Abstract
The epidermal growth factor receptor (EGFR) is expressed in numerous cell types in the adult mammalian kidney and is activated by a family of EGF-like ligands. EGFR activation has been implicated in a variety of physiologic and pathophysiologic functions. There is increasing evidence that aberrant EGFR activation is a mediator of progressive kidney injury in diabetic kidney disease. This review will highlight recent studies indicating its potential role and mechanisms of injury of both glomerular and tubular cells in development and progression of diabetic kidney disease.
1. The Epidermal Growth Factor Receptor and Its Ligands
The epidermal growth factor receptor (EGFR; aka ErbB1 or HER1) is a member of the ErbB/HER family of receptor tyrosine kinases, which also includes ErbB2 (HER2), ErbB3 (HER3) and ErbB4 (HER4). All four ErbBs have a common structure, with an extracellular ligand-binding domain, a single membrane-spanning region, a homologic cytoplasmic protein tyrosine kinase domain and a C-terminal tail with multiple phosphorylation sites. EGFR is activated by the ligands of the EGF-related peptide growth factor family [1]. These ligands include EGF, amphiregulin, transforming growth factor-alpha (TGF-α) betacellulin, heparin-binding EGF (HB-EGF) epiregulin and epigen [2,3]. In addition, a study has suggested that connecting tissue growth factor (CTGF) can bind to and activate EGFR [4].
2. Expression of EGFR in the Kidney
The EGFR is expressed in numerous cell types in the adult mammalian kidney, including podocytes, endothelial cells and mesangial cells in the glomerulus and in multiple tubule segments [3]. It is also expressed in cells in the interstitium, including medullary interstitial cells as well as in resident and infiltrating immune cells. The EGFR has been implicated in regulation of both physiologic and pathophysiologic functions in the adult kidney [3,5,6,7,8].
3. Mechanisms of Dysfunctional Expression and Activation of EGFR in Diabetic Nephropathy
There is upregulation of expression and activation of EGFR in experimental models of diabetic kidney injury and in cultured renal cells exposed to high glucose [9,10,11,12,13]. A recent study has also identified a SNP of an enhancer located in the EGFR gene associated with upregulation of EGFR expression in type II diabetes [14]. In addition, a number of EGFR ligands, including TGF-α, HB-EGF and amphiregulin, have been reported to increase in experimental models of diabetic nephropathy [10,13,15].
4. Effect of EGFR Inhibition to Ameliorate Progression of Diabetic Nephropathy
In a streptozotocin (STZ) model of type I diabetes in mice, wild type mice developed mesangial expansion and moderate albuminuria after 24 weeks of diabetes, and mice with selective deletion of endothelial nitric oxide synthase (eNOS–/–) had markedly exacerbated development of diabetic nephropathy [16]. We treated both wild type and eNOS-deficient diabetic mice with the EGFR tyrosine kinase inhibitor, erlotinib, for 22 weeks. Erlotinib decreased both albuminuria and mesangial expansion and decreased glomerulosclerosis in the STZ-eNOS–/– mice. Similar studies in diabetic rats reported that a different EGFR tyrosine kinase inhibitor, PKI 166 also attenuated early glomerular enlargement, decreased proteinuria and preserved podocyte number [17].
The eNOS–/– db/db mouse model is an accelerated model of diabetic nephropathy in an obese type 2 model of diabetes secondary to leptin receptor deficiency. These mice develop significant functional and structural kidney injury within the first 20 weeks of life [18]. Inhibition of EGFR activation by erlotinib in the kidney of eNOS–/– db/db mice was confirmed by inhibition of EGFR phosphorylation and inhibition of activation of ERK1/2 [19]. The eNOS–/– db/db mice have increased blood pressure compared to wild type, and erlotinib administration did not decrease blood pressure. However, erlotinib did prevent further increases in albuminuria and resulted in significantly less glomerulosclerosis. In addition, there was relative preservation of podocyte number in response to erlotinib treatment. Furthermore, there was decreased tubulointerstitial injury and fibrosis, indicated by deceased expression of the proximal tubule injury marker, KIM-1, decreased fibrillar extracellular collagen indicated by less Sirius red and collagen I staining and decreased expression of mRNA for components of fibrosis (collagens I&III, fibronectin), decreased myofibroblasts, indicated by less α-SMA immunostaining and decreased expression of profibrotic factors (transforming growth factor beta (TGF-ß), CTGF). There was a marked decrease in renal macrophage infiltration with erlotinib treatment, indicated by decreased F4/80 mRNA expression and decreased F4/80 staining. Erlotinib also decreased T cell infiltration, as indicated by decreased CD3 mRNA and CD8α staining. Erlotinib treatment led to a significant decrease in mRNA for IRF5, a mediator of proinflammatory M1 macrophage phenotype [20] and decreased mRNA for proinflammatory cytokines (iNOS, TNF-α, INF-γ, IL-6).
