The Primary Cilium and Neuronal Migration
Abstract
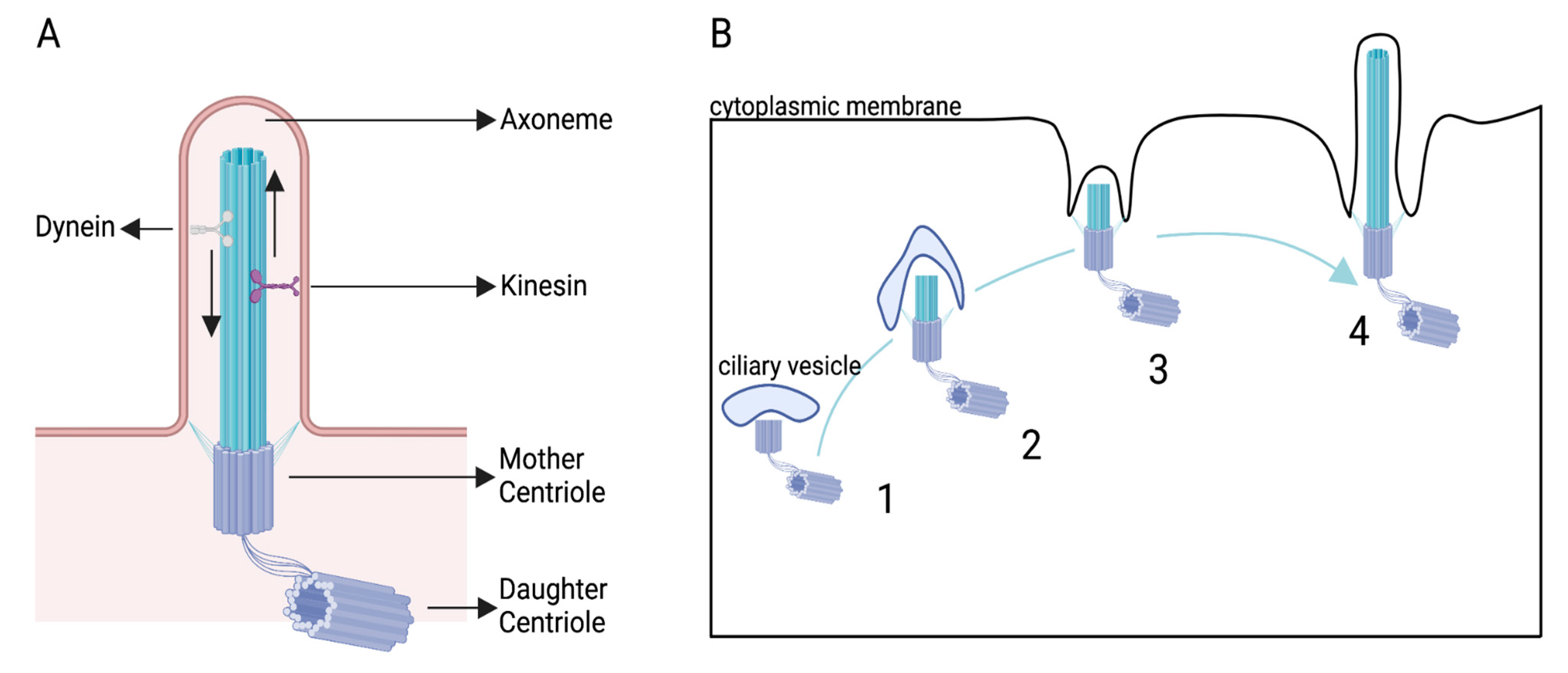
:1. Introduction
2. The PC as a Migration Guide in Non-Neuronal Cells
3. The PC: A Guide and Beat Maker in Migrating Neurons
3.1. Tangential Migration in the Cortex
3.2. Tangential Migration in the Rostral Migratory Stream
3.3. Radial Migration in the Cortex
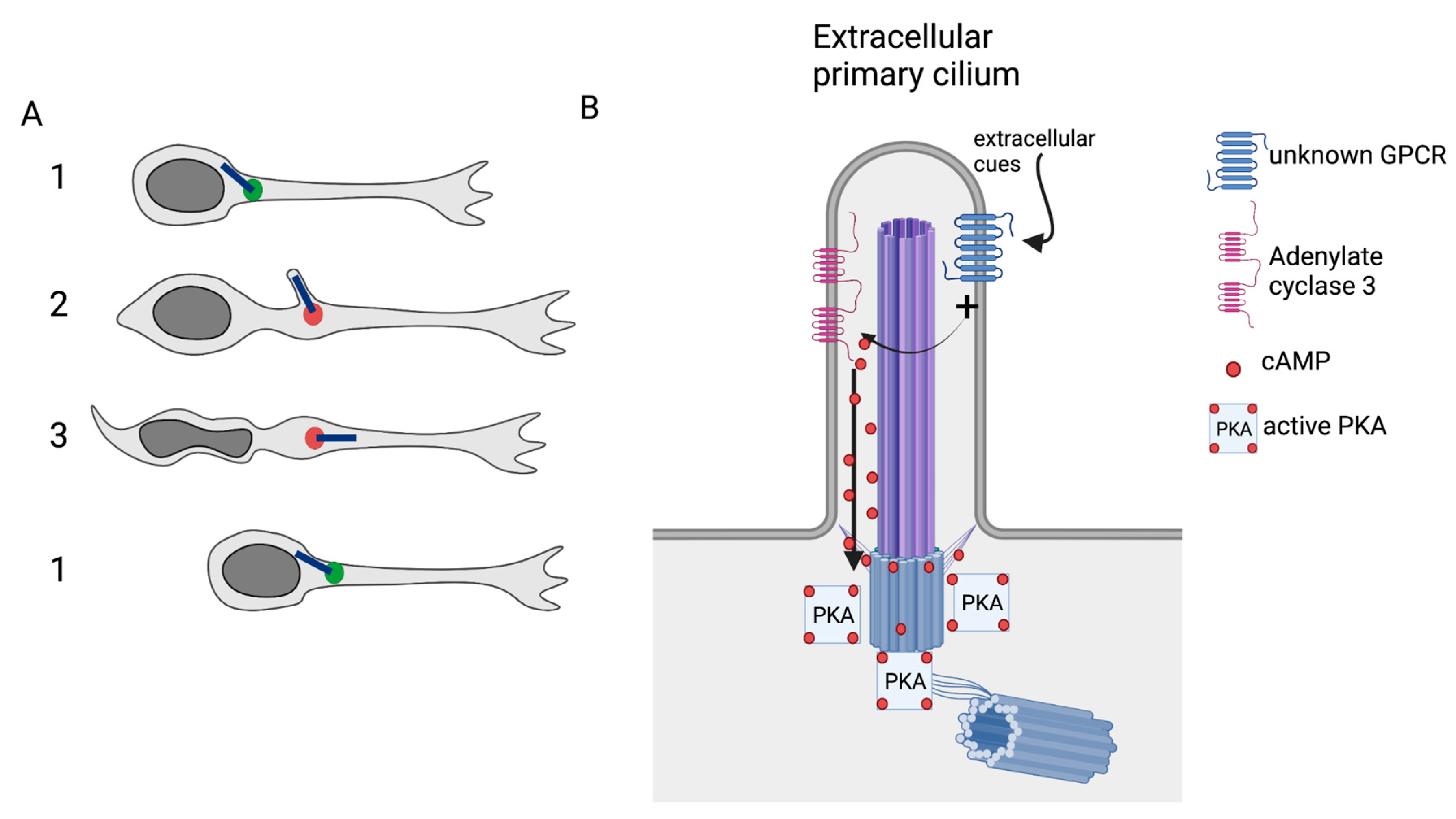
4. The PC and the Intracellular Control of Migration
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nachury, M.V.; Mick, D.U. Establishing and Regulating the Composition of Cilia for Signal Transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, Q.; Zhang, Y.; Li, Y.; Zhang, Q.; Hu, Z.; Harris, P.C.; Torres, V.E.; Ling, K.; Hu, J. Transition Fibre Protein FBF1 Is Required for the Ciliary Entry of Assembled Intraflagellar Transport Complexes. Nat. Commun. 2013, 4, 2750. [Google Scholar] [CrossRef] [Green Version]
- Ecker, A. Flimmerbewegung Im Gehörorgan von Petromyzon Marinus. Arch. Anat. Physiol. Wiss. Med. 1844, 520–521. [Google Scholar]
- Sorokin, S. Centrioles and the Formation of Rudimentary Cilia by Fibroblasts and Smooth Muscle Cells. J. Cell Biol. 1962, 15, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, S.P. Reconstructions of Centrile Formation and Ciliogenesis in Mammalian Lungs. J. Cell Sci. 1968, 3, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Guemez-Gamboa, A.; Coufal, N.G.; Gleeson, J.G. Primary Cilia in the Developing and Mature Brain. Neuron 2014, 82, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazour, G.J.; Witman, G.B. The Vertebrate Primary Cilium Is a Sensory Organelle. Curr. Opin. Cell Biol. 2003, 15, 105–110. [Google Scholar] [CrossRef]
- Sattar, S.; Gleeson, J.G. The Ciliopathies in Neuronal Development: A Clinical Approach to Investigation of Joubert Syndrome and Joubert Syndrome-Related Disorders: Review. Dev. Med. Child Neurol. 2011, 53, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Trupiano, M.X.; Simon, J.; Guo, J.; Anton, E.S. The Essential Role of Primary Cilia in Cerebral Cortical Development and Disorders. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 142, pp. 99–146. ISBN 978-0-12-815281-2. [Google Scholar]
- Guo, J.; Higginbotham, H.; Li, J.; Nichols, J.; Hirt, J.; Ghukasyan, V.; Anton, E.S. Developmental Disruptions Underlying Brain Abnormalities in Ciliopathies. Nat. Commun. 2015, 6, 7857. [Google Scholar] [CrossRef] [Green Version]
