Autologous Stem Cell Transplantation in Multiple Myeloma: Where Are We and Where Do We Want to Go?
Abstract
:1. Introduction
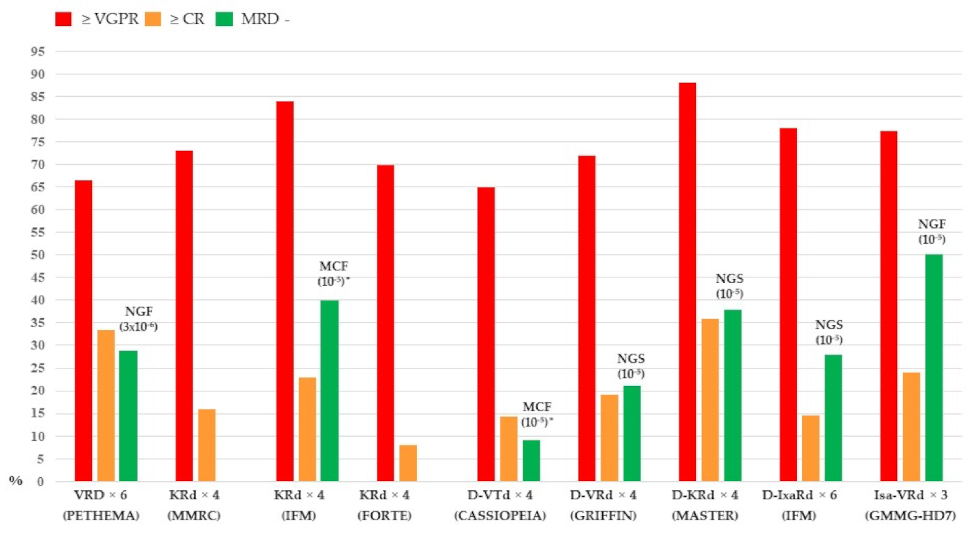
2. Where We Are with Induction Therapy
3. Considerations with Stem Cell Mobilization and Harvesting
4. Where We Are with Consolidation Therapy Post-ASCT
5. A Look at ASCT in the Era of Novel Drugs, New Regimens, and CAR-T Cell Therapies
6. Where We Are with Maintenance Therapy
7. The Role of ASCT in High-Risk Patients
8. The Role of ASCT in Relapsed MM Patients
9. Perspectives for Tailored Therapies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, staging, and management of multiple myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Gondos, A.; Pulte, D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 2008, 111, 2521–2526. [Google Scholar] [CrossRef]
- Nishimura, K.K.; Barlogie, B.; van Rhee, F.; Zangari, M.; Walker, B.A.; Rosenthal, A.; Schinke, C.; Thanendrarajan, S.; Davies, F.E.; Hoering, A.; et al. Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood Adv. 2020, 4, 422–431. [Google Scholar] [CrossRef]
- Corre, J.; Perrot, A.; Hulin, C.; Caillot, D.; Stoppa, A.M.; Facon, T.; Leleu, X.; Dib, M.; Karlin, L.; Moreau, P.; et al. Improved survival in multiple myeloma during the 2005–2009 and 2010–2014 periods. Leukemia 2021, 35, 3600–3603. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Chen, Y.; Wu, J.; Hageman, L.; Richman, J.; Francisco, L.; Landier, W.; Costa, L.; McDonald, A.; Murdaugh, D.; et al. Reduction in late mortality among patients with multiple myeloma treated with autologous peripheral blood stem cell transplantation—A Blood or Marrow Transplant Survivor Study report. Transpl. Cell Ther. 2021, 27, 840.e1–840.e7. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. J. Clin. Oncol. 2019, 37, 1228–1263. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. EHA Guidelines Committee. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Harousseau, J.L.; Stoppa, A.M.; Sotto, J.J.; Fuzibet, J.G.; Rossi, J.F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Child, J.A.; Morgan, G.J.; Davies, F.E.; Owen, R.G.; Bell, S.E.; Hawkins, K.; Brown, J.; Drayson, M.T.; Selby, P.J.; Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003, 348, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, A.; Bringhen, S.; Petrucci, M.T.; Musto, P.; Rossini, F.; Nunzi, M.; Lauta, V.M.; Bergonzi, C.; Barbui, A.; Caravita, T.; et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: Results of a randomized controlled trial. Blood 2004, 104, 3052–3057. [Google Scholar] [CrossRef]
- Fermand, J.P.; Katsahian, S.; Divine, M.; Leblond, V.; Dreyfus, F.; Macro, M.; Arnulf, B.; Royer, B.; Mariette, X.; Pertuiset, E.; et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: Long-term results of a randomized control trial from the Group Myelome-Autogreffe. J. Clin. Oncol. 2005, 23, 9227–9233. [Google Scholar] [CrossRef]
- Bladé, J.; Rosiñol, L.; Sureda, A.; Ribera, J.M.; Díaz-Mediavilla, J.; García-Laraña, J.; Mateos, M.V.; Palomera, L.; Fernández-Calvo, J.; Martí, J.M.; et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: Long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood 2005, 106, 3755–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlogie, B.; Kyle, R.A.; Anderson, K.C.; Greipp, P.R.; Lazarus, H.M.; Hurd, D.D.; McCoy, J.; Moore, D.F., Jr.; Dakhil, S.R.; Lanier, K.S.; et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: Final results of phase III US Intergroup Trial S9321. J. Clin. Oncol. 2006, 24, 929–936. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef]
