Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors
Abstract
1. Introduction
1.1. Incidence and Etiology
1.2. Genetics of Canine MCTs
1.3. Biological Behavior
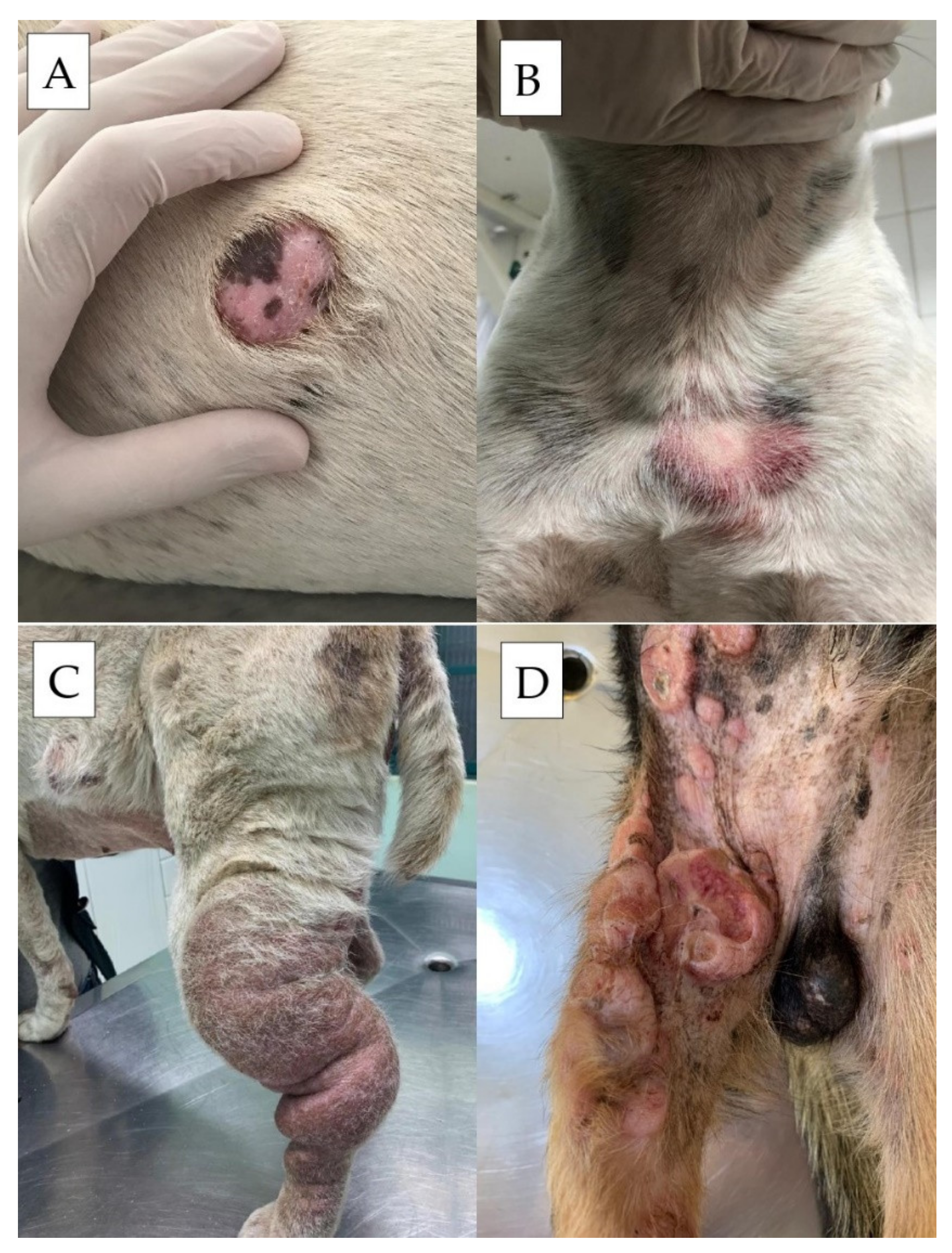
1.4. Clinical Signs and Paraneoplastic Syndromes
2. Diagnostic Approach
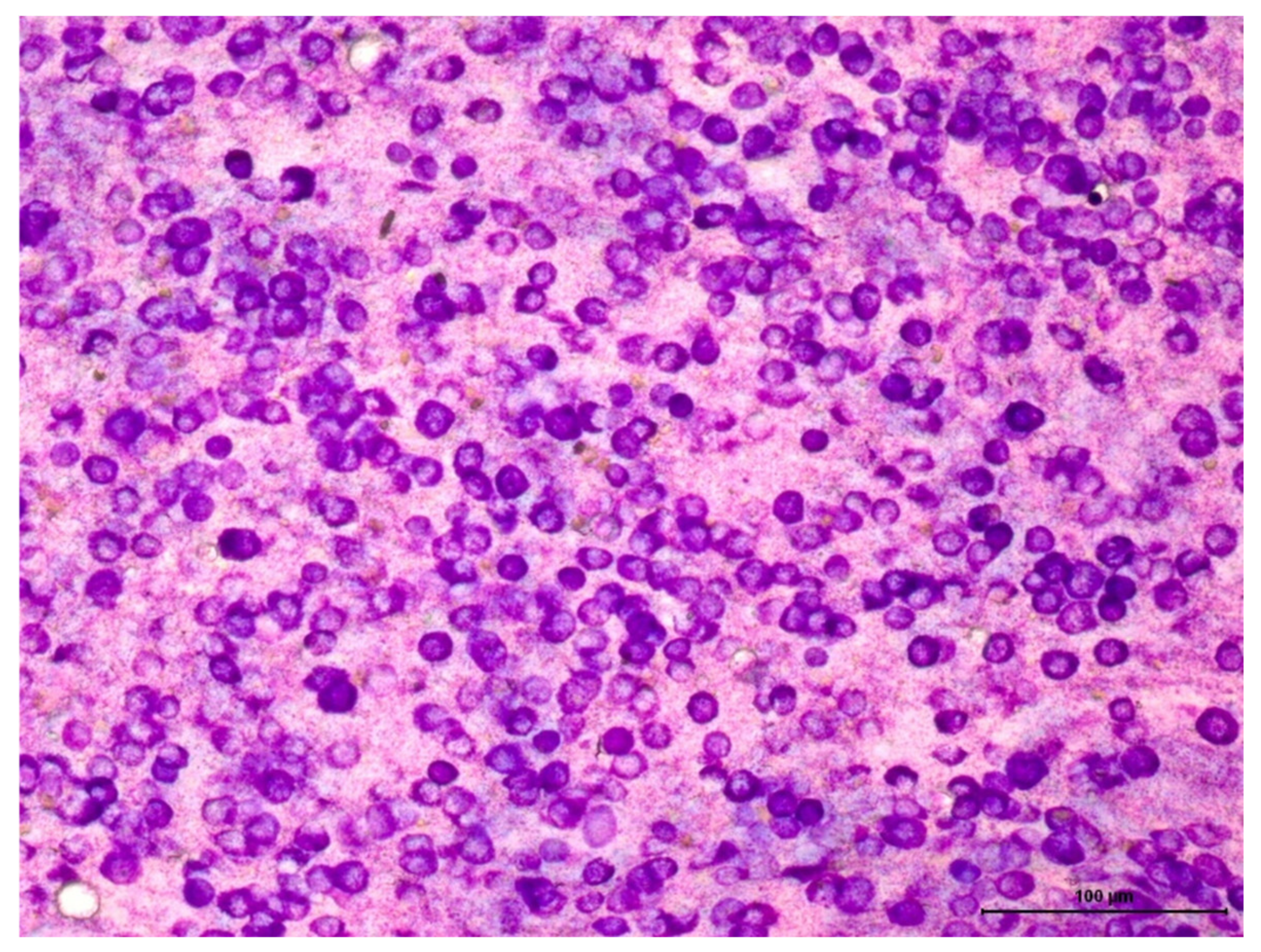
2.1. Cytological Analysis
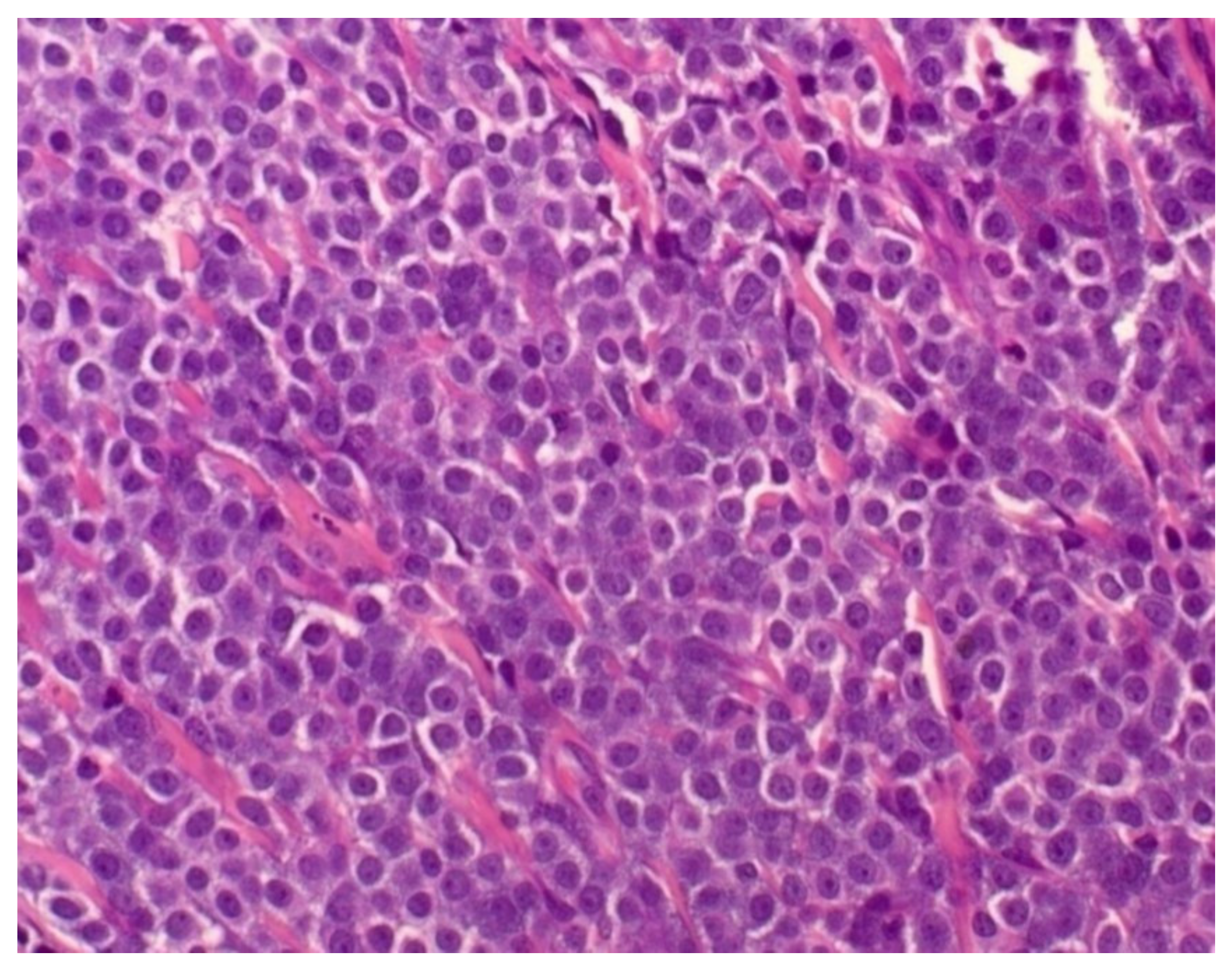
2.2. Histopathological Analysis
- When it is necessary to perform cytoreductive therapy with the use of corticosteroids or chemotherapy, it is recommended that material first be collected by incisional biopsy, considering that cytoreductive protocols can change the assessment of the cell proliferation index (mitotic count and immunohistochemical analysis for Ki67), and when possible, the excised formation should be reassessed;
- When an incisional biopsy is necessary, maximum caution is recommended in handling the tumor, in order to avoid degranulation of mast cells and risk to the patient;
- For a better assessment of the surgical margins, the use of India ink or suture stitches is recommended to identify the lateral and deep margins;
- When there are multiple nodules, the submission must be completed separately with the appropriate identifications, allowing individual analysis of each lesion;
- A minimum of seven mitotic figures counted in 10 fields of higher magnification, and evaluated in the areas with the greatest number of mitotic figures;
- At least three multinucleated cells (three or more nuclei) in 10 high-power fields;
- At least three bizarre nuclei or markedly pleomorphic nuclei in 10 fields of higher magnification; or
- Circumscribed;
- Combined (infiltrative/circumscribed); and
- Infiltrative.