The Erlotinib treated mice had a slower rate to increase body weight, which was secondary to relatively less increase in the fat tissue mass. Accompanying this decreased weight gain, erlotinib also decreased fasting blood glucose levels and improved glucose tolerance and insulin tolerance tests. The erlotinib-treated mice had preserved pancreatic islet insulin staining, higher fasting blood insulin levels and decreased islet macrophage infiltration. Erlotinib administration also prevented decreases in serum levels of adiponectin, an adipocyte-derived hormone that increases insulin sensitivity.
As a confirmation of the role of EGFR activation in mediating diabetic kidney injury, we also studied waved 2 mice, which have a point mutation in EGFR that reduces intrinsic tyrosine kinase activity by >90% [21]. Similar to erlotinib inhibition of EGFR, homozygous waved 2 mice crossed to eNOS–/–db/db mice exhibited decreased gain of body weight, lower fasting blood glucose, preserved pancreatic islet insulin levels, less islet macrophage infiltration, and less glomerulosclerosis. waved 1 mice have markedly decreased expression of the EGFR ligand, TGF-α [22]. Similar to erlotinib-treated eNOS–/– db/db and waved 2 eNOS–/– db/db mice, waved 1 mice crossed to eNOS–/– db/db mice also had marked decreases in gain of body weight, fasting blood glucose, islet macrophage infiltration, and glomerulosclerosis and preserved pancreatic insulin levels. Serum and urine TGF-α increases in human diabetic kidney disease, and neutralizing antibodies to TGF-α were found to slow progression in models of accelerated diabetic kidney disease [15].
5. The Role of EGFR Activation in Podocyte Injury in Diabetic Nephropathy
Glomerular podocytes are highly specialized cells characterized by formation of foot processes that are interconnected by the slit diaphragm, which is a critical component of the glomerular filtration barrier. In both experimental animals [23] and type II diabetes patients [24], reduction in the number of podocytes per glomerulus is associated with broadening of podocyte foot processes and is thought to contribute to the progression of diabetic nephropathy. Moreover, it is now widely recognized that proteinuria, specifically microalbuminuria, is one of the earliest clinically identifiable markers of diabetes-induced renal damage, and appearance of proteinuria indicates a compromised glomerular filtration barrier. A metaanalysis of glomerular transcriptomic characteristics of human and mouse samples of kidney diseases indicated a central role for the EGFR in both species [25].
We developed a podocyte-specific EGFR knockout mouse (EGFRpodKO) by crossing EGFRflox/flox mice [26] with Podocin Cre mice [27] (Figure 1). Effective cleavage of the EGFR gene was verified by PCR analysis of isolated glomerular genomic DNA, and effective deletion of EGFR protein expression in podocytes was confirmed by immunoblotting of isolated glomerular lysates [12]. In a model of type I diabetes induced by streptozotocin injection, the diabetic EGFRpodKO mice had significantly less albuminuria compared with the WT mice. Electron microscopy revealed more severe segmental podocyte foot process effacement in the WT mice, and there was significantly more podocytes loss in WT diabetic mice compared with the EGFRpodKO diabetic mice. To determine the mechanism of podocyte loss, we isolated glomeruli and analyzed the expression levels of Bcl2, an anti-apoptotic protein, and cleaved caspase3, a pro-apoptotic protein and found that the expression of Bcl2 was down-regulated, and the expression of cleaved caspase3 was up-regulated in WT diabetic glomeruli, and these alterations were significantly less in EGFRpodKO mice. The expression levels of TGFβ and fibronectin were up-regulated in the glomeruli isolated from WT diabetic mice, but there was marked inhibition of TGF-ß and fibronectin expression in EGFRpodKO diabetic mice.