- Higginbotham, H.; Eom, T.-Y.; Mariani, L.E.; Bachleda, A.; Hirt, J.; Gukassyan, V.; Cusack, C.L.; Lai, C.; Caspary, T.; Anton, E.S. Arl13b in Primary Cilia Regulates the Migration and Placement of Interneurons in the Developing Cerebral Cortex. Dev. Cell 2012, 23, 925–938. [Google Scholar] [CrossRef] [Green Version]
- Baudoin, J.-P.; Viou, L.; Launay, P.-S.; Luccardini, C.; Espeso Gil, S.; Kiyasova, V.; Irinopoulou, T.; Alvarez, C.; Rio, J.-P.; Boudier, T.; et al. Tangentially Migrating Neurons Assemble a Primary Cilium That Promotes Their Reorientation to the Cortical Plate. Neuron 2012, 76, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Sawada, M.; García-González, D.; Herranz-Pérez, V.; Ogino, T.; Bang Nguyen, H.; Quynh Thai, T.; Narita, K.; Kumamoto, N.; Ugawa, S.; et al. Dynamic Changes in Ultrastructure of the Primary Cilium in Migrating Neuroblasts in the Postnatal Brain. J. Neurosci. 2019, 39, 9967–9988. [Google Scholar] [CrossRef] [PubMed]
- Stoufflet, J.; Chaulet, M.; Doulazmi, M.; Fouquet, C.; Dubacq, C.; Métin, C.; Schneider-Maunoury, S.; Trembleau, A.; Vincent, P.; Caille, I. Primary Cilium-Dependent CAMP/PKA Signaling at the Centrosome Regulates Neuronal Migration. Sci. Adv. 2020, 6, eaba3992. [Google Scholar] [CrossRef] [PubMed]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular Signalling by Primary Cilia in Development, Organ Function and Disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef]
- Ferreira, R.; Fukui, H.; Chow, R.; Vilfan, A.; Vermot, J. The Cilium as a Force Sensor−myth versus Reality. J. Cell Sci. 2019, 132, jcs213496. [Google Scholar] [CrossRef] [Green Version]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Mykytyn, K.; Askwith, C. G-Protein-Coupled Receptor Signaling in Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028183. [Google Scholar] [CrossRef] [Green Version]
- Nauli, S.M.; Pala, R.; Kleene, S.J. Calcium Channels in Primary Cilia. Curr. Opin. Nephrol. Hypertens. 2016, 25, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Y.; Falcone, J.L.; Curci, S.; Hofer, A.M. Direct Visualization of CAMP Signaling in Primary Cilia Reveals Up-Regulation of Ciliary GPCR Activity Following Hedgehog Activation. Proc. Natl. Acad. Sci. USA 2019, 116, 12066–12071. [Google Scholar] [CrossRef] [Green Version]