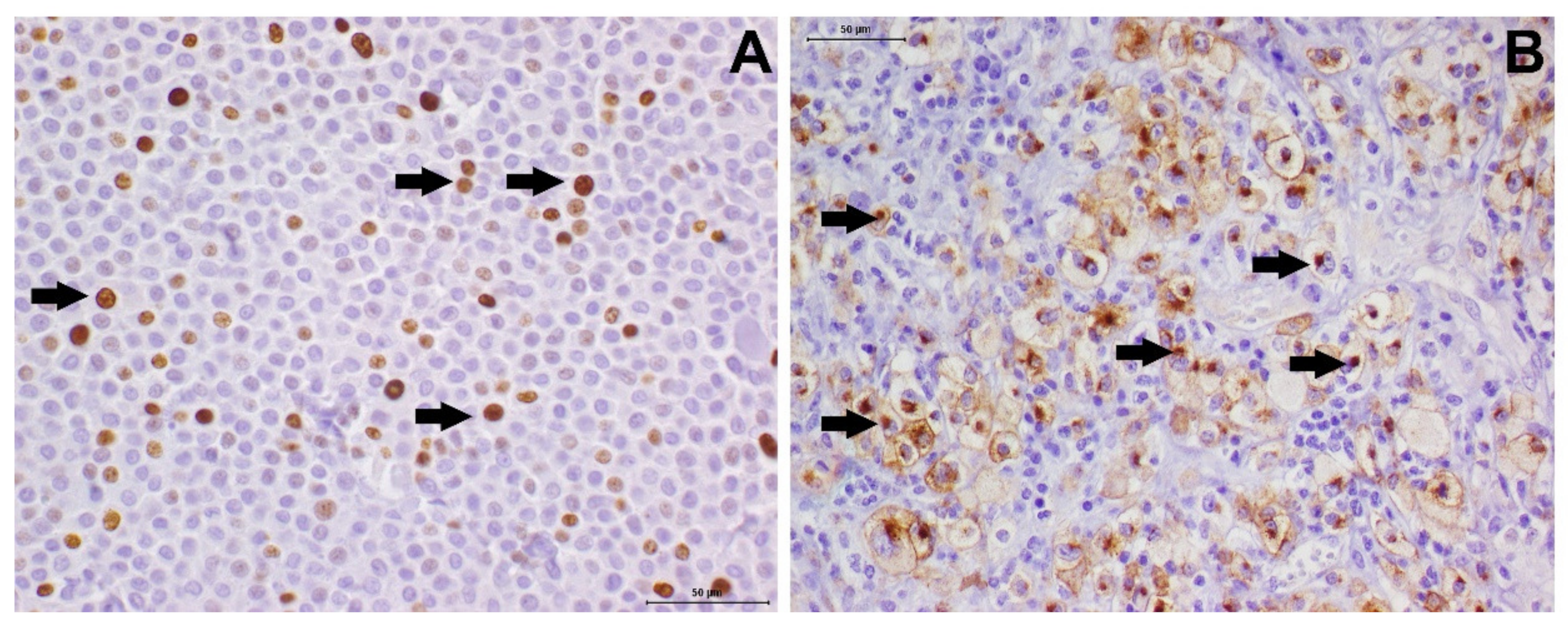
2.3. Immunohistochemistry and Histochemical Staining Methods
2.4. KIT Mutation
2.5. Staging
3. Prognostic Factors
4. Therapeutic Approach
4.1. Local Treatments
4.1.1. Surgery
4.1.2. Electrochemotherapy
4.1.3. Radiotherapy
4.1.4. Tigilanol Tiglate (Stelfonta®)
4.2. Systemic Treatments
4.2.1. Anticancer Chemotherapy
4.2.2. Tyrosine Kinase Inhibitors
4.3. Support and Palliative Treatment
- Manifest systemic and/or local clinical signs;
- Are larger;
- Will undergo considerable surgical manipulation or tumor section (cytoreductive surgery);
- With a greater probability of local degranulation (chemotherapy or radiotherapy in macroscopic disease); and/or
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macy, D.W. Canine Mast Cell Tumors. Vet. Clin. North Am. Small Anim. Pr. 1985, 15, 783–803. [Google Scholar] [CrossRef]
- Pakhrin, B.; Kang, M.-S.; Bae, I.-H.; Park, M.-S.; Jee, H.; You, M.-H.; Kim, J.-H.; Yoon, B.-I.; Choi, Y.-K.; Kim, D.-Y. Retrospective study of canine cutaneous tumors in Korea. J. Vet. Sci. 2007, 8, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Gamlem, H.; Nordstoga, K.; Glattre, E. Canine neoplasia—Introductory paper. APMIS 2008, 116, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Grabarević, Ž.; Bubić Špoljar, J.; Gudan Kurilj, A.; Šoštarić-Zuckermann, I.C.; Artuković, B.; Hohšteter, M.; Beck, A.; Džaja, P.; Maltar Strmečki, N. Mast cell tumor in dogs–incidence and histopathological characterization. Coll. Antropol. 2009, 33, 253–258. [Google Scholar]
- Villamil, J.A.; Henry, C.J.; Bryan, J.N.; Ellersieck, M.; Schultz, L.; Tyler, J.W.; Hahn, A.W. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. J. Am. Vet. Med. Assoc. 2011, 239, 960–965. [Google Scholar] [CrossRef]
- Kimura, K.C.; Gárate, A.P.; Dagli, M.L.Z. Retrospective Study of Neoplasms in Domestic Animals: A Survey Between 1993 and 2002 of the Service of Animal Pathology Department of Pathology School of Veterinary Medicine and Animal Science University of Sao Paulo Southeast Brazil. Braz. J. Vet. Pathol. 2012, 5, 60–69. [Google Scholar]
- Warland, J.; Dobson, J.M. Breed predispositions in canine mast cell tumour: A single centre experience in the United Kingdom. Vet. J. 2013, 197, 496–498. [Google Scholar] [CrossRef]
- Freeman, K.; Kirtz, G.; Hooijberg, E.H.; Sick, K.; Leidinger, E.F. Breed related odds ratio and anatomic distribution of canine mast cell tumours in Austria. Tierarztl. Prax. Ausg. K. 2014, 42, 367–373. [Google Scholar] [CrossRef]
- Shoop-Worrall, S.; Marlow, S.; Church, D.B.; English, K.; McGreevy, P.D.; Stell, A.J.; Thomson, P.C.; O’Neill, D.G.; Brodbelt, D.C. Prevalence and risk factors for mast cell tumours in dogs in England. Canine Genet. Epidemiol. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Baioni, E.; Scanziani, E.; Vincenti, M.C.; Leschiera, M.; Bozzetta, E.; Pezzolato, M.; Desiato, R.; Bertolini, S.; Maurella, C.; Ru, G. Estimating canine cancer incidence: Findings from a population-based tumour registry in northwestern Italy. BMC Vet. Res. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Horta, R.S.; LaValle, G.E.; Monteiro, L.N.; Souza, M.C.C.; Cassali, G.D.; Araújo, R.B. Assessment of Canine Mast Cell Tumor Mortality Risk Based on Clinical, Histologic, Immunohistochemical, and Molecular Features. Vet. Pathol. 2018, 55, 212–223. [Google Scholar] [CrossRef]
- Graf, R.; Pospischil, A.; Guscetti, F.; Meier, D.; Welle, M.; Dettwiler, M. Cutaneous Tumors in Swiss Dogs: Retrospective Data From the Swiss Canine Cancer Registry, 2008–2013. Vet. Pathol. 2018, 55, 809–820. [Google Scholar] [CrossRef]
- Kok, M.K.; Chambers, J.; Tsuboi, M.; Nishimura, R.; Tsujimoto, H.; Uchida, K.; Nakayama, H. Retrospective study of canine cutaneous tumors in Japan, 2008-2017. J. Vet. Med. Sci. 2019, 81, 1133–1143. [Google Scholar] [CrossRef]
- De Nardi, A.B.; Costa, M.T.; Amorim, R.L.; Vasconcelos, R.O.; Dagli, M.L.Z.; Rocha, N.S.; Neto, R.T.; Grandi, F.; Alessi, A.C.; Magalhães, G.M.; et al. Brazilian Consensus For The Diagnosis, Treatment And Prognosis Of Cutaneous Mast Cell Tumors In Dogs. Investigação 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Thamm, D.H. Miscellaneous tumors: Hemangiosarcoma. In Withrow and MacEwen’s Small Animal Clinical Oncology, 5th ed.; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: St. Louis, MI, USA, 2013; pp. 679–688. [Google Scholar]
- De Nardi, A.B.; Rodaski, S.; Sousa, R.S.; Costa, T.A.; Macedo, T.R.; Rodigheri, S.M.; Ríos, A.; Piekarz, C.H. Prevalência de neoplasias e modalidades de tratamentos em cães, atendidos no hospital veterinário da Universidade federal do Paraná. Arch. Vet. Sci. 2002, 7. [Google Scholar] [CrossRef]
- De Souza, T.M.; Fighera, R.A.; Irigoyen, L.F.; De Barros, C.S.L. Estudo retrospectivo de 761 tumores cutâneos em cães. CiÊNcia Rural 2006, 36, 555–560. [Google Scholar] [CrossRef]
- Meirelles, A.E.W.B.; Oliveira, E.C.; Rodrigues, B.A.; Costa, G.R.; Sonne, L.; Tesser, E.S.; Driemeier, D. Prevalência de neoplasmas cutâneos em cães da região metropolitana de Porto Alegre, RS: 1.017 casos (2002–2007). Pesqui. Vet. Bras. 2010, 30, 968–973. [Google Scholar] [CrossRef]
- Kiupel, M.; Webster, J.D.; Miller, R.A.; Kaneene, J.B. Impact of Tumour Depth, Tumour Location and Multiple Synchronous Masses on the Prognosis of Canine Cutaneous Mast Cell Tumours. J. Vet. Med. Ser. A 2005, 52, 280–286. [Google Scholar] [CrossRef]
- Murphy, S.; Sparkes, A.H.; Blunden, A.S.; Brearley, M.J.; Smith, K.C. Effects of stage and number of tumours on prognosis of dogs with cutaneous mast cell tumours. Vet. Rec. 2006, 158, 287–291. [Google Scholar] [CrossRef]
- Mullins, M.N.; Dernell, W.S.; Withrow, S.J.; Ehrhart, E.J.; Thamm, D.H.; Lana, S.E. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004). J. Am. Vet. Med. Assoc. 2006, 228, 91–95. [Google Scholar] [CrossRef]
- Costa-Casagrande, T.A.; Elias, D.S.; Melo, S.R.; Matera, J.M. ESTUDO RETROSPECTIVO DO MASTOCITOMA CANINO NO SERVIÇO DE CIRURGIA DE PEQUENOS ANIMAIS—HOSPITAL VETERINÁRIO DA FACULDADE DE MEDICINA VETERINÁRIA E ZOOTECNIA DA UNIVERSIDADE DE SÃO PAULO. Arch. Vet. Sci. 2008, 13. [Google Scholar] [CrossRef][Green Version]
- Furlani, J.M.; Daleck, C.R.; Vicenti, F.A.M.; De Nardi, A.B.; Pereira, G.T.; Santana, Á.E.; Eurides, D.; da Silva, L.A.F. Mastocitoma canino: Estudo retrospectivo. CiÊNcia Anim. Bras. 2008, 9, 242–250. [Google Scholar]
- Mochizuki, H.; Motsinger-Reif, A.; Bettini, C.; Moroff, S.; Breen, M. Association of breed and histopathological grade in canine mast cell tumours. Vet. Comp. Oncol. 2016, 15, 829–839. [Google Scholar] [CrossRef]
- Śmiech, A.; Ślaska, B.; Łopuszyński, W.; Jasik, A.; Bochyńska, D.; Dąbrowski, R. Epidemiological assessment of the risk of canine mast cell tumours based on the Kiupel two-grade malignancy classification. Acta Vet. Scand. 2018, 60, 1–9. [Google Scholar] [CrossRef]
- Pierini, A.; Lubas, G.; Gori, E.; Binanti, D.; Millanta, F.; Marchetti, V. Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy. Vet. Sci. 2019, 6, 53. [Google Scholar] [CrossRef]
- Śmiech, A.; Łopuszyński, W.; Ślaska, B.; Bulak, K.; Jasik, A. Occurrence and distribution of canine cutaneous mast cell tumour characteristics among predisposed breeds. J. Vet. Res. 2019, 63, 141–148. [Google Scholar] [CrossRef]
- London, C.; Galli, S.J.; Yuuki, T.; Hu, Z.-Q.; Helfand, S.C.; Geissler, E.N. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp. Hematol. 1999, 27, 689–697. [Google Scholar] [CrossRef]
- Reguera, M.J.; Rabanal, R.M.; Puigdemont, A.; Ferrer, L. Canine Mast Cell Tumors Express Stem Cell Factor Receptor. Am. J. Dermatopathol. 2000, 22, 49–54. [Google Scholar] [CrossRef]
- Zemke, D.; Yamini, B.; Yuzbasiyan-Gurkan, V. Mutations in the Juxtamembrane Domain of c-KIT Are Associated with Higher Grade Mast Cell Tumors in Dogs. Vet. Pathol. 2002, 39, 529–535. [Google Scholar] [CrossRef]
- Turin, L.; Acocella, F.; Stefanello, D.; Oseliero, A.; Fondrini, D.; Brizzola, S.; Riva, F. Expression of C-Kit Proto-Oncogene in Canine Mastocytoma: A Kinetic Study Using Real-Time Polymerase Chain Reaction. J. Vet. Diagn. Investig. 2006, 18, 343–349. [Google Scholar] [CrossRef]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Miller, R.A.; Kaneene, J.B.; Kiupel, M. Cellular Proliferation in Canine Cutaneous Mast Cell Tumors: Associations with c-KIT and Its Role in Prognostication. Vet. Pathol. 2007, 44, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Longley, B.J.; Wang, X.; Blount, J.L.; Langley, K.; Caughey, G.H. Clustering of Activating Mutations in c-KIT’s Juxtamembrane Coding Region in Canine Mast Cell Neoplasms. J. Investig. Dermatol. 1999, 112, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Chien, M.B.; Kass, P.H.; Moore, P.F.; London, C.A. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c- kit in mast cell tumors of dogs. Am. J. Vet. Res. 2002, 63, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Evaluation of the kinase domain of c-KIT in canine cutaneous mast cell tumors. BMC Cancer 2006, 6, 85–88. [Google Scholar] [CrossRef][Green Version]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Kaneene, J.B.; Miller, R.; Resau, J.H.; Kiupel, M. The Role of c-KIT in Tumorigenesis: Evaluation in Canine Cutaneous Mast Cell Tumors. Neoplasia 2006, 8, 104–111. [Google Scholar] [CrossRef]