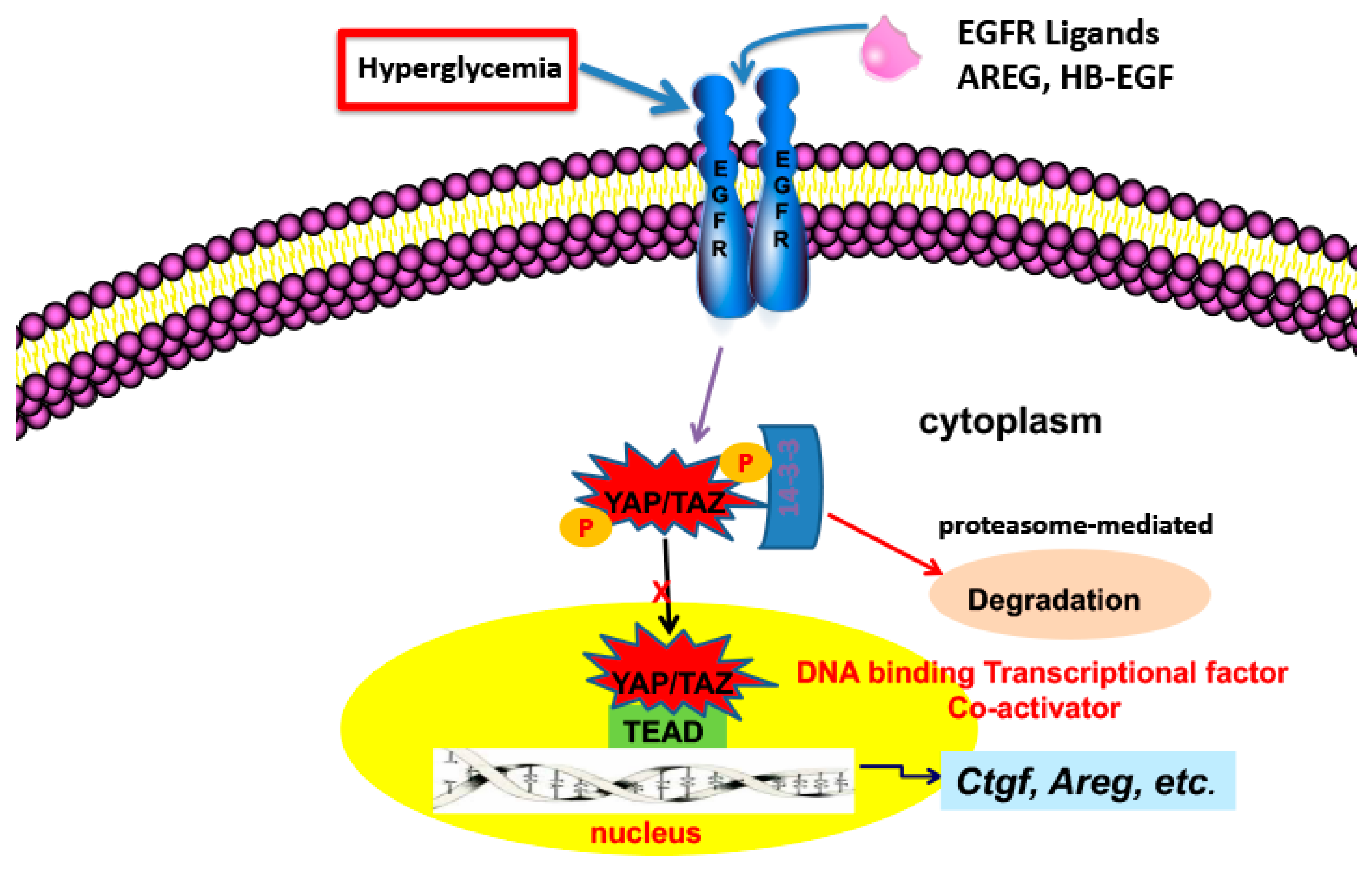
Figure 1.
EGFR activation leads to increased YAP translocation to the nucleus, association with TEAD and transcription of genes involved in progressive kidney injury.