- Gay, F.; Oliva, S.; Petrucci, M.T.; Conticello, C.; Catalano, L.; Corradini, P.; Siniscalchi, A.; Magarotto, V.; Pour, L.; Carella, A.; et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: A randomised, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 1617–1629. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Musto, P.; Rota-Scalabrini, D.; Bertamini, L.; Belotti, A.; Galli, M.; Offidani, M.; Zamagni, E.; Ledda, A.; Grasso, M.; et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): A randomised, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Tacchetti, P.; Patriarca, F.; Petrucci, M.T.; Pantani, L.; Galli, M.; Di Raimondo, F.; Crippa, C.; Zamagni, E.; Palumbo, A.; et al. GIMEMA Italian Myeloma Network Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem ell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet 2010, 376, 2075–2785. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Teruel, A.I.; Hernández, D.; López-Jiménez, J.; de la Rubia, J.; Granell, M.; Besalduch, J.; Palomera, L.; González, Y.; et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood 2012, 120, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Hulin, C.; Macro, M.; Caillot, D.; Chaleteix, C.; Roussel, M.; Garderet, L.; Royer, B.; Brechignac, S.; Tiab, M.; et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: Results of the prospective IFM2013-04 trial. Blood 2016, 127, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Viterbo, L.; Greil, R.; Masszi, T.; Spicka, I.; Shpilberg, O.; Hajek, R.; Dmoszynska, A.; Paiva, B.; Vidriales, M.B.; et al. Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. J. Clin. Oncol. 2013, 31, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; San Miguel, J.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. ESMO Guidelines Committee. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv52–iv61. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Sexton, R.; Abidi, M.H.; Epstein, J.; Rajkumar, S.V.; Dispenzieri, A.; Kahanic, S.P.; Thakuri, M.C.; Reu, F.J.; et al. Longer term follow-up of the randomized phase III trial SWOG S0777: Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020, 10, 53. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Joseph, N.S.; Kaufman, J.L.; Dhodapkar, M.V.; Hofmeister, C.C.; Almaula, D.K.; Heffner, L.T.; Gupta, V.A.; Boise, L.H.; Lonial, S.; Nooka, A.K. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J. Clin. Oncol. 2020, 38, 1928–1937. [Google Scholar] [CrossRef]
- Gaballa, M.R.; Ma, J.; Tanner, M.R.; Al-Juhaishi, T.; Bashir, Q.; Srour, S.A.; Saini, N.Y.; Ramdial, J.L.; Nieto, Y.; Murphy, R.; et al. Real-world long-term outcomes in multiple myeloma with VRD induction, Mel200-conditioned auto-HCT, and lenalidomide maintenance. Leuk. Lymphoma 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rosinol, L.; Hebraud, B.; Oriol, A.; Colin, A.-L.; Rios, R.; Hulin, C.; Blanchard, M.J.; Cailllot, D.; Sureda, A.; Hernandez, M.T.; et al. Integrated analysis of randomized controlled trials evaluating bortezomib + lenalidomide + dexamethasone or bortezomib + thalidomide + dexamethasoneinduction in transplant-eligible newly diagnosed multiple myeloma. Blood 2018, 132 (Suppl. S1), 3245. [Google Scholar] [CrossRef]
- Kumar, S.K.; Jacobus, S.J.; Cohen, A.D.; Weiss, M.; Callander, N.; Singh, A.K.; Parker, T.L.; Menter, A.; Yang, X.; Parsons, B.; et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 1317–1330. [Google Scholar] [CrossRef]