- Letard, S.; Yang, Y.; Hanssens, K.; Palmérini, F.; Leventhal, P.S.; Guéry, S.; Moussy, A.; Kinet, J.-P.; Hermine, O.; Dubreuil, P. Gain-of-Function Mutations in the Extracellular Domain of KIT Are Common in Canine Mast Cell Tumors. Mol. Cancer Res. 2008, 6, 1137–1145. [Google Scholar] [CrossRef]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, Placebo-controlled, Double-blind, Randomized Study of Oral Toceranib Phosphate (SU11654), a Receptor Tyrosine Kinase Inhibitor, for the Treatment of Dogs with Recurrent (Either Local or Distant) Mast Cell Tumor Following Surgical Excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fujino, Y.; Watanabe, M.; Takahashi, M.; Nakagawa, T.; Takeuchi, A.; Bonkobara, M.; Kobayashi, T.; Ohno, K.; Uchida, K.; et al. Validation of the prognostic value of histopathological grading or c-kit mutation in canine cutaneous mast cell tumours: A retrospective cohort study. Vet. J. 2013, 196, 492–498. [Google Scholar] [CrossRef]
- Tamlin, V.S.; Bottema, C.D.K.; Peaston, A.E. Comparative aspects of mast cell neoplasia in animals and the role of KIT in prognosis and treatment. Vet. Med. Sci. 2019, 6, 3–18. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Fictum, P.; Fröhlich, J.; Jelinek, F.; Rubes, J. Prevalence and prognostic value of c-kit and TP53 mutations in canine mast cell tumours. Vet. J. 2019, 247, 71–74. [Google Scholar] [CrossRef]
- Brocks, B.A.W.; Bertram, C.A.; Bartel, A.; Kirpensteijn, J.; Collins-Webb, A.; Catlin, C.; Thaiwong, T.; Kiupel, M. Internal Tandem Duplication of Exon 8 of c-kit Is Associated With Longer Total Survival in Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2020, 58, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Okuda, M.; Toyama, N.; Oikawa, T.; Inokuma, H.; Morimoto, M.; Hayashi, T.; Une, S.; Nakaichi, M.; Taura, Y.; et al. Detection of the Anti-P53 Antibodies in Dogs with Tumors. J. Vet. Med. Sci. 2002, 64, 973–979. [Google Scholar] [CrossRef][Green Version]
- Zorzan, E.; Hanssens, K.; Giantin, M.; Dacasto, M.; Dubreuil, P. Mutational Hotspot of TET2, IDH1, IDH2, SRSF2, SF3B1, KRAS, and NRAS from Human Systemic Mastocytosis Are Not Conserved in Canine Mast Cell Tumors. PLoS ONE 2015, 10, e0142450. [Google Scholar] [CrossRef]
- Yoda, A.; Adelmant, G.; Tamburini, J.; Chapuy, B.; Shindoh, N.; Yoda, Y.; Weigert, O.; Kopp, N.; Wu, S.-C.; Kim, S.S.; et al. Mutations in G protein β subunits promote transformation and kinase inhibitor resistance. Nat. Med. 2014, 21, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Thomas, R.; Moroff, S.; Breen, M. Genomic profiling of canine mast cell tumors identifies DNA copy number aberrations associated with KIT mutations and high histological grade. Chromosom. Res. 2017, 25, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Jark, P.C.; Mundin, D.B.; de Carvalho, M.; Ferioli, R.B.; Anai, L.A.; Marchi, F.A.; Rogatto, S.R.; Laufer-Amorim, R.; Tinucci-Costa, M. Genomic copy number variation associated with clinical outcome in canine cutaneous mast cell tumors. Res. Vet. Sci. 2017, 111, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef]
- Bostock, D. Neoplasms of the skin and subcutaneous tissues in dogs and cats. Br. Vet. J. 1986, 142, 1–19. [Google Scholar] [CrossRef]
- Weisse, C.; Shofer, F.S.; Sorenmo, K. Recurrence Rates and Sites for Grade II Canine Cutaneous Mast Cell Tumors Following Complete Surgical Excision. J. Am. Anim. Hosp. Assoc. 2002, 38, 71–73. [Google Scholar] [CrossRef]
- Hume, C.T.; Kiupel, M.; Rigatti, L.; Shofer, F.S.; Skorupski, K.A.; Sorenmo, K.U. Outcomes of Dogs with Grade 3 Mast Cell Tumors: 43 Cases (1997–2007). J. Am. Anim. Hosp. Assoc. 2011, 47, 37–44. [Google Scholar] [CrossRef]
- Daleck, C.R.; De Nardi, A.B. Oncologia em cães e gatos; Grupo Gen-Editora Roca Ltda: Rio de Janeiro, Brazil, 2016; Volume 2, pp. 971–995. [Google Scholar]
- O’Connell, K.; Thomson, M. Evaluation of prognostic indicators in dogs with multiple, simultaneously occurring cutaneous mast cell tumours: 63 cases. Vet. Comp. Oncol. 2011, 11, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Berlato, D.; Bulman-Fleming, J.; Clifford, C.A.; Garrett, L.; Intile, J.; Jones, P.; Kamstock, D.A.; Liptak, J.M.; Pavuk, A.; Powell, R.; et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet. Pathol. 2021, 58, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Frimberger, A.E.; Taylor, D.; Sullivan, N. Retrospective outcome evaluation for dogs with surgically excised, solitary Kiupel high-grade, cutaneous mast cell tumours. Vet. Comp. Oncol. 2020, 18, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.; Yuzbasiyan-Gurkan, V.; Marconato, L.; Dacasto, M.; Hadzijusufovic, E.; Hermine, O.; Sadovnik, I.; Gamperl, S.; Schneeweiss-Gleixner, M.; Gleixner, K.V.; et al. Proposed Diagnostic Criteria and Classification of Canine Mast Cell Neoplasms: A Consensus Proposal. Front. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Macy, D.; Tams, T. Canine Mast Cell Tumors. Vet. Clin. North Am. Small Anim. Pract. 1981, 3, 869–877. [Google Scholar] [CrossRef]
- Turrel, J.M.; Kitchell, B.E.; Miller, L.M.; Théon, A. Prognostic factors for radiation treatment of mast cell tumor in 85 dogs. J. Am. Vet. Med. Assoc. 1988, 193. [Google Scholar]
- Fox, L.E. Mast Cell Tumors. In Cancer in Dogs and Cats Medical and Surgical Management; Morrison, W.B., Ed.; Linppincott Williams & Wilkins: Philadelphia, PA, USA, 1998; pp. 479–488. [Google Scholar]
- Nelson, R.; Couto, C.G. Medicina interna de pequenos animais; Elsevier: Centro Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Sfiligoi, G.; Rassnick, K.M.; Scarlett, J.M.; Northrup, N.C.; Gieger, T.L. Outcome of Dogs with Mast Cell Tumors in the Inguinal or Perineal Region Versus Other Cutaneous Locations: 124 Cases (1990–2001). J. Am. Vet. Med. Assoc. 2005, 226, 1368–1374. [Google Scholar] [CrossRef]
- Hillman, L.A.; Garrett, L.D.; De Lorimier, L.-P.; Charney, S.C.; Borst, L.; Fan, T.M. Biological behavior of oral and perioral mast cell tumors in dogs: 44 cases (1996–2006). J. Am. Vet. Med. Assoc. 2010, 237, 936–942. [Google Scholar] [CrossRef]
- Stockham, S.L.; Basel, D.L.; Schmidt, D.A. Mastocytemia in Dogs With Acute Inflammatory Diseases. Vet. Clin. Pathol. 1986, 15, 16–21. [Google Scholar] [CrossRef]
- Hikasa, Y.; Morita, T.; Futaoka, Y.; Sato, K.; Shimada, A.; Kagota, K.; Matsuda, H. Connective Tissue-Type Mast Cell Leukemia in a Dog. J. Vet. Med. Sci. 2000, 62, 187–190. [Google Scholar] [CrossRef]
- Plier, M.L.; MacWilliams, P.L. Schalm’s Veterinary Hematology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; Volume 5, pp. 747–754. [Google Scholar]
- Welle, M.M.; Bley, C.R.; Howard, J.; Rüfenacht, S. Canine mast cell tumours: A review of the pathogenesis, clinical features, pathology and treatment. Vet. Dermatol. 2008, 19, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, L.; Murphy, S.; Buracco, P.; De Vos, J.P.; De Fornel-Thibaud, P.; Hirschberger, J.; Kessler, M.; Pastor, J.; Ponce, F.; Savary-Bataille, K.; et al. European consensus document on mast cell tumours in dogs and cats. Vet. Comp. Oncol. 2012, 10, e1–e29. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kadosawa, T.; Nagase, M.; Matsunaga, S.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Visceral mast cell tumors in dogs: 10 cases (1982-1997). J. Am. Vet. Med. Assoc. 2000, 216, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Endicott, M.M.; Charney, S.C.; McKnight, J.A.; Loar, A.S.; Barger, A.M.; Bergman, P.J. Clinicopathological findings and results of bone marrow aspiration in dogs with cutaneous mast cell tumours: 157 cases (1999?2002). Vet. Comp. Oncol. 2007, 5, 31–37. [Google Scholar] [CrossRef]
- Musser, M.; Berger, E.; Flaherty, H.A.; Fox, L.; Johannes, C.M. Marked paraneoplastic hypereosinophilia associated with a low-grade, metastatic canine mast cell tumour. Vet. Rec. Case Rep. 2018, 6, e000563. [Google Scholar] [CrossRef]
- Cifuentes-Arias, S.; Osorio-Morales, L.; Pedraza-Ordóñez, F. Clinical follow-up of canine mast cell tumour cases diagnosed by cytology and histopathology. Vet. Stanica 2021, 52, 397–403. [Google Scholar] [CrossRef]
- Cowell, R.L.; Tyler, R.D.; Meinkoth, J.H.; DeNicola, D.B. Diagnóstico citológico e hematologia de cães e gatos. Medvet. São Paulo 2009, 68–77. [Google Scholar]
- De Strefezzi, R.F.; Xavier, J.G.; Kleeb, S.R.; Catão-Dias, J.L. Nuclear Morphometry in Cytopathology: A Prognostic Indicator for Canine Cutaneous Mast Cell Tumors. J. Vet. Diagn. Investig. 2009, 21, 821–825. [Google Scholar] [CrossRef]