To confirm the potential role of podocyte EGFR in the development of type II diabetic nephropathy, podocyte EGFR was selectively deleted in two mouse lines [28]. The first mouse line was db/db mice with selective podocyte EGFR deletion: nphs2-Cre; EGFRf/f; db/db (egfrpodKO; db/db) mice and their corresponding controls, egfrf/f; db/db mice (db/db) mice. The second mouse line was an accelerated type II diabetic model with selective podocyte EGFR deletion: nphs2-Cre; egfrf/f; nos3–/–; db/db (egfrpodKO; nos3–/–; db/db) mice and their corresponding controls, egfrf/f; nos3–/–; db/db (nos3–/–; db/db) mice. The effectiveness of EGFR deletion in podocytes was confirmed by determining genomic DNA), immunoblotting of glomerular lysates and immunofluorescent staining of EGFR expression. Most WT1 positive (Wilms Tumor Protein 1, a podocyte nuclear marker) cells were also EGFR positive in 20 weeks old nos3–/–; db/db mice. In contrast, most WT1 positive podocytes were devoid of EGFR staining or exhibited only faint EGFR staining in 20 weeks old egfrpodKO; nos3–/–; db/db mice. Selective podocyte deletion reduced albuminuria in both models of type II DN and preserved podocyte number.
Podocytes have high basal levels of autophagy [29], and autophagy in podocytes has been implicated as important contributor to preserve podocyte structure and function [30], since genetic inhibition of autophagy in podocytes led to glomerulopathy [29,31,32]. Beclin-1 is an essential component of the autophagic machinery [33,34]. Glomeruli of nos3–/–; db/db mice had low levels of expression of beclin-1, but selective deletion of podocyte EGFR increased beclin-1 expression in podocytes as well as increasing LC3B puncta, a hallmark of autophagy. In addition, the autophagy substrate SQSTM1/p62, was decreased in glomeruli of nos3–/–; db/db mice, indicating increased autophagic activity. Rubicon binds to, and inactivates beclin-1, and rubicon expression was high and beclin-1 expression was low in glomeruli from nos3–/–; db/db mice while rubicon expression was lower and beclin-1 expression was higher in egfrpodKO; nos3–/–; db/db mice. Knocking down rubicon in cultured podocytes using siRNA and treating with high glucose led to increased beclin-1 expression and autophagosome accumulation and decreased SQSTM1 expression. Immunofluorescent staining also confirmed that high glucose-induced rubicon upregulation and beclin-1 downregulation were abolished with inhibition of rubicon expression in podocytes.
mTOR activity increases in podocytes in diabetic mice, and correlates with increased ER stress and progressive glomerulosclerosis [35]. In addition to glomeruli, persistent mTOR activation has also been associated with apoptosis of renal tubule cells in diabetes [36]. Renal mTOR activation in poorly controlled diabetes may result from a combination of AKT inhibition of TSC2, hyperglycemia-induced AMP kinase inhibition and increased glucose uptake through glucose transporter 1 (GLUT1), in which the resulting increased glycolysis and activation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) can lead directly to Rheb activation of mTOR by reducing Rheb binding to GAPDH [37,38]. EGFR activation is a well-described mediator of mTOR activity through activation of the PI3K/AKT pathway [39,40]. In addition, EGFR activation inhibits renal gluconeogenesis and stimulates glycolysis in proximal tubules [41,42] and increases GLUT1 expression in mesangial cells [43]. Phosphorylation of mTOR and its partner raptor were markedly lower in erlotinib-treated than vehicle-treated STZ-eNOS–/– kidney. In addition, erlotinib treatment led to decreases in phosphorylated p70 S6K and eIF-4B, downstream targets of mTOR signaling, and erlotinib treatment led to increased AMPK kinase activity. Immunolocalization indicated that phosphorylated AMPKα due to erlotinib treatment was increased in both glomeruli and in renal epithelial cells. Selective EGFR deletion in podocytes also led to decreases in renal mTORC activation, as indicated by decreased phosphorylation of the p-RPS6KB/p70S6k substrate RPS6 (ribosomal proteinS6) levels in egfrpodKO; nos3–/–; db/db mice. Inhibition of EGFR activation or expression podocyte increased autophagy secondary to inhibition of mTORC1 and stimulation of AMP kinase [16,28].