- Jasielec, J.K.; Kubicki, T.; Raje, N.; Vij, R.; Reece, D.; Berdeja, J.; Derman, B.A.; Rosenbaum, C.A.; Richardson, P.; Gurbuxani, S.; et al. Carfilzomib, lenalidomide, and dexamethasone plus transplant in newly diagnosed multiple myeloma. Blood 2020, 136, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Lauwers-Cances, V.; Wuilleme, S.; Belhadj, K.; Manier, S.; Garderet, L.; Escoffre-Barbe, M.; Mariette, C.; Benboubker, L.; Caillot, D.; et al. Up-front carfilzomib, lenalidomide, and dexamethasone with transplant for patients with multiple myeloma: The IFM KRd final results. Blood 2021, 138, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Chhabra, S.; Medvedova, E.; Dholaria, B.R.; Schmidt, T.M.; Godby, K.N.; Silbermann, R.; Dhakal, B.; Bal, S.; Giri, S.; et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J. Clin. Oncol. 2021, 13, 01935. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Hultcrantz, M.; Diamond, B.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; Tan, C.; Shah, U.A.; Lu, S.X.; Salcedo, M.; et al. Safety and effectiveness of weekly carfilzomib, lenalidomide, dexamethasone, and daratumumab combination therapy for patients with newly diagnosed multiple myeloma: The MANHATTAN nonrandomized clinical trial. JAMA Oncol. 2021, 7, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Touzeau, C.; Decaux, O.; Hulin, C.; Macro, M.; Stoppa, A.-M.; Chretien, M.L.; Karlin, L.; Mariette, C.; et al. Daratumumab plus ixazomib, lenalidomide, and dexamethasone as extended induction and consolidation followed by lenalidomide maintenance in standard-risk transplant-eligible newly diagnosed multiple myeloma (NDMM) patients (IFM 2018-01): A phase II study of the Intergroupe Francophone Du Myélome (IFM). Blood 2021, 138 (Suppl. S1), 79. [Google Scholar]
- Goldschmidt, H.; Mai, E.K.; Nievergall, E.; Fenk, R.; Bertsch, U.; Tichy, D.; Besemer, B.; Dürig, J.; Schroers, R.; Metzler, I.V.; et al. Addition of isatuximab to lenalidomide, bortezomib and dexamethasone as induction therapy for newly-diagnosed, transplant-eligible multiple myeloma patients: The phase III GMMG-HD7 trial. Blood 2021, 138 (Suppl. S1), 463. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Mai, E.K.; Bertsch, U.; Besemer, B.; Haenel, M.; Miah, K.; Fenk, R.; Schlenzka, J.; Munder, M.; Dürig, J.; et al. Elotuzumab in combination with lenalidomide, bortezomib, dexamethasone and autologous transplantation for newly-diagnosed multiple myeloma: Results from the randomized phase III GMMG-HD6 trial. Blood 2021, 138 (Suppl. S1), 486. [Google Scholar] [CrossRef]
- Giralt, S.; Costa, L.; Schriber, J.; Dipersio, J.; Maziarz, R.; McCarty, J.; Shaughnessy, P.; Snyder, E.; Bensinger, W.; Copelan, E.; et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: Consensus guidelines and recommendations. Biol. Blood Marrow Transpl. 2014, 20, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Prakash, V.S.; Malik, P.S.; Sahoo, R.K.; Pramanik, R.; Choudhary, P.; Varshney, A.N.; Kumar, L. Multiple Myeloma: Risk adapted use of plerixafor for stem cell mobilization prior to autologous stem cell transplantation is effective and cost efficient. Clin. Lymphoma Myeloma Leuk. 2022, 22, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Bucci, T.G.; Shaw, J.R.; Alexander, M.D.; Grgic, T.; Riches, M.; Ptachcinski, J.R. Plerixafor strategies for autologous hematopoietic cell transplant mobilization: A comparison of efficacy and cost. Transfus. Apher. Sci. 2021, 2021, 103303. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, D.; Marcacci, G.P.; Martino, M.; Radice, D.; Rabascio, C.; Lucchetti, B.; Magarò, A.; Caime, A.; Menna, S.; Lionetti, M.T.; et al. A comparison of chemo-free strategy with G-CSF plus plerixafor on demand versus intermediate-dose cyclophosphamide and G-CSF as PBSC mobilization in newly diagnosed multiple myeloma patients: An Italian explorative cost analysis. Transfus. Apher. Sci. 2020, 5, 102819. [Google Scholar] [CrossRef]