- Sherpa, R.T.; Mohieldin, A.M.; Pala, R.; Wachten, D.; Ostrom, R.S.; Nauli, S.M. Sensory Primary Cilium Is a Responsive CAMP Microdomain in Renal Epithelia. Sci. Rep. 2019, 9, 6523. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.S.; Stepanchick, A.N.; Tewson, P.H.; Hartle, C.M.; Zhang, J.; Quinn, A.M.; Hughes, T.E.; Mirshahi, T. Cilia Have High CAMP Levels That Are Inhibited by Sonic Hedgehog-Regulated Calcium Dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 13069–13074. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Guevarra, M.D.; Nguyen, A.M.; Chua, M.C.; Wang, Y.; Jacobs, C.R. The Primary Cilium Functions as a Mechanical and Calcium Signaling Nexus. Cilia 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht-Buehler, G. Phagokinetic Tracks of 3T3 Cells: Parallels between the Orientation of Track Segments and of Cellular Structures Which Contain Actin or Tubulin. Cell 1977, 12, 333–339. [Google Scholar] [CrossRef]
- Katsumoto, T.; Higaki, K.; Ohno, K.; Onodera, K. The Orientation of Primary Cilia during the Wound Response in 3Y1 Cells. Biol. Cell 1994, 81, 17–21. [Google Scholar] [CrossRef]
- McGowan, S.E.; McCoy, D.M. Platelet-Derived Growth Factor-A and Sonic Hedgehog Signaling Direct Lung Fibroblast Precursors during Alveolar Septal Formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L229–L239. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.J.; Du, H.; Wu, J.; Jansen, D.A.; Jordan, K.L.; Xu, N.; Sieck, G.C.; Qian, Q. Non-Random Distribution and Sensory Functions of Primary Cilia in Vascular Smooth Muscle Cells. Kidney Blood Press Res. 2008, 31, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Clement, D.L.; Mally, S.; Stock, C.; Lethan, M.; Satir, P.; Schwab, A.; Pedersen, S.F.; Christensen, S.T. PDGFRα Signaling in the Primary Cilium Regulates NHE1-Dependent Fibroblast Migration via Coordinated Differential Activity of MEK1/2-ERK1/2-P90RSK and AKT Signaling Pathways. J. Cell Sci. 2012, 126, 953–965. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Clement, C.A.; Teilmann, S.C.; Pazour, G.J.; Hoffmann, E.K.; Satir, P.; Christensen, S.T. PDGFRαα Signaling Is Regulated through the Primary Cilium in Fibroblasts. Curr. Biol. 2005, 15, 1861–1866. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Cammer, M.; Lehman, J.; Nielsen, S.; Guerra, C.; Veland, I.; Stock, C.; Hoffmann, E.; Yoder, B.; Schwab, A.; et al. Directional Cell Migration and Chemotaxis in Wound Healing Response to PDGF-AA Are Coordinated by the Primary Cilium in Fibroblasts. Cell Physiol. Biochem. 2010, 25, 279–292. [Google Scholar] [CrossRef]