6. The Role of EGFR in Tubulointerstitial Injury in Diabetic Nephropathy
In addition to the glomerulus, there is increasing evidence that the renal tubules and specifically the proximal tubules, are a target for diabetes and mediate the increased tubulointerstitial injury seen in diabetic nephropathy [44]. Diabetes-induced increased expression of EGFR and their ligands and subsequent EGFR activation have been previously reported both in in vivo models and in cultured renal proximal tubule epithelial cells [9,10]. Proximal tubule hypertrophy is an early response seen with the onset of diabetes, and in a streptozotocin model of type I diabetes, either erlotinib or selective proximal tubule deletion of EGFR expression reduced this early kidney hypertrophy.
The Hippo signaling pathway is a kinase cascade conserved from Drosophila to mammals that controls the balance of cell proliferation, cell differentiation and cell death to define organ size via regulating the phosphorylation and activation of YAP (Yes-associated protein) and/or TAZ (transcriptional co-activator with PDZ-binding motif), which serve as transcriptional co-activators for numerous target genes in the nucleus primarily by interacting with the TEAD family of transcription factors. Upon activation of the Hippo pathway in response to different extracellular cues, YAP and TAZ are phosphorylated at specific serine/threonine residues, which results in their inactivation by cytoplasmic sequestration and/or proteasome-mediated degradation, thereby inactivating downstream target genes expression, as indicated in Figure 1 [45]. YAP has moderate expression in normal adult kidney but very low expression in normal adult proximal tubule. TAZ, sharing 45% amino acid identity with YAP has higher expression in the normal adult kidney. YAP and TAZ play some redundant roles in the morula stage of mouse development and in controlling adult cardiac growth, but they also show differential functions in many aspects, as evidenced by embryonic lethality (at E8.5) with global deletion of YAP, whereas global mutation of TAZ caused early mortality in only a subset of homozygous mice, while surviving adult mice developed bilateral kidney cyst formation and a pulmonary emphysema-like phenotype.
YAP expression and activation (nuclear localization) increased in the proximal tubule in experimental models of both type I and type II diabetes and in kidney samples from patients with type II diabetes [13,46] while TAZ expression was either unchanged or actually decreased. The increased proximal tubule YAP expression and activation was inhibited in mice with proximal tubule EGFR deletion or by administration of the EGFR tyrosine kinase inhibitor, erlotinib. In cultured proximal tubule cells, high glucose increased nuclear association with TEAD and in the streptozotocin model increased renal expression of kidney expression of two known targets of YAP/TEAD gene transcription, CTGF and amphiregulin. Both CTGF and amphiregulin have been implicated in development of tubulointerstitial fibrosis [47,48]. Expression of both of these profibrotic factors was attenuated in EGFRptKO mice or by administration of erlotinib. EGFR-mediated nuclear translocation of YAP was dependent on RhoA/ROCK activation and subsequent activation of a PI3-kinase/AKT pathway.
To investigate the role of EGFR activation of YAP in mediation of proximal tubule injury in diabetic nephropathy. YapPTiKO (inducible conditional proximal tubule-specific knockout of YAP) were utilized in the type I model of diabetes. Kidney expression of CTGF was decreased by proximal tubule selective deletion of YAP as well as the YAP/TEAD activation inhibitor verteporfin. Verteporfin decreased proteinuria, but there were no differences in proteinuria with selective proximal tubule Yap deletion. Either verteporfin or selective proximal tubule deletion of YAP significantly inhibited development of tubulointerstitial fibrosis. In vitro studies indicated that proximal tubule YAP activation resulted in secreted CTGF, which induced myofibroblast transformation in cultured fibroblasts.