- Vaxman, I.; Muchtar, E.; Jacob, E.; Kapoor, P.; Kumar, S.; Dispenzieri, A.; Buadi, F.; Dingli, D.; Gonsalves, W.; Kourelis, T.; et al. The efficacy and safety of chemotherapy-based stem cell mobilization in multiple myeloma patients who are poor responders to induction: The Mayo Clinic Experience. Transpl. Cell Ther. 2021, 27, 770.e1–770.e7. [Google Scholar] [CrossRef]
- Sarmiento, M.; Ramírez, P.; Parody, R.; Salas, M.Q.; Beffermannm, N.; Jara, V.; Bertín, P.; Pizarro, I.; Lorca, C.; Rivera, E.; et al. Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy. Bone Marrow Transpl. 2018, 53, 960–966. [Google Scholar] [CrossRef]
- Joseph, J.; Wookey, V.; Randolph, B.; Chandler, J.C.; Marjoncu, D.; Holman, K.; Armstrong, M.; Khaled, Y. Fresh versus cryopreserved peripheral stem cell for autologous transplantation in multiple myeloma: An analysis of short-term outcomes. Blood 2020, 136 (Suppl. S1), 9–10. [Google Scholar] [CrossRef]
- Kulkarni, U.; Devasia, A.J.; Korula, A.; Fouzia, N.A.; Nisham, P.N.; Samoon, Y.J.; Lakshmi, K.M.; Abraham, A.; Srivastava, A.; Mathews, V.; et al. Use of Non-Cryopreserved Peripheral Blood Stem Cells Is Associated with Adequate Engraftment in Patients with Multiple Myeloma Undergoing an Autologous Transplant. Biol. Blood Marrow Transplant. 2018, 24, e31–e35. [Google Scholar] [CrossRef] [Green Version]
- Bekadja, M.A.; Boumendil, A.; Blaise, D.; Chevallier, P.; Peggs, K.S.; Salles, G.; Giebel, S.; Marks, R.; Arcese, W.; Milpied, N.; et al. Non-cryopreserved hematopoietic stem cells in autograft patients with lymphoma: A matched-pair analysis comparing a single center experience with the use of cryopreserved stem cells reported to the European Society for Blood and Marrow Transplantation registry. Cytotherapy 2021, 23, 483–487. [Google Scholar]
- Sánchez-Salinas, A.; Cabañas-Perianes, V.; Blanquer, M.; Majado, M.J.; Insausti, C.L.; Monserrat, J.; Sánchez-Ibáñez, M.V.; Menchón, P.; García-Hernández, A.; Gómez-Espuch, J.; et al. An automatic wash method for dimethyl sulfoxide removal in autologous hematopoietic stem cell transplantation decreases the adverse effects related to infusion. Transfusion 2012, 52, 2382–2386. [Google Scholar] [CrossRef]
- Wannesson, L.; Panzarella, T.; Mikhael, J.; Keating, A. Feasibility and safety of autotransplants with noncryopreserved marrow or peripheral blood stem cells: A systematic review. Ann. Oncol. 2007, 18, 623–632. [Google Scholar] [CrossRef]
- Kayal, S.; Sharma, A.; Iqbal, S.; Tejomurtula, T.; Cyriac, S.L.; Raina, V. High-dose chemotherapy and autologous stem cell transplantation in multiple myeloma: A single institution experience at All India Institute of Medical Sciences, New Delhi, using non-cryopreserved peripheral blood stem cells. Clin. Lymphoma Myeloma Leuk. 2014, 14, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kardduss-Urueta, A.; Gale, R.P.; Gutierrez-Aguirre, C.H.; Herrera-Rojas, M.A.; Murrieta-Álvarez, I.; Perez-Fontalvo, R.; Ruiz-Delgado, G.J.; Ruiz-Rojas, G.; Jaimovich, G.; Feldman, L. Freezing the graft is not necessary for autotransplants for plasma cell myeloma and lymphomas. Bone Marrow Transpl. 2018, 53, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Naithani, R.; Dayal, N.; Pathak, S.; Rai, R. Hematopoietic stem cell transplantation using non-cryopreserved peripheral blood stem cells graft is effective in multiple myeloma and lymphoma. Bone Marrow Transpl. 2018, 53, 1198–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacchetti, P.; Pantani, L.; Patriarca, F.; Petrucci, M.T.; Zamagni, E.; Dozza, L.; Galli, M.; Di Raimondo, F.; Crippa, C.; Boccadoro, M.; et al. Bortezomib, thalidomide, and dexamethasone followed by double autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma (GIMEMA-MMY-3006): Long-term follow-up analysis of a randomised phase 3, open-label study. Lancet Haematol. 2020, 7, e861–e873. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, H.; Londhe, A.; Ataman, O.; Johns, H.L.; Hill, S.; Landers, E.; Berlin, J.A. Association between complete response and outcomes in transplant-eligible myeloma patients in the era of novel agents. Eur. J. Haematol. 2017, 98, 269–279. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef]