- Schneider, L.; Stock, C.-M.; Dieterich, P.; Jensen, B.H.; Pedersen, L.B.; Satir, P.; Schwab, A.; Christensen, S.T.; Pedersen, S.F. The Na+/H+ Exchanger NHE1 Is Required for Directional Migration Stimulated via PDGFR-α in the Primary Cilium. J. Cell Biol. 2009, 185, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.J.; Adapala, R.K.; Geldenhuys, W.J.; Bursley, C.; AbouAlaiwi, W.A.; Nauli, S.M.; Thodeti, C.K. Primary Cilia Regulates the Directional Migration and Barrier Integrity of Endothelial Cells through the Modulation of Hsp27 Dependent Actin Cytoskeletal Organization. J. Cell. Physiol. 2012, 227, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.L.; Panagis, L.; Gusella, G.L.; Danias, J.; Mlodzik, M.; Iomini, C. Primary Cilia Dynamics Instruct Tissue Patterning and Repair of Corneal Endothelium. Proc. Natl. Acad. Sci. USA 2011, 108, 2819–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labour, M.-N.; Riffault, M.; Christensen, S.T.; Hoey, D.A. TGFβ1—Induced Recruitment of Human Bone Mesenchymal Stem Cells Is Mediated by the Primary Cilium in a SMAD3-Dependent Manner. Sci. Rep. 2016, 6, 35542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.N.; Song, J.H.; Oh, S.-H.; Tham, N.T.; Kim, J.-W.; Yang, J.-W.; Kim, E.-S.; Koh, J.-T. The Primary Cilium Directs Osteopontin-Induced Migration of Mesenchymal Stem Cells by Regulating CD44 Signaling and Cdc42 Activation. Stem Cell Res. 2020, 45, 101799. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.M.; Bahi-Buisson, N.; Francis, F. Genetics and Mechanisms Leading to Human Cortical Malformations. Semin. Cell Dev. Biol. 2018, 76, 33–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellion, A.; Baudoin, J.-P.; Alvarez, C.; Bornens, M.; Métin, C. Nucleokinesis in Tangentially Migrating Neurons Comprises Two Alternating Phases: Forward Migration of the Golgi/Centrosome Associated with Centrosome Splitting and Myosin Contraction at the Rear. J. Neurosci. 2005, 25, 5691–5699. [Google Scholar] [CrossRef]
- Schaar, B.T.; McConnell, S.K. Cytoskeletal Coordination during Neuronal Migration. Proc. Natl. Acad. Sci. USA 2005, 102, 13652–13657. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.; Simms, R.J.; Abdelhamed, Z.; Dawe, H.R.; Szymanska, K.; Logan, C.V.; Wheway, G.; Pitt, E.; Gull, K.; Knowles, M.A.; et al. A Meckelin–Filamin A Interaction Mediates Ciliogenesis. Hum. Mol. Genet. 2012, 21, 1272–1286. [Google Scholar] [CrossRef]