7. Adverse Effects of EGFR Inhibitors
Although EGFR blocking antibodies and tyrosine kinase inhibitors are widely used in cancer therapy, there are serious concerns about whether they could ever be employed as therapeutic agents in kidney diseases, and specifically in diabetic nephropathy. The most common side effect seen in cancer patients treated with EGFR inhibitors is an acneiform rash, seen in between 50 and 100% of treated patients. In fact, the occurrence of the rash correlates with effectiveness of treatment. Although this side effect is deemed acceptable for the short-term treatment of life-threatening cancers, it would not be an acceptable side effect for what would be chronic continuous therapy in patients with diabetic nephropathy. In addition, magnesium wasting due to inhibition of magnesium reabsorption by the thick ascending limb is a rare complication of EGFR inhibition, especially with blocking EGFR antibodies
In summary, there is strong evidence for an important pathological role of persistent EGFR receptor activation in the development and progression of diabetic kidney disease. That inhibiting EGFR expression or activity can ameliorate progression of diabetic kidney injury indicates that the direct inhibition of EGFR activity and/or inhibition of signaling pathways activated by the receptor may be viable targets for prevention of progressive kidney injury resulting from diabetes.
Funding
This research was funded by NIH grand DK51265 and funds from the Department of Veterans Affairs.
Conflicts of Interest
The author declares no conflict of interest.
References
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Chung, E.; Coffey, R.J. EGF receptor ligands. Exp. Cell Res. 2003, 284, 2–13. [Google Scholar] [CrossRef]
- Zeng, F.; Singh, A.B.; Harris, R.C. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009, 315, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Díez, R.R.; Morgado-Pascual, J.L.; Díez, R.R.R.; Mas, S.; Lavoz, C.; Alique, M.; Pato, J.; Keri, G.; Ortiz, A.; et al. Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J. Mol. Cell Biol. 2013, 5, 323–335. [Google Scholar] [CrossRef]
- Harris, R.C. The epidermal growth factor receptor axis and kidney fibrosis. Curr. Opin. Nephrol. Hypertens. 2021, 30, 275–279. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage. Mediat. Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef]
- Harskamp, L.R.; Gansevoort, R.T.; Van Goor, H.; Meijer, E. The epidermal growth factor receptor pathway in chronic kidney diseases. Nat. Rev. Nephrol. 2016, 12, 496–506. [Google Scholar] [CrossRef]
- Melenhorst, W.B.; Mulder, G.M.; Xi, Q.; Hoenderop, J.G.; Kimura, K.; Eguchi, S.; van Goor, H. Epidermal growth factor receptor signaling in the kidney: Key roles in physiology and disease. Hypertension 2008, 52, 987–993. [Google Scholar] [CrossRef]
- Asakawa, H.; Miyagawa, J.; Higashiyama, S.; Goishi, K.; Hanafusa, T.; Kuwajima, M.; Taniguchi, N.; Matsuzawa, Y. High glucose and hyperosmolarity increase heparin-binding epidermal growth factor-like growth factor (HB-EGF) production in cultured human aortic endothelial cells. Cell Biochem. Funct. 1996, 14, 181–186. [Google Scholar] [CrossRef]
- Miyazawa, T.; Zeng, F.; Wang, S.; Fan, X.; Cheng, H.; Yang, H.; Bian, A.; Fogo, A.B.; Harris, R.C. Low nitric oxide bioavailability upregulates renal heparin binding EGF-like growth factor expression. Kidney Int. 2013, 84, 1176–1188. [Google Scholar] [CrossRef]
- Sayed-Ahmed, N.; Besbas, N.; Mundy, J.; Muchaneta-Kubara, E.; Cope, G.; Pearson, C.; El Nahas, M. Upregulation of epidermal growth factor and its receptor in the kidneys of rats with streptozotocin-induced diabetes. Exp. Nephrol. 1996, 46, 330–339. [Google Scholar]