- Paiva, B.; Puig, N.; Cedena, M.T.; Rosiñol, L.; Cordón, L.; Vidriales, M.B.; Burgos, L.; Flores-Montero, J.; Sanoja-Flores, L.; Lopez-Anglada, L.; et al. GEM (Grupo Español de Mieloma)/PETHEMA (Programa Para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Group. Measurable residual disease by next-generation flow cytometry in multiple myeloma. J. Clin. Oncol. 2020, 38, 784–792. [Google Scholar] [CrossRef]
- Puig, N.; Contreras Sanfeliciano, T.; Paiva, B.; Cedena, M.T.; Rosinol, L.; Garcia-Sanz, R.; Martinez-Lopez, J.; Oriol, A.; Blanchard, M.J.; Rios, R.; et al. Assessment of treatment response by ife, next generation flow cytometry and mass spectrometry coupled with liquid chromatography in the GEM2021MENOS65 clinical trial. Blood 2021, 138 (Suppl. S1), 544. [Google Scholar] [CrossRef]
- Avet Loiseau, H.; Sonneveld, P.; Moreau, P.; Offner, F.; van der Velden, V.H.J.; Caillot, D.; Hulin, C.; Arnulf, B.; Corre, J.; Mohty, M.; et al. Daratumumab (DARA) with bortezomib, thalidomide, and dexamethasone (VTd) in transplant-eligible patients (Pts) with newly diagnosed multiple myeloma (NDMM): Analysis of minimal residual disease (MRD) negativity in Cassiopeia Part 1 and Part 2. Blood 2021, 138 (Suppl. S1), 82. [Google Scholar] [CrossRef]
- Laubach, J.P.; Kaufman, J.L.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.W.; Costa, L.J.; Anderson, L.D., Jr.; Nathwani, N.; et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma: Updated analysis of Griffin after 24 months of maintenance. Blood 2021, 138 (Suppl. S1), 79. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Beksac, M.; van der Holt, B.; Aquino, S.; Ludwig, H.; Zweegman, S.; Zander; Zamagni, E.; Wester, R.; et al. Consolidation and maintenance in newly diagnosed multiple myeloma. J. Clin. Oncol. 2021, 39, 3613–3622. [Google Scholar] [CrossRef]
- Oliva, S.; Bruinink, D.H.O.; Rihova, L.; D’Agostino, M.; Pantani, L.; Capra, A.; van der Holt, B.; Troia, R.; Petrucci, M.T.; Villanova, T.; et al. Minimal residual disease assessment by multiparameter flow cytometry in transplant-eligible myeloma in the EMN02/HOVON 95 MM trial. Blood Cancer J. 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Pasquini, M.C.; Blackwell, B.; Hari, P.; Bashey, A.; Devine, S.; Efebera, Y.; Ganguly, S.; Gasparetto, C.; Geller, N.; et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 Trial. J. Clin. Oncol. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Parameswaran, H.; Pasquini, M.C.; Stadtmauer, E.A.; Fraser, R.; Fei, M.; Devine, S.M.; Adeduni Efebera, Y.; Geller, N.; Horowitz, M.M.; Koreth, J.; et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). J. Clin. Oncol. 2020, 38 (Suppl. S15), 8506. [Google Scholar]
- Perrot, A.; Lauwers-Cances, V.; Cazaubiel, T.; Facon, T.; Caillot, D.; Clement-Filliatre, L.; Macro, M.; Decaux, O.; Belhadj, K.; Mohty, M.; et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: Long-term follow-up analysis of the IFM 2009 trial. Blood 2020, 136 (Suppl. S1), 143. [Google Scholar] [CrossRef]