- Juric-Sekhar, G.; Adkins, J.; Doherty, D.; Hevner, R.F. Joubert Syndrome: Brain and Spinal Cord Malformations in Genotyped Cases and Implications for Neurodevelopmental Functions of Primary Cilia. Acta Neuropathol. 2012, 123, 695–709. [Google Scholar] [CrossRef]
- Poretti, A.; Huisman, T.A.G.M.; Scheer, I.; Boltshauser, E. Joubert Syndrome and Related Disorders: Spectrum of Neuroimaging Findings in 75 Patients. AJNR Am. J. Neuroradiol. 2011, 32, 1459–1463. [Google Scholar] [CrossRef] [Green Version]
- Vilboux, T.; Malicdan, M.C.V.; Roney, J.C.; Cullinane, A.R.; Stephen, J.; Yildirimli, D.; Bryant, J.; Fischer, R.; Vemulapalli, M.; Mullikin, J.C.; et al. CELSR2, Encoding a Planar Cell Polarity Protein, Is a Putative Gene in Joubert Syndrome with Cortical Heterotopia, Microophthalmia, and Growth Hormone Deficiency. Am. J. Med. Genet. 2017, 173, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Specification of Cerebral Cortical Areas. Science 1988, 241, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Eisenstat, D.D.; Shi, L.; Rubenstein, J.L.R. Interneuron Migration from Basal Forebrain to Neocortex: Dependence on Dlx Genes. Science 1997, 278, 474–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, L.-H.; Gleeson, J.G. Nucleokinesis in Neuronal Migration. Neuron 2005, 46, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Caspary, T.; Larkins, C.E.; Anderson, K.V. The Graded Response to Sonic Hedgehog Depends on Cilia Architecture. Dev. Cell 2007, 12, 767–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, J. Autoradiographic and Histological Studies of Postnatal Neurogenesis. III. Dating the Time of Production and Onset of Differentiation of Cerebellar Microneurons in Rats. J. Comp. Neurol. 1969, 136, 269–293. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-BuyIIa, A. Long-Distance Neuronal Migration in the Adult Mammalian Brain. Science 1994, 264, 5. [Google Scholar] [CrossRef]
- Peretto, P.; Giachino, C.; Aimar, P.; Fasolo, A.; Bonfanti, L. Chain Formation and Glial Tube Assembly in the Shift from Neonatal to Adult Subventricular Zone of the Rodent Forebrain. J. Comp. Neurol. 2005, 487, 407–427. [Google Scholar] [CrossRef]
- Bozoyan, L.; Khlghatyan, J.; Saghatelyan, A. Astrocytes Control the Development of the Migration-Promoting Vasculature Scaffold in the Postnatal Brain via VEGF Signaling. J. Neurosci. 2012, 32, 1687–1704. [Google Scholar] [CrossRef] [Green Version]