- MacNeill, A.L. Cytology of Canine and Feline Cutaneous and Subcutaneous Lesions and Lymph Nodes. Top. Companion Anim. Med. 2011, 26, 62–76. [Google Scholar] [CrossRef]
- Grandi, F.; Beserra, H.E.O.; Costa, L.D. Citopatologia veterinária diagnóstica. São Paulo: Medvet 2014, 80–90. [Google Scholar]
- Camus, M.S.; Priest, H.L.; Koehler, J.W.; Driskell, E.A.; Rakich, P.M.; Ilha, M.R.; Krimer, P.M. Cytologic Criteria for Mast Cell Tumor Grading in Dogs With Evaluation of Clinical Outcome. Vet. Pathol. 2016, 53, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Hergt, F.; Von Bomhard, W.; Kent, M.; Hirschberger, J. Use of a 2-tier histologic grading system for canine cutaneous mast cell tumors on cytology specimens. Vet. Clin. Pathol. 2016, 45, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Bostock, D.E. The prognosis following surgical removal of mastocytomas in dogs. J. Small Anim. Pr. 1973, 14, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, R.; Morini, M.; Sarli, G. Expression of the KIT Protein (CD117) in Primary Cutaneous Mast Cell Tumors of the Dog. J. Vet. Diagn. Investig. 2004, 16, 554–561. [Google Scholar] [CrossRef]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-Tier Histologic Grading System for Canine Cutaneous Mast Cell Tumors to More Accurately Predict Biological Behavior. Vet. Pathol. 2010, 48, 147–155. [Google Scholar] [CrossRef]
- Thompson, J.J.; Pearl, D.L.; Yager, J.A.; Best, S.J.; Coomber, B.L.; Foster, R.A. Canine Subcutaneous Mast Cell Tumor. Vet. Pathol. 2010, 48, 156–168. [Google Scholar] [CrossRef]
- Fulcher, R.P.; Ludwig, L.L.; Bergman, P.J.; Newman, S.J.; Simpson, A.M.; Patnaik, A.K. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2006, 228, 210–215. [Google Scholar] [CrossRef]
- Kamstock, D.A.; Ehrhart, E.J.; Getzy, D.M.; Bacon, N.J.; Rassnick, K.M.; Moroff, S.D.; Liu, S.M.; Straw, R.C.; McKnight, C.A.; Amorim, R.L.; et al. Recommended Guidelines for Submission, Trimming, Margin Evaluation, and Reporting of Tumor Biopsy Specimens in Veterinary Surgical Pathology. Vet. Pathol. 2010, 48, 19–31. [Google Scholar] [CrossRef]
- Thompson, J.J.; Yager, J.A.; Best, S.J.; Pearl, D.L.; Coomber, B.L.; Torres, R.N.; Kiupel, M.; Foster, R.A. Canine Subcutaneous Mast Cell Tumors. Vet. Pathol. 2010, 48, 169–181. [Google Scholar] [CrossRef]
- Scarpa, F.; Sabattini, S.; Marconato, L.; Capitani, O.; Morini, M.; Bettini, G. Use of histologic margin evaluation to predict recurrence of cutaneous malignant tumors in dogs and cats after surgical excision. J. Am. Vet. Med. Assoc. 2012, 240, 1181–1187. [Google Scholar] [CrossRef]
- Sledge, D.G.; Webster, J.; Kiupel, M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet. J. 2016, 215, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Strefezzi, R.D.F.; Xavier, J.G.; Catão-Dias, J.L. Morphometry of Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2003, 40, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Northrup, N.C.; Howerth, E.W.; Harmon, B.G.; Brown, C.A.; Carmicheal, K.P.; Garcia, A.P.; Latimer, K.S.; Munday, J.S.; Rakich, P.M.; Richey, L.J.; et al. Variation among Pathologists in the Histologic Grading of Canine Cutaneous Mast Cell Tumors with Uniform Use of a Single Grading Reference. J. Vet. Diagn. Investig. 2005, 17, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Pinczowski, P.; Torres-Neto, R.; Fabris, V.E.; Laufer-Amorim, R. Mastocitoma cutâneo canino: Variação da graduação histopatológica entre patologistas. ClÍNica VeterinÁRia 2008, 77, 76–78. [Google Scholar]
- Shaw, T.; Kudnig, S.T.; Firestone, S.M. Diagnostic accuracy of pre-treatment biopsy for grading cutaneous mast cell tumours in dogs. Vet. Comp. Oncol. 2017, 16, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Meuten, D.J. (Ed.) Tumors in Domestic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2020; p. 1008. [Google Scholar]
- Romansik, E.M.; Reilly, C.M.; Kass, P.H.; Moore, P.F.; London, C.A. Mitotic Index Is Predictive for Survival for Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2007, 44, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Elston, L.B.; Sueiro, F.A.; Cavalcanti, J.N.; Metze, K. Letter to the Editor: The Importance of the Mitotic Index as a Prognostic Factor for Survival of Canine Cutaneous Mast Cell Tumors: A Validation Study. Vet. Pathol. 2009, 46, 362–364. [Google Scholar] [CrossRef]
- Bertram, C.A.; Aubreville, M.; Gurtner, C.; Bartel, A.; Corner, S.M.; Dettwiler, M.; Kershaw, O.; Noland, E.L.; Schmidt, A.; Sledge, D.G.; et al. Computerized Calculation of Mitotic Count Distribution in Canine Cutaneous Mast Cell Tumor Sections: Mitotic Count Is Area Dependent. Vet. Pathol. 2019, 57, 214–226. [Google Scholar] [CrossRef]
- Bertram, C.A.; Aubreville, M.; Donovan, T.A.; Bartel, A.; Wilm, F.; Marzahl, C.; Assenmacher, C.-A.; Becker, K.; Bennett, M.; Corner, S.; et al. Computer-assisted mitotic count using a deep learning–based algorithm improves interobserver reproducibility and accuracy. Vet. Pathol. 2021. [Google Scholar] [CrossRef]
- Newman, S.; Mrkonjich, L.; Walker, K.; Rohrbach, B. Canine Subcutaneous Mast Cell Tumour: Diagnosis and Prognosis. J. Comp. Pathol. 2007, 136, 231–239. [Google Scholar] [CrossRef]
- Gill, V.; Leibman, N.; Monette, S.; Craft, D.M.; Bergman, P.J. Prognostic Indicators and Clinical Outcome in Dogs with Subcutaneous Mast Cell Tumors Treated with Surgery Alone: 43 Cases. J. Am. Anim. Hosp. Assoc. 2020, 56, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, K.; Thamm, D.; Worley, D.; Kamstock, D. Correlation of Nodal Mast Cells with Clinical Outcome in Dogs with Mast Cell Tumour and a Proposed Classification System for the Evaluation of Node Metastasis. J. Comp. Pathol. 2014, 151, 329–338. [Google Scholar] [CrossRef]
- Simoes, J.P.C.; Schoning, P. Canine Mast Cell Tumors: A Comparison of Staining Techniques. J. Vet. Diagn. Investig. 1994, 6, 458–465. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Riaz, R.; Zia, S.; Shahid, H.; Malik, U.A.; Khan, R.; Irfan, M.; Shamail, F.; Zia, F.; Asif, M.G. Impact of Histological Type and Grade on the Diagnostic Accuracy of Intraoperative Frozen Section for Detecting Breast Cancer Metastasis to Axillary Sentinel Lymph Nodes. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Kaneko, H.; Deguchi, M.; Yano, H. Submandibular lymph node metastasis of occult thyroid carcinoma first suspected to be a salivary gland tumor: A case report. J. Med. Case Rep. 2021, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stern, A. Frozen sections compared with paraffin-embedded sections: A retrospective veterinary autopsy study. Braz. J. Vet. Pathol. 2020, 13. [Google Scholar] [CrossRef]
- Whitehair, J.G.; Griffey, S.M.; Olander, H.J.; Vasseur, P.B.; Naydan, D. The Accuracy of Intraoperative Diagnoses Based on Examination of Frozen Sections A Prospective Comparison with Paraffin-Embedded Sections. Vet. Surg. 1993, 22, 255–259. [Google Scholar] [CrossRef]
- Puebla-Osorio, N.; Sarchio, S.N.E.; Ullrich, S.E.; Byrne, S.N. Detection of Infiltrating Mast Cells Using a Modified Toluidine Blue Staining. Methods Mol. Biol. 2017, 1627, 213–222. [Google Scholar] [CrossRef]
- Abadie, J.J.; Amardeilh, M.A.; Delverdier, M. Immunohistochemical detection of proliferating cell nuclear antigen and Ki-67 in mast cell tumors from dogs. J. Am. Vet. Med. Assoc. 1999, 215. [Google Scholar]
- Kiupel, M.; Webster, J.D.; Kaneene, J.B.; Miller, R.; Yuzbasiyan-Gurkan, V. The Use of KIT and Tryptase Expression Patterns as Prognostic Tools for Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2004, 41, 371–377. [Google Scholar] [CrossRef]
- Scase, T.J.; Edwards, D.; Miller, J.; Henley, W.; Smith, K.; Blunden, A.; Murphy, S. Canine mast cell tumors: Correlation of apoptosis and proliferation markers with prognosis. J. Vet. Intern. Med. 2006, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Maglennon, G.A.; Murphy, S.; Adams, V.; Miller, J.; Smith, K.; Blunden, A.; Scase, T.J. Association of Ki67 index with prognosis for intermediate-grade canine cutaneous mast cell tumours*. Vet. Comp. Oncol. 2008, 6, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Strefezzi, R.D.F.; Kleeb, S.R.; Xavier, J.G.; Dias, J.L.C. Avaliação da proliferação celular como indicador prognóstico para mastocitomas cutâneos caninos. Pesq. Vet. Bras. 2010, 30, 559–565. [Google Scholar] [CrossRef][Green Version]
- Fonseca-Alves, C.E.; Palmieri, C.; Dagli, M.L.Z.; Laufer-Amorim, R. Editorial: Precision Medicine in Veterinary Oncology. Front. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Thompson, J.J.; Morrison, J.A.; Pearl, D.L.; Boston, S.E.; Wood, G.; Foster, R.A.; Coomber, B.L. Receptor Tyrosine Kinase Expression Profiles in Canine Cutaneous and Subcutaneous Mast Cell Tumors. Vet. Pathol. 2015, 53, 545–558. [Google Scholar] [CrossRef]
