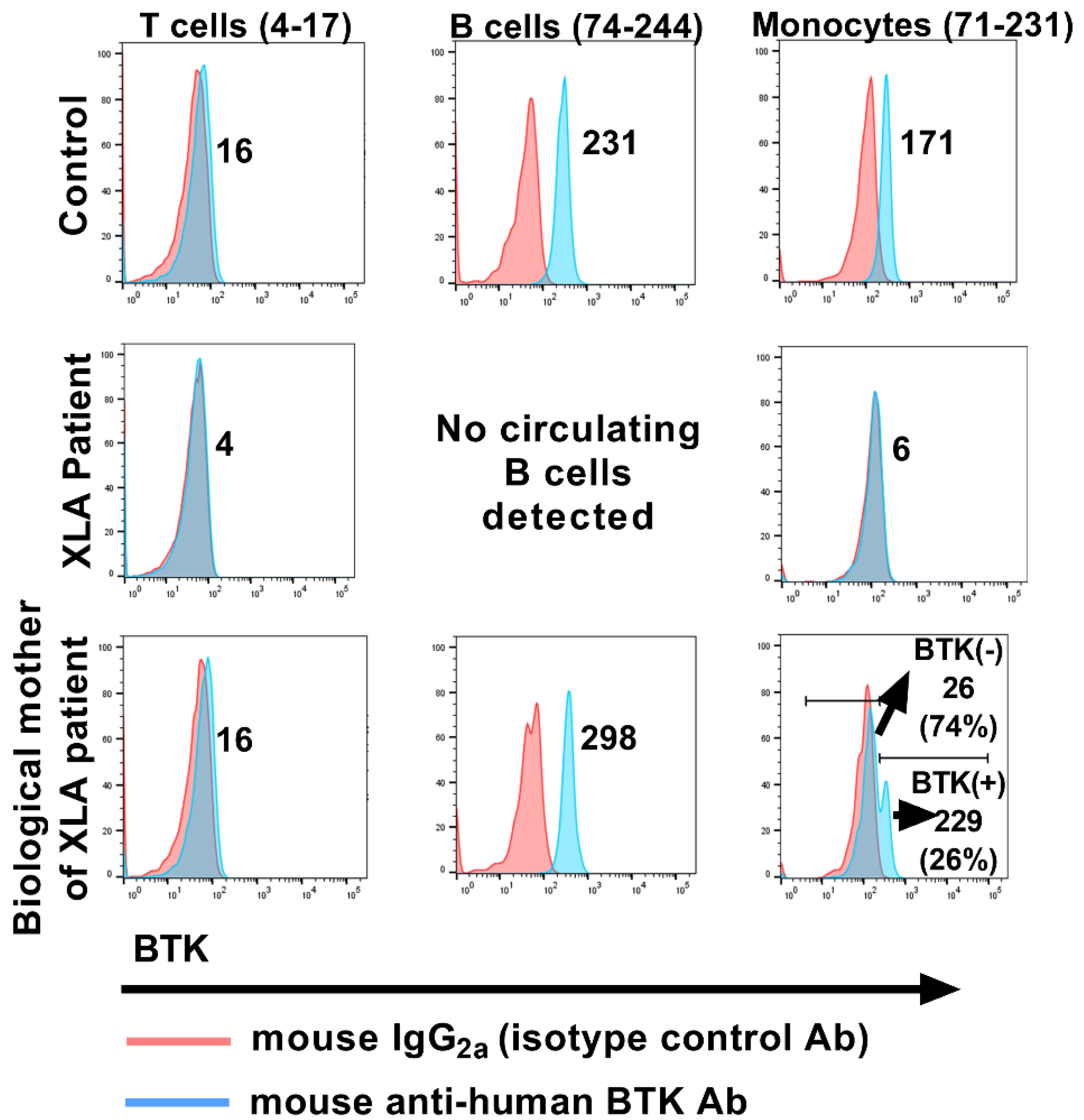
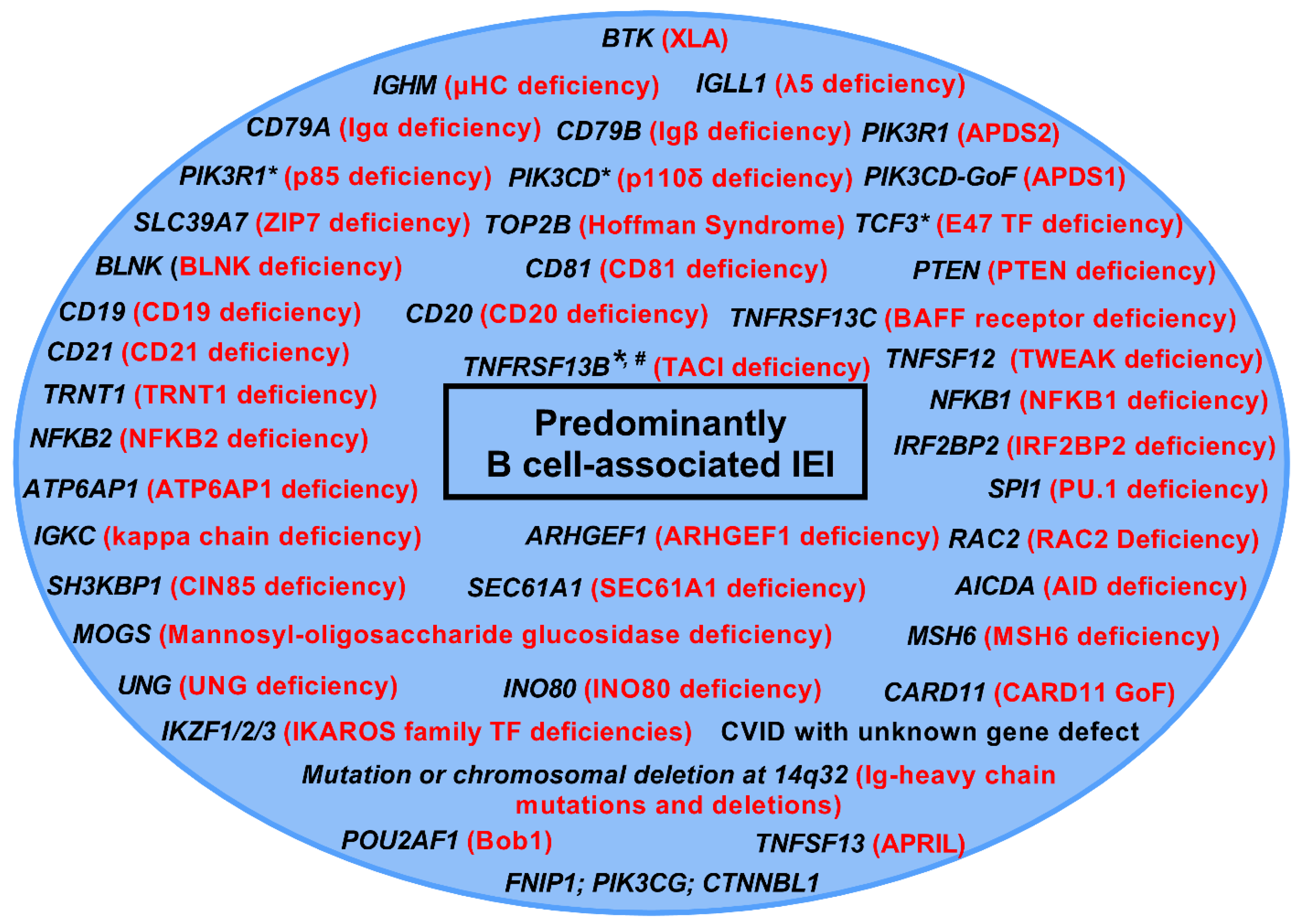
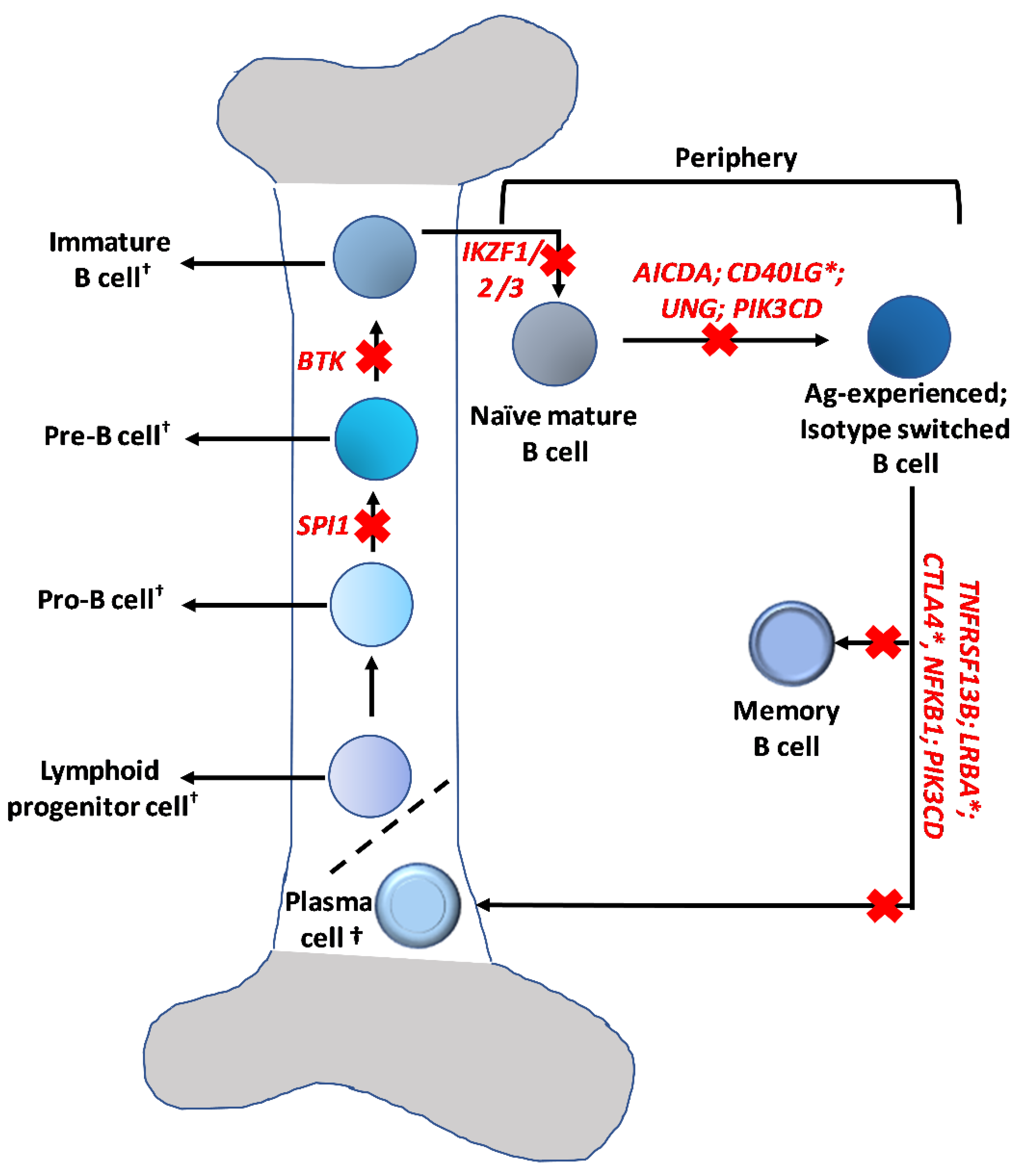
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Lippner, E.; Khanolkar, A. Clinical Aspects of B Cell Immunodeficiencies: The Past, the Present and the Future. Cells 2022, 11, 3353. https://doi.org/10.3390/cells11213353
Ahmed A, Lippner E, Khanolkar A. Clinical Aspects of B Cell Immunodeficiencies: The Past, the Present and the Future. Cells. 2022; 11(21):3353. https://doi.org/10.3390/cells11213353
Chicago/Turabian StyleAhmed, Aisha, Elizabeth Lippner, and Aaruni Khanolkar. 2022. "Clinical Aspects of B Cell Immunodeficiencies: The Past, the Present and the Future" Cells 11, no. 21: 3353. https://doi.org/10.3390/cells11213353
APA StyleAhmed, A., Lippner, E., & Khanolkar, A. (2022). Clinical Aspects of B Cell Immunodeficiencies: The Past, the Present and the Future. Cells, 11(21), 3353. https://doi.org/10.3390/cells11213353