- Wichterle, H.; García-Verdugo, J.M.; Alvarez-Buylla, A. Direct Evidence for Homotypic, Glia-Independent Neuronal Migration. Neuron 1997, 18, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Arellano, J.I.; Guadiana, S.M.; Breunig, J.J.; Rakic, P.; Sarkisian, M.R. Development and Distribution of Neuronal Cilia in Mouse Neocortex. J. Comp. Neurol. 2012, 520, 848–873. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, H.R.; Gleeson, J.G. The Centrosome in Neuronal Development. Trends Neurosci. 2007, 30, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Pruski, M.; Rajnicek, A.; Yang, Z.; Clancy, H.; Ding, Y.-Q.; McCaig, C.D.; Lang, B. The Ciliary GTPase Arl13b Regulates Cell Migration and Cell Cycle Progression. Cell Adhes. Migr. 2016, 10, 393–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.M.; Lim, J.S.; Ramakrishina, S.; Kim, S.H.; Kim, W.K.; Lee, J.; Kang, H.-C.; Reiter, J.F.; Kim, D.S.; Kim, H.; et al. Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination. Neuron 2018, 99, 83–97.e7. [Google Scholar] [CrossRef] [Green Version]
- Tobin, J.L.; Di Franco, M.; Eichers, E.; May-Simera, H.; Garcia, M.; Yan, J.; Quinlan, R.; Justice, M.J.; Hennekam, R.C.; Briscoe, J.; et al. Inhibition of Neural Crest Migration Underlies Craniofacial Dysmorphology and Hirschsprung’s Disease in Bardet-Biedl Syndrome. Proc. Natl. Acad. Sci. USA 2008, 105, 6714–6719. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, N.; Ramahi, J.S.; Karakaya, M.; Howell, D.; Kerekes, R.A.; Solecki, D.J. Leading-Process Actomyosin Coordinates Organelle Positioning and Adhesion Receptor Dynamics in Radially Migrating Cerebellar Granule Neurons. Neural Dev. 2014, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Saade, M.; Gonzalez-Gobartt, E.; Escalona, R.; Usieto, S.; Martí, E. Shh-Mediated Centrosomal Recruitment of PKA Promotes Symmetric Proliferative Neuroepithelial Cell Division. Nat. Cell Biol. 2017, 19, 493–503. [Google Scholar] [CrossRef]
- Pruski, M.; Hu, L.; Yang, C.; Wang, Y.; Zhang, J.-B.; Zhang, L.; Huang, Y.; Rajnicek, A.M.; St Clair, D.; McCaig, C.D.; et al. Roles for IFT172 and Primary Cilia in Cell Migration, Cell Division, and Neocortex Development. Front. Cell Dev. Biol. 2019, 7, 287. [Google Scholar] [CrossRef]