- Thamm, D.H.; Weishaar, K.M.; Charles, J.B.; Ehrhart, E.J. Phosphorylated KIT as a predictor of outcome in canine mast cell tumours treated with toceranib phosphate or vinblastine. Vet. Comp. Oncol. 2019, 18, 169–175. [Google Scholar] [CrossRef]
- Smith, J.; Kiupel, M.; Farrelly, J.; Cohen, R.; Olmsted, G.; Kirpensteijn, J.; Brocks, B.; Post, G. Recurrence rates and clinical outcome for dogs with grade II mast cell tumours with a low AgNOR count and Ki67 index treated with surgery alone. Vet. Comp. Oncol. 2015, 15, 36–45. [Google Scholar] [CrossRef]
- Longley, B.; Reguera, M.J.; Ma, Y. Classes of c-KIT activating mutations: Proposed mechanisms of action and implications for disease classification and therapy. Leuk. Res. 2001, 25, 571–576. [Google Scholar] [CrossRef]
- Yamada, O.; Kobayashi, M.; Sugisaki, O.; Ishii, N.; Ito, K.; Kuroki, S.; Sasaki, Y.; Isotani, M.; Ono, K.; Washizu, T.; et al. Imatinib elicited a favorable response in a dog with a mast cell tumor carrying a c-kit c.1523A>T mutation via suppression of constitutive KIT activation. Vet. Immunol. Immunopathol. 2011, 142, 101–106. [Google Scholar] [CrossRef]
- Reguera, M.J.; Ferrer, L.; Rabanal, R.M. Evaluation of an intron deletion in the c-kit gene of canine mast cell tumors. Am. J. Vet. Res. 2002, 63, 1257–1261. [Google Scholar] [CrossRef]
- Cameron, L.R.J.; Grahn, R.A.; Chien, M.B.; Lyons, L.A.; London, C.A. Detection of c-kit Mutations in Canine Mast Cell Tumors using Fluorescent Polyacrylamide Gel Electrophoresis. J. Vet. Diagn. Investig. 2004, 16, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Brizzola, S.; Stefanello, D.; Crema, S.; Turin, L. A Study of Mutations in the c-kit Gene of 32 Dogs with Mastocytoma. J. Vet. Diagn. Investig. 2005, 17, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, K.; Kawarai, S.; Yasuda, N.; Tanaka, A.; Matsuda, H.; Nishimura, R.; Sasaki, N.; Tsujimoto, H.; Masuda, K. Identification of c-kit mutations-independent neoplastic cell proliferation of canine mast cells. Vet. Immunol. Immunopathol. 2008, 126, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.C. Molecular Diagnostics of Hematologic Malignancies in Small Animals. Vet. Clin. North Am. Small Anim. Pr. 2012, 42, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Book, A.P.; Fidel, J.; Wills, T.; Bryan, J.; Sellon, R.; Mattoon, J. CORRELATION OF ULTRASOUND FINDINGS, LIVER AND SPLEEN CYTOLOGY, AND PROGNOSIS IN THE CLINICAL STAGING OF HIGH METASTATIC RISK CANINE MAST CELL TUMORS. Vet. Radiol. Ultrasound 2011, 52, 548–554. [Google Scholar] [CrossRef]
- Warland, J.; Amores-Fuster, I.; Newbury, W.; Brearley, M.; Dobson, J.M. The utility of staging in canine mast cell tumours. Vet. Comp. Oncol. 2012, 12, 287–298. [Google Scholar] [CrossRef]
- Hughes, J.R.; Szladovits, B.; Drees, R. Abdominal CT evaluation of the liver and spleen for staging mast cell tumors in dogs yields nonspecific results. Vet. Radiol. Ultrasound 2019, 60, 306–315. [Google Scholar] [CrossRef]
- Tuohy, J.L.; Milgram, J.; Worley, D.R.; Dernell, W.S. A review of sentinel lymph node evaluation and the need for its incorporation into veterinary oncology. Vet. Comp. Oncol. 2009, 7, 81–91. [Google Scholar] [CrossRef]
- Krick, E.L.; Billings, A.P.; Shofer, F.S.; Watanabe, S.; Sorenmo, K.U. Cytological lymph node evaluation in dogs with mast cell tumours: Association with grade and survival*. Vet. Comp. Oncol. 2009, 7, 130–138. [Google Scholar] [CrossRef]
- Ku, C.; Kass, P.H.; Christopher, M.M. Cytologic-histologic concordance in the diagnosis of neoplasia in canine and feline lymph nodes: A retrospective study of 367 cases. Vet. Comp. Oncol. 2016, 15, 1206–1217. [Google Scholar] [CrossRef]
- Ferrari, R.; Marconato, L.; Buracco, P.; Boracchi, P.; Giudice, C.; Iussich, S.; Grieco, V.; Chiti, L.E.; Favretto, E.; Stefanello, D. The impact of extirpation of non-palpable/normal-sized regional lymph nodes on staging of canine cutaneous mast cell tumours: A multicentric retrospective study. Vet. Comp. Oncol. 2018, 16, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Worley, D.R. Incorporation of sentinel lymph node mapping in dogs with mast cell tumours: 20 consecutive procedures. Vet. Comp. Oncol. 2012, 12, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lapsley, J.; Hayes, G.M.; Janvier, V.; Newman, A.W.; Peters-Kennedy, J.; Balkman, C.; Sumner, J.P.; Johnson, P. Influence of locoregional lymph node aspiration cytology vs sentinel lymph node mapping and biopsy on disease stage assignment in dogs with integumentary mast cell tumors. Vet. Surg. 2020, 50, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Fournier, Q.; Thierry, F.; Longo, M.; Malbon, A.; Cazzini, P.; Bisson, J.; Woods, S.; Liuti, T.; Bavcar, S. Contrast-enhanced ultrasound for sentinel lymph node mapping in the routine staging of canine mast cell tumours: A feasibility study. Vet. Comp. Oncol. 2020, 19, 451–462. [Google Scholar] [CrossRef]
- Stefanello, D.; Buracco, P.; Sabattini, S.; Finotello, R.; Giudice, C.; Grieco, V.; Iussich, S.; Tursi, M.; Scase, T.; Di Palma, S.; et al. Comparison of 2- and 3-category histologic grading systems for predicting the presence of metastasis at the time of initial evaluation in dogs with cutaneous mast cell tumors: 386 cases (2009–2014). J. Am. Vet. Med. Assoc. 2015, 246, 765–769. [Google Scholar] [CrossRef]
- London, C.A.; Seguin, B. Mast cell tumors in the dog. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 473–489. [Google Scholar] [CrossRef]
- Ozaki, K.; Yamagami, T.; Nomura, K.; Narama, I. Prognostic Significance of Surgical Margin, Ki-67 and Cyclin D1 Protein Expression in Grade II Canine Cutaneous Mast Cell Tumor. J. Vet. Med. Sci. 2007, 69, 1117–1121. [Google Scholar] [CrossRef]
- Hahn, K.A.; King, G.K.; Carreras, J.K. Efficacy of radiation therapy for incompletely resected grade-III mast cell tumors in dogs: 31 cases (1987–1998). J. Am. Vet. Med. Assoc. 2004, 224, 79–82. [Google Scholar] [CrossRef]
- Hayes, A.; Adams, V.; Smith, K.; Maglennon, G.; Murphy, S. Vinblastine and prednisolone chemotherapy for surgically excised grade III canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2007, 5, 168–176. [Google Scholar] [CrossRef]
- Pizzoni, S.; Sabattini, S.; Stefanello, D.; Dentini, A.; Ferrari, R.; Dacasto, M.; Giantin, M.; LaGanga, P.; Amati, M.; Tortorella, G.; et al. Features and prognostic impact of distant metastases in 45 dogs with de novo stage IV cutaneous mast cell tumours: A prospective study. Vet. Comp. Oncol. 2017, 16, 28–36. [Google Scholar] [CrossRef]
- Jaffe, M.H.; Hosgood, G.; Taylor, H.W.; Kerwin, S.C.; Hedlund, C.S.; Lopez, M.K.; Davidson, J.R.; Miller, D.M.; Paranjpe, M. Immunohistochemical and Clinical Evaluation of p53 in Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2000, 37, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cruz, V.S.; Borges, J.C.A.; Nepomuceno, L.L.; Gonçalves, P.A.M.; Prado, Y.C.L.; Bianchi, C.; Fioravanti, M.C.S.; Araújo, E.G. Histological classification and expression of markers of canine mast cell tumors. Vet. World 2020, 13, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Thamm, D.H.; Hamilton, E.; Kiupel, M. Evaluation of prognostic markers for canine mast cell tumors treated with vinblastine and prednisone. BMC Vet. Res. 2008, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Giantin, M.; Capello, K.; Carminato, A.; Morello, E.M.; Vercelli, A.; Granato, A.; Buracco, P.; Dacasto, M.; Mutinelli, F. Expression of Ki67, BCL-2, and COX-2 in Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2012, 50, 110–121. [Google Scholar] [CrossRef]
- Casagrande, T.A.C.; Barros, L.M.D.O.; Fukumasu, H.; Cogliati, B.; Chaible, L.M.; Dagli, M.L.Z.; Matera, J.M. The value of molecular expression of KIT and KIT ligand analysed using real-time polymerase chain reaction and immunohistochemistry as a prognostic indicator for canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Bento, D.D.; Torres-Neto, R.; Werner, J.; Kitchell, B.; Laufer-Amorim, R. Ki67/KIT double immunohistochemical staining in cutaneous mast cell tumors from Boxer dogs. Res. Vet. Sci. 2015, 102, 122–126. [Google Scholar] [CrossRef]
- Bergman, P.J.; Craft, D.M.; Newman, S.J.; Baer, K.; Camps-Palau, M.A.; McKnight, J.A.; Leibman, N.F.; Brenn, S.; Finora, K.; Hohenhaus, A.E.; et al. Correlation of histologic grading of canine mast cell tumors with Ki67/PCNA/AgNOR/c-Kit scores: 38 cases (2002-2003). Vet. Comp. Oncol. 2004, 2, 98. [Google Scholar] [CrossRef]
- Barra, C.N.; Macedo, B.M.; Cadrobbi, K.G.; Pulz, L.H.; Huete, G.C.; Kleeb, S.R.; Xavier, J.G.; Catão-Dias, J.L.; Nishiya, A.T.; Fukumasu, H.; et al. Apoptotic intrinsic pathway proteins predict survival in canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2017, 16, E38–E44. [Google Scholar] [CrossRef]
- Melo, S.R.; Januário, E.V.; Zanuto, E.; De Castro Miranda, B.; Macedo, T.R.; Cogliati, B.; Matera, J.M. Immunohistochemical Expression of Vascular Endothelial Growth Factor as a Prognostic Marker for Canine Mast Cell Tumor. Med. Top. Comp. Anim. 2021, 43, 100506. [Google Scholar] [CrossRef]