- Chen, J.; Chen, J.K.; Harris, R.C. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Harris, R.C. Interaction of the EGF Receptor and the Hippo Pathway in the Diabetic Kidney. J. Am. Soc. Nephrol. 2015, 27, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, S.; Ding, J.-M.; Chen, W.; Guo, Y. Enhancer-Gene Interaction Analyses Identified the Epidermal Growth Factor Receptor as a Susceptibility Gene for Type 2 Diabetes Mellitus. Diabetes Metab. J. 2021, 45, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Heuer, J.G.; Harlan, S.M.; Yang, D.D.; Jaqua, D.L.; Boyles, J.S.; Wilson, J.M.; Heinz-Taheny, K.M.; Sullivan, J.M.; Wei, T.; Qian, H.-R.; et al. Role of TGF-alpha in the progression of diabetic kidney disease. Am. J. Physiol. Physiol. 2017, 312, F951–F962. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Wang, Y.; Paueksakon, P.; Harris, R.C. Epidermal Growth Factor Receptor Inhibition Slows Progression of Diabetic Nephropathy in Association With a Decrease in Endoplasmic Reticulum Stress and an Increase in Autophagy. Diabetes 2014, 63, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Advani, A.; Wiggins, K.J.; Cox, A.J.; Zhang, Y.; Gilbert, E.R.; Kelly, D.J. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology 2011, 16, 573–581. [Google Scholar] [CrossRef]
- Zhao, H.J.; Wang, S.; Cheng, H.; Zhang, M.-Z.; Takahashi, T.; Fogo, A.B.; Breyer, D.M.; Harris, R.C. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 2006, 17, 2664–2669. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Overstreet, J.M.; Chung, S.; Niu, A.; Fan, X.; Wang, S.; Wang, Y.; Zhang, M.-Z.; Harris, R.C. Inhibition of Epidermal Growth Factor Receptor Activation Is Associated With Improved Diabetic Nephropathy and Insulin Resistance in Type 2 Diabetes. Diabetes 2018, 67, 1847–1857. [Google Scholar] [CrossRef]
- Weiss, M.; Byrne, A.J.; Blazek, K.; Saliba, D.G.; Pease, J.E.; Perocheau, D.; Feldmann, M.; Udalova, I.A. IRF5 controls both acute and chronic inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, 11001–11006. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.-K.; Wang, S.-W.; Moeckel, G.; Harris, R.C. Importance of Functional EGF Receptors in Recovery from Acute Nephrotoxic Injury. J. Am. Soc. Nephrol. 2003, 14, 3147–3154. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A.E.; Seroogy, K.B.; Schroeder, A.J.; Russell, E.W.; Evans, E.P.; Riedel, R.F.; Phillips, H.K.; Harrison, A.C.; Lee, D.C.; Luetteke, N.C. Characterization of the mouse transforming growth factor alpha gene: Its expression during eyelid development and in waved 1 tissues. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1996, 7, 12711282. [Google Scholar]
- Reidy, K.; Susztak, K. Epithelial-Mesenchymal Transition and Podocyte Loss in Diabetic Kidney Disease. Am. J. Kidney Dis. 2009, 54, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Pagtalunan, E.M.; Miller, P.L.; Jumping-Eagle, S.; Nelson, R.G.; Myers, B.D.; Rennke, H.G.; Coplon, N.S.; Sun, L.; Meyer, T.W. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Investig. 1997, 99, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Abdalla, M.; Siddiqi, F.; Geldenhuys, L.; Batchu, S.N.; Tolosa, M.F.; Yuen, D.A.; Dos Santos, C.C.; Advani, A. A common glomerular transcriptomic signature distinguishes diabetic kidney disease from other kidney diseases in humans and mice. Curr. Res. Transl. Med. 2020, 68, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-C.; Threadgill, D.W. Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis 2009, 47, 85–92. [Google Scholar] [CrossRef]
- Moeller, M.J.; Sanden, S.K.; Soofi, A.; Wiggins, R.; Holzman, L.B. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 2003, 35, 39–42. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Cao, S.; Sasaki, K.; Wang, Y.; Niu, A.; Fan, X.; Wang, S.; Zhang, M.-Z.; Harris, R.C. Podocyte EGFR Inhibits Autophagy Through Upregulation of Rubicon in Type 2 Diabetic Nephropathy. Diabetes 2021, 70, 562–576. [Google Scholar] [CrossRef]
- Hartleben, B.; Godel, M.; Meyer-Schwesinger, C.; Liu, S.; Ulrich, T.; Kobler, S.; Wiech, T.; Grahammer, F.; Arnold, S.J.; Lindernmeter, M.T.; et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Investig. 2010, 120, 1084–1096. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, Y.; Cao, H.; Wen, P.; Jiang, L.; He, W.; Dai, C.; Yang, J. Autophagy Attenuates Diabetic Glomerular Damage through Protection of Hyperglycemia-Induced Podocyte Injury. PLoS ONE 2013, 8, e60546. [Google Scholar] [CrossRef]