| References | Cell Type | Type of Migration | Ciliary Gene Depleted | Experiment to Study Migration | In Vivo, Ex Vivo, In Vitro | Cell Staining | Effects or Not |
|---|---|---|---|---|---|---|---|
| Higginbotham et al., 2012 [11] | Cortical Interneurons | Tangential migration | Arl13b | Live-imaging and fixed tissues | In vivo and ex vivo | Genetic labelling | Altered migration and directionality |
| Baudoin et al., 2012 [12] | Cortical Interneurons | Tangential migration | kif3a, IFT88 | Live-imaging and fixed tissues | In vivo and ex vivo | Genetic labelling or acute brain slices electroporation | Altered migration and directionality |
| Matsumoto et al., 2019 [13] | RMS Neuroblast | Tangential migration | kif3a, IFT88 | Live-imaging | In vitro | Nucleofection on V-SVZ explants | Altered migration |
| Stoufflet et al., 2020 [14] | RMS Neuroblast | Tangential migration | kif3a, Rpgrip1L | Live-imaging | Ex vivo | Postnatal electroporation | Altered migration |
| Higginbotham et al., 2012 [11] | Cortical projection neurons | Radial migration | Arl13b | Fixed tissues | In vivo | Genetic labelling | No effects |
| Guo et al., 2015 [10] | Cortical projection neurons | Radial migration | AHI1, ALMS1, BBS1, BBS4, BBS7, BBS9, BBS10, BBS11, BBS12, BUBR1, IFT80, KIF7, NPHP1, NPHP8, TCTN2, TMEM216, TUB | Fixed tissues | In vivo | In utero electroporation | Retarded migration |
| Pruski et al., 2019 [15] | Cortical projection neurons | Radial migration | IFT172 | Fixed tissues | In vivo | In utero electroporation | Retarded migration |
| Park et al., 2018 [16] | Cortical projection neurons | Radial migration | ATG5 | Fixed tissues | In vivo | In utero electroporation | Retarded migration |
| Tobin et al., 2008 [17] | Neural Crest cells | NA | BBS8 | Fixed tissues | In vivo | In utero electroporation | Retarded migration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoufflet, J.; Caillé, I. The Primary Cilium and Neuronal Migration. Cells 2022, 11, 3384. https://doi.org/10.3390/cells11213384
Stoufflet J, Caillé I. The Primary Cilium and Neuronal Migration. Cells. 2022; 11(21):3384. https://doi.org/10.3390/cells11213384
Chicago/Turabian StyleStoufflet, Julie, and Isabelle Caillé. 2022. "The Primary Cilium and Neuronal Migration" Cells 11, no. 21: 3384. https://doi.org/10.3390/cells11213384
APA StyleStoufflet, J., & Caillé, I. (2022). The Primary Cilium and Neuronal Migration. Cells, 11(21), 3384. https://doi.org/10.3390/cells11213384