- Freytag, J.O.; Queiroz, M.R.; Govoni, V.M.; Pereira, I.V.A.; Pulz, L.H.; Strefezzi, R.D.F.; Queiroga, F.L.; Cogliati, B. Prognostic value of immunohistochemical markers in canine cutaneous mast cell tumours: A systematic review and meta-analysis. Vet. Comp. Oncol. 2021, 19, 529–540. [Google Scholar] [CrossRef]
- Da Silva, L.; Fonseca-Alves, C.E.; Thompson, J.J.; Foster, R.A.; Wood, G.A.; Amorim, R.L.; Coomber, B.L. Pilot assessment of vascular endothelial growth factor receptors and trafficking pathways in recurrent and metastatic canine subcutaneous mast cell tumours. Vet. Med. Sci. 2017, 3, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Camus, M. Diagnosis and Prognosis of Canine Cutaneous Mast Cell Tumors. Vet. Clin. North. Am. Small Anim. Pr. 2019, 49, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; Campos, M.; Lamego, L.; Magalhães, D.; Menezes, R.; Oliveira, R.; Patanita, F.; Ferreira, D.A. Canine and feline cutaneous mast cell tumour: A comprehensive review of treatments and outcomes. Top. Companion Anim. Med. 2020, 41, 100472. [Google Scholar] [CrossRef] [PubMed]
- Pratschke, K.M.; Atherton, M.J.; Sillito, J.A.; Lamm, C.G. Evaluation of a modified proportional margins approach for surgical resection of mast cell tumors in dogs: 40 cases (2008–2012). J. Am. Vet. Med. Assoc. 2013, 243, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.L.; Hayes, G.M.; Henry, J.G.; Oblak, M.L. Comparison of lateral surgical margins of up to two centimeters with margins of three centimeters for achieving tumor-free histologic margins following excision of grade I or II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2020, 256, 567–572. [Google Scholar] [CrossRef]
- Saunders, H.; Thomson, M.J.; O’Connell, K.; Bridges, J.P.; Chau, L. Evaluation of a modified proportional margin approach for complete surgical excision of canine cutaneous mast cell tumours and its association with clinical outcome. Vet. Comp. Oncol. 2020, 19, 604–615. [Google Scholar] [CrossRef]
- Selmic, L.E.; Ruple, A. A systematic review of surgical margins utilized for removal of cutaneous mast cell tumors in dogs. BMC Vet. Res. 2020, 16, 1–6. [Google Scholar] [CrossRef]
- Itoh, T.; Kojimoto, A.; Uchida, K.; Chambers, J.; Shii, H. Long-term postsurgical outcomes of mast cell tumors resected with a margin proportional to the tumor diameter in 23 dogs. J. Vet. Med. Sci. 2021, 83, 230–233. [Google Scholar] [CrossRef]
- Simpson, A.M.; Ludwig, L.L.; Newman, S.J.; Bergman, P.J.; Hottinger, H.A.; Patnaik, A.K. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 236–240. [Google Scholar] [CrossRef]
- Donnelly, L.; Mullin, C.; Balko, J.; Goldschmidt, M.; Krick, E.; Hume, C.; Brown, D.C.; Sorenmo, K. Evaluation of histological grade and histologically tumour-free margins as predictors of local recurrence in completely excised canine mast cell tumours. Vet. Comp. Oncol. 2013, 13, 70–76. [Google Scholar] [CrossRef]
- Ferrari, R.; Chiti, L.E.; Manfredi, M.; Ravasio, G.; De Zani, D.; Zani, D.D.; Giudice, C.; Gambini, M.; Dvm, D.S. Biopsy of sentinel lymph nodes after injection of methylene blue and lymphoscintigraphic guidance in 30 dogs with mast cell tumors. Vet. Surg. 2020, 49, 1099–1108. [Google Scholar] [CrossRef]
- Marconato, L.; Polton, G.; Stefanello, D.; Morello, E.; Ferrari, R.; Henriques, J.; Tortorella, G.; Benali, S.L.; Bergottini, R.; Vasconi, M.E.; et al. Therapeutic impact of regional lymphadenectomy in canine stage II cutaneous mast cell tumours. Vet. Comp. Oncol. 2018, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Fossum, T.W. Cirurgia de pequenos animais; Elsevier: Centro Rio de Janeiro, Brazil, 2021; pp. 546–596. [Google Scholar]
- Milovancev, M.; Townsend, K.L.; Tuohy, J.L.; Gorman, E.; Bracha, S.; Curran, K.M.; Russell, D.S. Long-term outcomes of dogs undergoing surgical resection of mast cell tumors and soft tissue sarcomas: A prospective 2-year-long study. Vet. Surg. 2019, 49, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Karbe, G.T.; Davis, E.; Runge, J.J.; Brown, D.C.; Holt, D.E. Evaluation of scar revision after inadequate primary excision of cutaneous mast cell tumors in 85 dogs (2000–2013). Vet. Surg. 2021, 50, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Belehradek, J.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer Clin. Oncol. 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Mir, L.; Glass, L.; Serša, G.; Teissié, J.; Domenge, C.; Miklavčič, D.; Jaroszeski, M.; Orlowski, S.; Reintgen, D.; Rudolf, Z.; et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br. J. Cancer 1998, 77, 2336–2342. [Google Scholar] [CrossRef]
- Tozon, N.; Tratar, U.L.; Znidar, K.; Sersa, G.; Teissie, J.; Cemazar, M. Operating Procedures of the Electrochemotherapy for Treatment of Tumor in Dogs and Cats. J. Vis. Exp. 2016, e54760. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Suzuki, D.O.; Anselmo, J.; de Oliveira, K.D.; Freytag, J.O.; Rangel, M.M.; Marques, J.L.; Ramos, A. Numerical Model of Dog Mast Cell Tumor Treated by Electrochemotherapy. Artif. Organs 2014, 39, 192–197. [Google Scholar] [CrossRef]
- Pintarelli, G.; Berkenbrock, J.; Rassele, A.; Rangel, M.; Suzuki, D. Computer simulation of commercial conductive gels and their application to increase the safety of electrochemotherapy treatment. Med. Eng. Phys. 2019, 74, 99–105. [Google Scholar] [CrossRef]
- Gerlini, G.; Di Gennaro, P.; Borgognoni, L. Enhancing anti-melanoma immunity by electrochemotherapy and in vivo dendritic-cell activation. OncoImmunology 2012, 1, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. OncoImmunology 2014, 3, e28131. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Baldi, A. Electrochemotherapy in Veterinary Oncology. Vet. Clin. North. Am. Small Anim. Pr. 2019, 49, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Vincenzi, B.; Baldi, F.; Citro, G.; Baldi, A. Adjuvant electrochemotherapy for the treatment of incompletely resected canine mast cell tumors. Anticancer Res. 2007, 26, 4585–4589. [Google Scholar]
- Kodre, V.; Cemazar, M.; Pecar, J.; Sersa, G.; Cor, A.; Tozon, N. Electrochemotherapy Compared to Surgery for Treatment of Canine Mast Cell Tumours. Vivo 2009, 23, 55–62. [Google Scholar]
- Spugnini, E.; Vincenzi, B.; Citro, G.; Dotsinsky, I.; Mudrov, T.; Baldi, A. Evaluation of Cisplatin as an Electrochemotherapy Agent for the Treatment of Incompletely Excised Mast Cell Tumors in Dogs. J. Vet. Intern. Med. 2011, 25, 407–411. [Google Scholar] [CrossRef]
- Lowe, R.; Gavazza, A.; Impellizeri, J.A.; Soden, D.M.; Lubas, G. The treatment of canine mast cell tumours with electrochemotherapy with or without surgical excision. Vet. Comp. Oncol. 2016, 15, 775–784. [Google Scholar] [CrossRef]
- Cemazar, M.; Avgustin, J.A.; Pavlin, D.; Sersa, G.; Poli, A.; Levacic, A.K.; Tesic, N.; Tratar, U.L.; Rak, M.; Tozon, N. Efficacy and safety of electrochemotherapy combined with peritumoral IL-12 gene electrotransfer of canine mast cell tumours. Vet. Comp. Oncol. 2016, 15, 641–654. [Google Scholar] [CrossRef]
- Salvadori, C.; Svara, T.; Rocchigiani, G.; Millanta, F.; Pavlin, D.; Cemazar, M.; Tratar, U.L.; Sersa, G.; Tozon, N.; Poli, A. Effects of electrochemotherapy with cisplatin and peritumoral IL-12 gene electrotransfer on canine mast cell tumors: A histopathologic and immunohistochemical study. Radiol. Oncol. 2017, 51, 286–294. [Google Scholar] [CrossRef]
- Dobson, J.M.; Scase, T.J. Advances in the diagnosis and management of cutaneous mast cell tumours in dogs. J. Small Anim. Pr. 2007, 48, 424–431. [Google Scholar] [CrossRef]
- Dobson, J.; Cohen, S.; Gould, S. Treatment of canine mast cell tumours with prednisolone and radiotherapy. Vet. Comp. Oncol. 2004, 2, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraf, R.; Mauldin, G.N.; Patnaik, A.K.; Meleo, K.A. A Prospective Study of Radiation Therapy for the Treatment of Grade 2 Mast Cell Tumors in 32 Dogs. J. Vet. Intern. Med. 1996, 10, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Frimberger, A.; Moore, A.S.; LaRue, S.M.; Gliatto, J.M.; Bengtson, A.E. Radiotherapy of incompletely resected, moderately differentiated mast cell tumors in the dog: 37 cases (1989-1993). J. Am. Anim. Hosp. Assoc. 1997, 33, 320–324. [Google Scholar] [CrossRef] [PubMed]
- LaDue, T.; Price, G.S.; Dodge, R.; Page, R.L.; Thrall, D.E. Radiation therapy for incompletely resected canine mast cell tumors. Vet. Radiol. Ultrasound 1998, 39, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Poirier, V.J.; Adams, W.M.; Forrest, L.J.; Green, E.M.; Dubielzig, R.R.; Vail, D.M. Radiation Therapy for Incompletely Excised Grade II Canine Mast Cell Tumors. J. Am. Anim. Hosp. Assoc. 2006, 42, 430–434. [Google Scholar] [CrossRef]