- Popat, R.; Wilson, W.; Camilleri, M.; De Tute, R.; Pang, G.; Jenner, R.; Dagada, T.; Kamora, S.; Streetly, M.; Ramasamy, K.; et al. CARDAMON: Carfilzomib (K) maintenance following autologous stem cell transplant (ASCT) or carfilzomib-cyclophosphamide-dexamethasone (KCd) consolidation for newly diagnosed (NDTE) multiple myeloma (MM). Clin. Lymphoma Myeloma Leuk. 2021, 21 (Suppl. S2), OAB-003. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Jakubowiak, A.; Agha, M.; Cohen, A.C.; Hari, P.; Avigan, A.; Deol, A.; Htut, M.; et al. Updated results from CARTITUDE-1: Phase 1b/2 study of ciltacabtagene autoleucel, a B-Cell Maturation Antigen-directed Chimeric Antigen Receptor T cell therapy, in patients with relapsed/refractory multiple myeloma. Blood 2021, 138 (Suppl. S1), 549. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holstein, S.A.; Jung, S.H.; Richardson, P.G.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: A randomised, double-blind, phase 3 trial. Lancet Haematol. 2017, 4, e431–e442. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, H.; Mai, E.K.; Dürig, J.; Scheid, C.; Weisel, K.C.; Kunz, C.; Bertsch, U.; Hielscher, T.; Merz, M.; Munder, M.; et al. Response-adapted lenalidomide maintenance in newly diagnosed myeloma: Results from the phase III GMMG-MM5 trial. Leukemia 2020, 34, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- De Tute, R.M.; Pawlyn, C.; Cairns, D.A.; Davies, F.E.; Menzies, T.; Rawstron, A.; Jones, J.R.; Hockaday, A.; Henderson, R.; Cook, G.; et al. Minimal residual disease following autologous cell transplant for myeloma patients in the Myeloma XI trial: Prognostic significance and the impact of lenalidomide maintenance and molecular risk. Clin. Lymphoma Myeloma Leuk. 2021, 21 (Suppl. S2), OAB-015. [Google Scholar] [CrossRef]
- Diamond, B.; Korde, N.; Lesokhin, A.M.; Smith, E.L.; Shah, U.; Mailankody, S.; Hultcrantz, M.; Hassoun, H.; Lu, S.X.; Tan, C.; et al. Dynamics of minimal residual disease in patients with multiple myeloma on continuous lenalidomide maintenance: A single-arm, single-centre, phase 2 trial. Lancet Haematol. 2021, 8, e422–e432. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Lokhorst, H.M.; Mai, E.K.; van der Holt, B.; Blau, I.W.; Zweegman, S.; Weisel, K.C.; Vellenga, E.; Pfreundschuh, M.; Kersten, M.J.; et al. Bortezomib before and after high-dose therapy in myeloma: Long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 2018, 32, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Baertsch, M.A.; Mai, E.K.; Hielscher, T.; Bertsch, U.; Salwender, H.J.; Munder, M.; Fuhrmann, S.; Dührsen, U.; Brossart, P.; Neben, K.; et al. Lenalidomide versus bortezomib maintenance after frontline autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2021, 11, 1. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Rosinol, L.; Oriol, A.; Tamayo, R.R.; Blanchard, M.J.; Jarque, I.; Bargay, J.; Hernandez, M.-T.; Moraleda, J.M.; Carrillo-Cruz, E.; Sureda, A.; et al. Ixazomib plus lenalidomide/dexamethasone (IRd) versus lenalidomide/dexamethasone (Rd) maintenance after autologous stem cell transplant in patients with newly diagnosed multiple myeloma: Results of the Spanish GEM2014MAIN Trial. Blood 2021, 138 (Suppl. S1), 466. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Perrot, A.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Zweegman, S.; Caillon, H.; Caillot, D.; et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Usmani, S.Z. Defining and managing high-risk multiple myeloma: Current concepts. J. Natl. Compr. Cancer Netw. 2020, 18, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Derman, B.A.; Kosuri, S.; Jakubowiak, A. Knowing the unknowns in high risk multiple myeloma. Blood Rev. 2022, 51, 100887. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C. High-risk myeloma: A challenge to define and to determine the optimal treatment. Lancet Haematol. 2021, 8, e4–e6. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Kapoor, P.; Jacobus, S.J.; Wei, Z.; Cohen, A.D.; Fonseca, R.; Callander, N.S.; Lonial, S.; Rajkumar, S.V.; Kumar, S.K. Impact of chromosome 1 abnormalities among patients with newly diagnosed multiple myeloma: A subgroup analysis from the Endurance (ECOG-ACRIN E1A11) trial. Blood 2021, 138 (Suppl. S1), 467. [Google Scholar]