- Bechtel, W.; Helmstädter, M.; Balica, J.; Hartleben, B.; Kiefer, B.; Hrnjic, F.; Schell, C.; Kretz, O.; Liu, S.; Geist, F.; et al. Vps34 Deficiency Reveals the Importance of Endocytosis for Podocyte Homeostasis. J. Am. Soc. Nephrol. 2013, 24, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, M.X.; Fogo, A.B.; Harris, R.C.; Chen, J.-K. mVps34 Deletion in Podocytes Causes Glomerulosclerosis by Disrupting Intracellular Vesicle Trafficking. J. Am. Soc. Nephrol. 2013, 24, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Saitoh, T.; Tabata, K.; Omori, H.; Satoh, T.; Kurotori, N.; Maejima, I.; Shirahama-Noda, K.; Ichimura, T.; Isobe, T.; et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 2009, 11, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Wang, Q.; Yue, Z. Atg14L and Rubicon: Yin and yang of Beclin 1-mediated autophagy control. Autophagy 2009, 5, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Godel, M.; Hartleben, B.; Herbach, N.; Liu, S.; Zschiedrich, S.; Lu, S.; Derecznei-Mor, A.; Lindenmeyer, M.T.; Maria-Pia, R.; Hartleben, G.; et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Investig. 2011, 121, 2197–2209. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, C.; Bhandari, B.S.; Abboud-Werner, S.; Simone, S.; Abboud, H.E.; Habib, S.L. The Tuberin/mTOR Pathway Promotes Apoptosis of Tubular Epithelial Cells in Diabetes. J. Am. Soc. Nephrol. 2011, 22, 262–273. [Google Scholar] [CrossRef]
- Brosius, F.C.; Khoury, C.C.; Buller, C.L.; Chen, S. Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev. Endocrinol. Metab. 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Buller, C.L.; Heilig, C.W.; Brosius, F.C., 3rd. GLUT1 enhances mTOR activity independently of TSC2 and AMPK. Am. J. Physiol. Renal. Physiol. 2011, 301, F588–F596. [Google Scholar] [CrossRef]
- Chiu, T.; Santiskulvong, C.; Rozengurt, E. EGF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway. Am. J. Physiol. Liver Physiol. 2005, 288, G182–G194. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Neilson, E.G.; Harris, R.C. Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J. Am. Soc. Nephrol. 2006, 17, 1615–1623. [Google Scholar] [CrossRef]
- Harris, R.C.; Daniel, T.O. Epidermal growth factor binding, stimulation of phosphorylation, and inhibition of gluconeogenesis in rat proximal tubule. J. Cell. Physiol. 1989, 139, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G.; Schnellmann, R.G. Integrative effects of EGF on metabolism and proliferation in renal proximal tubular cells. Am. J. Physiol. Physiol. 1995, 269, C1317–C1325. [Google Scholar] [CrossRef] [PubMed]
- Nose, A.; Mori, Y.; Uchiyama-Tanaka, Y.; Kishimoto, N.; Maruyama, K.; Matsubara, H.; Iwasaka, T. Regulation of Glucose Transporter (GLUT1) Gene Expression by Angiotensin II in Mesangial Cells: Involvement of HB-EGF and EGF Receptor Transactivation. Hypertens. Res. 2003, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yan, J.; Li, X.; Liu, N.; Zheng, R.; Zhong, Y. Update on the Mechanisms of Tubular Cell Injury in Diabetic Kidney Disease. Front. Med. 2021, 8, 661076. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; He, Q.; Bulus, N.; Fogo, A.B.; Zhang, M.-Z.; Harris, R.C. YAP Activation in Renal Proximal Tubule Cells Drives Diabetic Renal Interstitial Fibrogenesis. Diabetes 2020, 69, 2446–2457. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, H. Connective Tissue Growth Factor and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 365–380. [Google Scholar]
- Kefaloyianni, E.; Raja, M.R.K.; Schumacher, J.; Muthu, M.L.; Krishnadoss, V.; Waikar, S.S.; Herrlich, A. Proximal Tubule–Derived Amphiregulin Amplifies and Integrates Profibrotic EGF Receptor Signals in Kidney Fibrosis. J. Am. Soc. Nephrol. 2019, 30, 2370–2383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).