- Kry, K.L.; Boston, S.E. Additional Local Therapy With Primary Re-Excision or Radiation Therapy Improves Survival and Local Control After Incomplete or Close Surgical Excision of Mast Cell Tumors in Dogs. Vet. Surg. 2014, 43, 182–189. [Google Scholar] [CrossRef]
- Chaffin, K.; Thrall, D.E. Results of radiation therapy in 19 dogs with cutaneous mast cell tumor and regional lymph node metastasis. Vet. Radiol. Ultrasound 2002, 43, 392–395. [Google Scholar] [CrossRef]
- Lejeune, A.; Skorupski, K.; Frazier, S.; Vanhaezebrouck, I.; Rebhun, R.B.; Reilly, C.M.; Rodriguez, C.O., Jr. Aggressive local therapy combined with systemic chemotherapy provides long-term control in grade II stage 2 canine mast cell tumour: 21 cases (1999–2012). Vet. Comp. Oncol. 2013, 13, 267–280. [Google Scholar] [CrossRef]
- Mendez, S.E.; Drobatz, K.J.; Duda, L.E.; White, P.; Kubicek, L.; Sorenmo, K.U. Treating the locoregional lymph nodes with radiation and/or surgery significantly improves outcome in dogs with high-grade mast cell tumours. Vet. Comp. Oncol. 2019, 18, 239–246. [Google Scholar] [CrossRef]
- Carlsten, K.; London, C.A.; Haney, S.; Burnett, R.C.; Avery, A.C.; Thamm, D. Multicenter Prospective Trial of Hypofractionated Radiation Treatment, Toceranib, and Prednisone for Measurable Canine Mast Cell Tumors. J. Vet. Intern. Med. 2011, 26, 135–141. [Google Scholar] [CrossRef]
- Stiborova, K.; Treggiari, E.; Amores-Fuster, I.; Del Busto, I.; Killick, D.; Maddox, T.; Marrington, M.; Mason, S.L.; Blackwood, L. Haematologic toxicity in dogs with mast cell tumours treated with vinblastine/prednisolone chemotherapy with/without radiotherapy. J. Small Anim. Pr. 2019, 60, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Boyle, G.M.; D’Souza, M.M.A.; Pierce, C.J.; Adams, R.A.; Cantor, A.S.; Johns, J.P.; Maslovskaya, L.; Gordon, V.A.; Reddell, P.; Parsons, P. Intra-Lesional Injection of the Novel PKC Activator EBC-46 Rapidly Ablates Tumors in Mouse Models. PLoS ONE 2014, 9, e108887. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Campbell, J.; Blum, A.; Reddell, P.; Gordon, V.; Schmidt, P.; Lowden, S. Dose Characterization of the Investigational Anticancer Drug Tigilanol Tiglate (EBC-46) in the Local Treatment of Canine Mast Cell Tumors. Front. Vet. Sci. 2019, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.D.; Campbell, J.E.; Brown, G.; Johannes, C.M.; Reddell, P. Recurrence-free interval 12 months after local treatment of mast cell tumors in dogs using intratumoral injection of tigilanol tiglate. J. Vet. Intern. Med. 2020, 35, 451–455. [Google Scholar] [CrossRef]
- Reddell, P.; De Ridder, T.R.; Morton, J.M.; Jones, P.D.; Campbell, J.E.; Brown, G.; Johannes, C.M.; Schmidt, P.F.; Gordon, V. Wound formation, wound size, and progression of wound healing after intratumoral treatment of mast cell tumors in dogs with tigilanol tiglate. J. Vet. Intern. Med. 2021, 35, 430–441. [Google Scholar] [CrossRef]
- De Ridder, T.R.; Campbell, J.E.; Burke-Schwarz, C.; Clegg, D.; Elliot, E.L.; Geller, S.; Kozak, W.; Pittenger, S.T.; Pruitt, J.B.; Riehl, J.; et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC -46). J. Vet. Intern. Med. 2020, 35, 415–429. [Google Scholar] [CrossRef]
- Brown, G.K.; Campbell, J.E.; Jones, P.D.; De Ridder, T.R.; Reddell, P.; Johannes, C.M. Intratumoural Treatment of 18 Cytologically Diagnosed Canine High-Grade Mast Cell Tumours With Tigilanol Tiglate. Front. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Burton, J.; Venable, R.; Vail, D.; Williams, L.; Clifford, C.; Axiak-Bechtel, S.; Avery, A.; Thamm, D. Pulse-Administered Toceranib Phosphate Plus Lomustine for Treatment of Unresectable Mast Cell Tumors in Dogs. J. Vet. Intern. Med. 2015, 29, 1098–1104. [Google Scholar] [CrossRef]
- Stanclift, R.M.; Gilson, S.D. Evaluation of neoadjuvant prednisone administration and surgical excision in treatment of cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 53–62. [Google Scholar] [CrossRef]
- Taylor, F.; Gear, R.; Hoather, T.; Dobson, J. Chlorambucil and prednisolone chemotherapy for dogs with inoperable mast cell tumours: 21 cases. J. Small Anim. Pr. 2009, 50, 284–289. [Google Scholar] [CrossRef]
- Rassnick, K.M.; Bailey, D.B.; Russell, D.S.; Flory, A.B.; Kiselow, M.A.; Intile, J.; Malone, E.K.; Balkman, C.E.; Barnard, S.M. A phase II study to evaluate the toxicity and efficacy of alternating CCNU and high-dose vinblastine and prednisone (CVP) for treatment of dogs with high-grade, metastatic or nonresectable mast cell tumours. Vet. Comp. Oncol. 2010, 8, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Govier, S.M. Principles of treatment for mast cell tumors. Clin. Tech. Small Anim. Pr. 2003, 18, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Horta, R.; LaValle, G.; Costa, M.; Moura, L.; Marcinowska, A.; Araújo, R. Outcome of adjuvant chemotherapy with lomustine, vinblastine and chlorambucil on management of canine mast cell tumour of high to intermediate risk. Arq. Bras. Med. Vet. Zootec. 2017, 69, 1426–1436. [Google Scholar] [CrossRef]
- Davies, D.R.; Wyatt, K.M.; Jardine, J.E.; Robertson, I.D.; Irwin, P. Vinblastine and Prednisolone as Adjunctive Therapy for Canine Cutaneous Mast Cell Tumors. J. Am. Anim. Hosp. Assoc. 2004, 40, 124–130. [Google Scholar] [CrossRef]
- Trumel, C.; Bourgès-Abella, N.; Touron, C.; Lanore, D.; Geffré, A.; Diquelou, A.; Guelfi, J.F.; Braun, J.P. Adverse Haematological Effects of Vinblastine, Prednisolone and Cimetidine Treatment: A Retrospective Study in Fourteen Dogs with Mast Cell Tumours. J. Vet. Med. Ser. A 2005, 52, 275–279. [Google Scholar] [CrossRef]
- Thamm, D.; Turek, M.; Vail, D. Outcome and Prognostic Factors Following Adjuvant Prednisone/Vinblastine Chemotherapy for High-Risk Canine Mast Cell Tumour: 61 Cases. J. Vet. Med. Sci. 2006, 68, 581–587. [Google Scholar] [CrossRef]
- Rosenthal, R.C. Clinical applications of Vinca alkaloids. J. Am. Vet. Med. Assoc. 1981, 179. [Google Scholar]
- Golden, D.L.; Langston, V. Uses of vincristine and vinblastine in dogs and cats. J. Am. Vet. Med. Assoc. 1988, 193. [Google Scholar]
- Rungsipipat, A.; Srichat, W.; Manachai, N.; Jearanai, W.; Wangnaitham, S.; Tangkawattana, P.; Tangkawattana, S. Clinical evaluation of canine mast cell tumor treatment between combined vinblastine and prednisolone and single prednisolone. Comp. Clin. Path. 2009, 18, 77–84. [Google Scholar] [CrossRef]
- Vickery, K.R.; Wilson, H.; Vail, D.M.; Thamm, D.H. Dose-escalating vinblastine for the treatment of canine mast cell tumour. Vet. Comp. Oncol. 2008, 6, 111–119. [Google Scholar] [CrossRef]
- Rassnick, K.; Bailey, D.; Flory, A.; Balkman, C.; Kiselow, M.; Intile, J.; Autio, K. Efficacy of Vinblastine for Treatment of Canine Mast Cell Tumors. J. Vet. Intern. Med. 2008, 22, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.C.S.; Pecceu, E.; Handel, I.; Lawrence, J. Tolerability of a rapid-escalation vinblastine-prednisolone protocol in dogs with mast cell tumours. Vet. Med. Sci. 2016, 2, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.; Rassnick, K.; Kristal, O.; Chretin, J.; Balkman, C. Phase I Dose Escalation of Single-Agent Vinblastine in Dogs. J. Vet. Intern. Med. 2008, 22, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Kluthcovsky, L.C.; Firmo, B.F.; Cassino, P.C.; De Nardi, A.B.; Castro, J.L.C.; Halila, R.L.; Filho, J.R.E. Comparison of Two Different Vinblastine Dosages for Treatment of Cutaneous Mast Cell Tumor in Dogs. Acta Sci. Vet. 2020, 48. [Google Scholar] [CrossRef]
- Hosoya, K.; Kisseberth, W.C.; Alvarez, F.J.; Lara-Garcia, A.; Beamer, G.L.; Stromberg, P.C.; Couto, C.G. Adjuvant CCNU (Lomustine) and Prednisone Chemotherapy for Dogs With Incompletely Excised Grade 2 Mast Cell Tumors. J. Am. Anim. Hosp. Assoc. 2009, 45, 14–18. [Google Scholar] [CrossRef]
- Hay, J.K.; Larson, V.S. Lomustine (CCNU) and prednisone chemotherapy for high-grade completely excised canine mast cell tumors. Can. Vet. J. = La Rev. Vet. Can. 2019, 60, 1326–1330. [Google Scholar]
- Cooper, M.; Tsai, X.; Bennett, P. Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Vet. Comp. Oncol. 2009, 7, 196–206. [Google Scholar] [CrossRef]
- Teng, S.-P.; Hsu, W.-L.; Chiu, C.-Y.; Wong, M.-L.; Chang, S.-C. Overexpression of P-glycoprotein, STAT3, phospho-STAT3 and KIT in spontaneous canine cutaneous mast cell tumours before and after prednisolone treatment. Vet. J. 2012, 193, 551–556. [Google Scholar] [CrossRef]
- Linde, K.J.; Stockdale, S.L.; Mison, M.B.; Perry, J.A. The effect of prednisone on histologic and gross characteristics in canine mast cell tumors. Can. Vet. J. = La Rev. Vet. Can. 2021, 62, 45–50. [Google Scholar]
- Horta, R.D.S.; Lavalle, G.E.; Monteiro, L.N.; dos Reis, F.A.; Costa, M.D.P.; Giuliano, A.; Cassali, G.D.; Dobson, J. Evaluation of Histological, Immunohistochemical, Clinical and Genetic Prognostic Factors Associated with the Response of Canine Mast Cell Tumours to Glucocorticotherapy. J. Comp. Pathol. 2018, 165, 72–81. [Google Scholar] [CrossRef]