- Mina, R.; Zamagni, E.; Rota-Scalabrini, D.; Corradini, P.; Grasso, M.; Ballanti, S.; Giuliani, N.; De Rosa, L.; Cellini, C.; Vincelli, I.-D.; et al. Carfilzomib-based induction/consolidation with or without autologous transplant and lenalidomide (R) or carfilzomib-lenalidomide (KR) maintenance: Efficacy in high-risk patients of the FORTE study. Clin. Lymphoma Myeloma Leuk. 2021, 21 (Suppl. S2), OAB-004. [Google Scholar] [CrossRef]
- Yong, K.; Camilleri, M.; Wilson, W.; Ramasamy, K.; Streetly, M.J.; Sive, J.; Bygrave, C.; Chapman, M.A.; De Tute, R.; Chavda, S.J.; et al. Upfront autologous stem cell transplantation (ASCT) versus carfilzomib-cyclophosphamide-dexamethasone (KCd) consolidation with K maintenance in transplant-eligible, newly diagnosed (NDTE) multiple myeloma (MM). J. Clin. Oncol. 2021, 39 (Suppl. S15), 8000. [Google Scholar] [CrossRef]
- Anderson, L.D., Jr.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.W.; Costa, L.J.; Nathwani, N.; et al. Daratumumab plus lenalidomide, bortezomib, and dexamethasone (D-VRd) in transplant-eligible newly diagnosed multiple myeloma patients: A subgroup analysis of Griffin. Blood 2021, 138 (Suppl. S1), 2723. [Google Scholar] [CrossRef]
- Costa, L.J.; Chhabra, S.; Callander, N.S.; Medvedova, E.; Dholaria, B.; Silbermann, R.W.; Godby, K.N.; Dhakal, B.; Bal, S.; Giri, S. Daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd), autologous transplantation and MRD response-adapted consolidation and treatment cessation. Final primary endpoint analysis of the Master trial. Blood 2021, 138 (Suppl. S1), 481. [Google Scholar] [CrossRef]
- Goicoechea, I.; Puig, N.; Cedena, M.T.; Burgos, L.; Cordón, L.; Vidriales, M.B.; Flores-Montero, J.; Gutierrez, N.C.; Calasanz, M.J.; Ramos, M.M.; et al. Deep MRD profiling defines outcome and unveils different modes of treatment resistance in standard- and high-risk myeloma. Blood 2021, 137, 49–60. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Hoering, A.; Ailawadhi, S.; Sexton, R.; Lipe, B.; Hita, S.F.; Valent, J.; Rosenzweig, M.; Zonder, J.A.; Dhodapkar, M.; et al. Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): Primary analysis of a randomised, phase 2 trial. Lancet Haematol. 2021, 8, e45–e54. [Google Scholar] [CrossRef]
- Leypoldt, L.; Besemer, B.; Asemissen, A.M.; Hanel, M.; Blau, I.W.; Gorner, M.; Ko, Y.-D.; Reinhardt, H.C.; Staib, P.; Mann, C.; et al. Updated interim analysis of the GMMG-CONCEPT trial investigating isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRD) in front-line treatment of high-risk multiple myeloma. Hemasphere 2021, 5, S183. [Google Scholar]
- Kaiser, M.F.; Hall, A.; Walker, K.; Newnham, N.; de Tute, R.M.; Roberts, S.; Ingleson, E.; Bowles, K.M.; Garg, M.; LOkare, A.; et al. Daratumumab, cyclophosphamide, bortezomib, lenalidomide, dexamethasone (Dara-CVRd), v-augmented autologous stem cell transplant (V-ASCT) and Dara-Vrd consolidation in ultra-high risk (UHiR) newly diagnosed myeloma (NDMM) and primary plasma cell leukemia (pPCL) compared with Myeloma XI/XI+ trial treatment for Uhir MM: The UK Optimum/Muknine Trial. Blood 2021, 138 (Suppl. S1), 465. [Google Scholar]
- Gagelmann, N.; Eikema, D.J.; de Wreede, L.C.; Rambaldi, A.; Iacobelli, S.; Koster, L.; Caillot, D.; Blaise, D.; Remémyi, P.; Bulabois, C.E.; et al. Upfront stem cell transplantation for newly diagnosed multiple myeloma with del(17p) and t(4;14): A study from the CMWP-EBMT. Bone Marrow Transpl. 2021, 56, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S.; Garderet, L.; Durie, B.; Cook, G.; Gahrton, G.; Bruno, B.; Hari, P.; Lokhorst, H.; McCarthy, P.; Krishnan, A.; et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol. Blood Marrow Transplant. 2015, 21, 2039–2051. [Google Scholar]
- Cook, G.; Ashcroft, A.J.; Cairns, D.A.; Williams, C.D.; Brown, J.M.; Cavenagh, J.; Snowden, J.A.; Parrish, C.; Yong, K.; Cavet, J.; et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): A randomised, open-label, phase 3 trial. Lancet Haematol. 2016, 3, e340–e351. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, H.; Baertsch, M.A.; Schlenzka, J.; Becker, N.; Habermehl, C.; Hielscher, T.; Raab, M.S.; Hillengass, J.; Sauer, S.; Müller-Tidow, C.; et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: The randomized GMMG phase III trial ReLApsE. German Myeloma Multicenter Group (GMMG). Leukemia 2021, 35, 1134–1144. [Google Scholar] [CrossRef]

| Trial | Phase | No Pts | Design | Follow-Up | PFS (Median) | OS | Ref. |
|---|---|---|---|---|---|---|---|
| GIMEMA-MMY-3006 | III | 480 | VTD vs. TD induction and consolidation + 2 ASCT | 124 months | 60 vs. 41 months | 10-yr 60% vs. 46% | [54] |
| IFM 2009 | III | 700 | VRD induction/consolidation + ASCT vs. VRD | 93 months | 47.3 vs. 35 months | NR vs. NR | [66] |
| MMRC | II | 76 | KRd induction/consolidation, + ASCT | 56 months | NR | NR | [31] |
| IFM KRd | II | 46 | KRd induction/consolidation, + ASCT | 60.5 months | 56.4 months | NR | [32] |
| FORTE | III | 474 | KRd induction/consolidation + ASCT vs. KCd induction/consolidation + ASCT vs. KRd12 | 50.9 months | NR vs. 53 months vs. 55.3 months | KRd + ASCT vs. KRd12 HR = 0.61 | [19] |
| CARDAMON | III | 278 | KCd induction + ASCT vs. KCd | 32.1 months | 2-yr 76.1% vs. 68.6% | NA | [67] |
| CASSIOPEIA | III | 1085 | D-VTd vs. VTd induction/consolidation + ASCT | 18.8 months | At 18 months 93% vs. 85% | NR vs. NR | [33] |
| MASTER | II | 123 | D-KRd induction/consolidation + ASCT | 23.8 months | 2-yr 87% | 2-yr 94% | [35] |
| IFM 2018-01 | II | 45 | D-IxaRD induction/consolidation + ASCT | 23.6 months | 2-yr 95.2% | NA | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morè, S.; Corvatta, L.; Manieri, V.M.; Saraceni, F.; Scortechini, I.; Mancini, G.; Fiorentini, A.; Olivieri, A.; Offidani, M. Autologous Stem Cell Transplantation in Multiple Myeloma: Where Are We and Where Do We Want to Go? Cells 2022, 11, 606. https://doi.org/10.3390/cells11040606
Morè S, Corvatta L, Manieri VM, Saraceni F, Scortechini I, Mancini G, Fiorentini A, Olivieri A, Offidani M. Autologous Stem Cell Transplantation in Multiple Myeloma: Where Are We and Where Do We Want to Go? Cells. 2022; 11(4):606. https://doi.org/10.3390/cells11040606
Chicago/Turabian StyleMorè, Sonia, Laura Corvatta, Valentina Maria Manieri, Francesco Saraceni, Ilaria Scortechini, Giorgia Mancini, Alessandro Fiorentini, Attilio Olivieri, and Massimo Offidani. 2022. "Autologous Stem Cell Transplantation in Multiple Myeloma: Where Are We and Where Do We Want to Go?" Cells 11, no. 4: 606. https://doi.org/10.3390/cells11040606
APA StyleMorè, S., Corvatta, L., Manieri, V. M., Saraceni, F., Scortechini, I., Mancini, G., Fiorentini, A., Olivieri, A., & Offidani, M. (2022). Autologous Stem Cell Transplantation in Multiple Myeloma: Where Are We and Where Do We Want to Go? Cells, 11(4), 606. https://doi.org/10.3390/cells11040606