- Ginn, P.E.; Fox, L.E.; Brower, J.C.; Gaskin, A.; Kurzman, I.D.; Kubilis, P.S. Immunohistochemical Detection of p53 Tumor-Suppressor Protein is a Poor Indicator of Prognosis for Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2000, 37, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Tojo, E.; Oishi, A.; Fujiki, M.; Misumi, K.; Sakamoto, H.; Kameyama, K.; Shimizu, T.; Yasuda, N. Immunohistochemical detection of P-glycoprotein (PGP) and multidrug resistance-associated protein (MRP) in canine cutaneous mast cell tumors. J. Vet. Med. Sci. 2002, 64, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Nakaichi, M.; Takeshita, Y.; Okuda, M.; Nakamoto, Y.; Itamoto, K.; Une, S.; Sasaki, N.; Kadosawa, T.; Takahashi, T.; Taura, Y. Expression of the MDR1 Gene and P-Glycoprotein in Canine Mast Cell Tumor Cell Lines. J. Vet. Med. Sci. 2007, 69, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Rocca, A.; Sandri, M.T.; Zorzino, L.; Masci, G.; Nolè, F.; Peruzzotti, G.; Robertson, C.; Orlando, L.; Cinieri, S.; et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: Antitumor activity and correlation with vascular endothelial growth factor levels. Ann. Oncol. 2002, 13, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Kavallaris, M.; André, N. Metronomic chemotherapy: New rationale for new directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef]
- Tripp, C.; Fidel, J.; Anderson, C.; Patrick, M.; Pratt, C.; Sellon, R.; Bryan, J. Tolerability of Metronomic Administration of Lomustine in Dogs with Cancer. J. Vet. Intern. Med. 2011, 25, 278–284. [Google Scholar] [CrossRef]
- Leach, T.N.; Childress, M.O.; Greene, S.N.; Mohamed, A.S.; Moore, G.E.; Schrempp, D.R.; Lahrman, S.R.; Knapp, D.W. Prospective trial of metronomic chlorambucil chemotherapy in dogs with naturally occurring cancer. Vet. Comp. Oncol. 2011, 10, 102–112. [Google Scholar] [CrossRef]
- Gaspar, T.B.; Henriques, J.; Marconato, L.; Queiroga, F.L. The use of low-dose metronomic chemotherapy in dogs-insight into a modern cancer field. Vet. Comp. Oncol. 2017, 16, 2–11. [Google Scholar] [CrossRef]
- Thamm, D.H.; Mauldin, E.A.; Vail, D.M. Prednisone and vinblastine chemotherapy for canine mast cell tumor—41 cases (1992–1997). J. Vet. Intern. Med. 1999, 13, 491–497. [Google Scholar]
- Camps-Palau, M.A.; Leibman, N.F.; Elmslie, R.; Lana, S.E.; Plaza, S.; McKnight, J.A.; Risbon, R.; Bergman, P.J. Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997?2004). Vet. Comp. Oncol. 2007, 5, 156–167. [Google Scholar] [CrossRef]
- Rassnick, K.M.; Moore, A.S.; Williams, L.E.; London, C.A.; Kintzer, P.P.; Engler, S.J.; Cotter, S.M. Treatment of Canine Mast Cell Tumors with CCNU (Lomustine). J. Vet. Intern. Med. 1999, 13, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.A.; Thomson, M.; O’Connell, K.; Wyatt, K. Combination vinblastine, prednisolone and toceranib phosphate for treatment of grade II and III mast cell tumours in dogs. Vet. Med. Sci. 2018, 4, 237–251. [Google Scholar] [CrossRef]
- London, C.A. Tyrosine Kinase Inhibitors in Veterinary Medicine. Top. Companion Anim. Med. 2009, 24, 106–112. [Google Scholar] [CrossRef]
- Soria, J.; Massard, C.; Magné, N.; Bader, T.; Mansfield, C.; Blay, J.; Bui, B.; Moussy, A.; Hermine, O.; Armand, J. Phase 1 dose-escalation study of oral tyrosine kinase inhibitor masitinib in advanced and/or metastatic solid cancers. Eur. J. Cancer 2009, 45, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.; Ahn, A. Masitinib–the efficacy of targeted therapy in veterinary medicine. Vet. Cancer Soc. Newsl. Summer 2010, 34, 6–11. [Google Scholar]
- Hahn, K.A.; Legendre, A.M.; Shaw, N.G.; Phillips, B.; Ogilvie, G.K.; Prescott, D.M.; Atwater, S.W.; Carreras, J.K.; Lana, S.E.; LaDue, T.; et al. Evaluation of 12- and 24-month survival rates after treatment with masitinib in dogs with nonresectable mast cell tumors. Am. J. Vet. Res. 2010, 71, 1354–1361. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin. Cancer Res. 2003, 9, 2755–2768. [Google Scholar]
- Weishaar, K.; Ehrhart, E.; Avery, A.; Charles, J.; Elmslie, R.; Vail, D.; London, C.; Clifford, C.; Eickhoff, J.; Thamm, D. c-Kit Mutation and Localization Status as Response Predictors in Mast Cell Tumors in Dogs Treated with Prednisone and Toceranib or Vinblastine. J. Vet. Intern. Med. 2017, 32, 394–405. [Google Scholar] [CrossRef]
- Mitchell, L.; Thamm, D.; Biller, B. Clinical and Immunomodulatory Effects of Toceranib Combined with Low-Dose Cyclophosphamide in Dogs with Cancer. J. Vet. Intern. Med. 2012, 26, 355–362. [Google Scholar] [CrossRef]
- Amagai, Y.; Tanaka, A.; Jung, K.; Matsuda, A.; Oida, K.; Nishikawa, S.; Jang, H.; Ishizaka, S.; Matsuda, H. Production of stem cell factor in canine mast cell tumors. Res. Vet. Sci. 2013, 96, 124–126. [Google Scholar] [CrossRef]
- Horta, R.D.S.; Giuliano, A.; LaValle, G.E.; Costa, M.D.P.; De Ara�Jo, R.; Constantino-Casas, F.; Dobson, J.M. Clinical, histological, immunohistochemical and genetic factors associated with measurable response of high-risk canine mast cell tumours to tyrosine kinase inhibitors. Oncol. Lett. 2017, 15, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Smrkovski, O.A.; Essick, L.; Rohrbach, B.W.; Legendre, A.M. Masitinib mesylate for metastatic and non-resectable canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2013, 13, 314–321. [Google Scholar] [CrossRef] [PubMed]
- London, C.; Mathie, T.; Stingle, N.; Clifford, C.; Haney, S.; Klein, M.K.; Beaver, L.; Vickery, K.; Vail, D.M.; Hershey, B.; et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet. Comp. Oncol. 2012, 10, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.; North, S.; Lanore, D. Clinical response of masitinib mesylate in the treatment of canine macroscopic mast cell tumours. J. Small Anim. Pr. 2016, 57, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Robat, C.; London, C.; Bunting, L.; McCartan, L.; Stingle, N.; Selting, K.; Kurzman, I.; Vail, D.M. Safety evaluation of combination vinblastine and toceranib phosphate (Palladia®) in dogs: A phase I dose-finding study. Vet. Comp. Oncol. 2011, 10, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Tsimbas, K.; Kurzman, I.D.; Vail, D.M. Safety evaluation of combination CCNU and continuous toceranib phosphate (Palladia®) in tumour-bearing dogs: A phase I dose-finding study. Vet. Comp. Oncol. 2014, 14, 202–209. [Google Scholar] [CrossRef]
- Bavcar, S.; de Vos, J.; Kessler, M.; de Fornel, P.; Buracco, P.; Murphy, S.; Hirschberger, J.; Argyle, D. Combination toceranib and lomustine shows frequent high grade toxicities when used for treatment of non-resectable or recurrent mast cell tumours in dogs: A European multicentre study. Vet. J. 2017, 224, 1–6. [Google Scholar] [CrossRef]
- Gieger, T.; Northrup, N.; Wall, M. Clinical management of mast cell tumors in dogs. Compend. Contin. Educ. Pract. Vet. 2005, 27, 56–68. [Google Scholar]
- Peters, L.J.; Kovacic, J.P. Histamine: Metabolism, physiology, and pathophysiology with applications in veterinary medicine. J. Vet. Emerg. Crit. Care 2009, 19, 311–328. [Google Scholar] [CrossRef]
- Guedes, A.G.; Rudé, E.P.; Rider, M.A. Evaluation of histamine release during constant rate infusion of morphine in dogs. Vet. Anaesth. Analg. 2006, 33, 28–35. [Google Scholar] [CrossRef]
- O’Keefe, D.A. Canine Mast Cell Tumors. Vet. Clin. North. Am. Small Anim. Pr. 1990, 20, 1105–1115. [Google Scholar] [CrossRef]
- Pavlin, D.; Cemazar, M.; Cör, A.; Sersa, G.; Pogacnik, A.; Tozon, N. Electrogene therapy with interleukin-12 in canine mast cell tumors. Radiol. Oncol. 2011, 45, 31–39. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Fenger, J.; Murahari, S.; Bear, M.D.; Kulp, S.K.; Wang, D.; Chen, C.-S.; Kisseberth, W.C.; London, C.A. AR-42, a novel HDAC inhibitor, exhibits biologic activity against malignant mast cell lines via down-regulation of constitutively activated Kit. Blood 2010, 115, 4217–4225. [Google Scholar] [CrossRef]
- Nagamine, M.K.; Sanches, D.S.; Pinello, K.C.; Torres, L.N.; Mennecier, G.; Latorre, A.O.; Fukumasu, H.; Dagli, M.L.Z. In vitro inhibitory effect of trichostatin A on canine grade 3 mast cell tumor. Vet. Res. Commun. 2011, 35, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Wingelhofer, B.; Peter, B.; Bauer, K.; Berger, D.; Gamperl, S.; Reifinger, M.; Cerny-Reiterer, S.; Moriggl, R.; Willmann, M.; et al. The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma. Vet. Comp. Oncol. 2017, 16, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Cletzer, E.; Klahn, S.; Dervisis, N.; LeRoith, T. Identification of the JAK-STAT pathway in canine splenic hemangiosarcoma, thyroid carcinoma, mast cell tumor, and anal sac adenocarcinoma. Vet. Immunol. Immunopathol. 2019, 220, 109996. [Google Scholar] [CrossRef]
| Stage | Description |
|---|---|
| 0 | One tumor incompletely excised from the dermis, identified histologically, without regional lymph node involvement. a. Without systemic signs. b. With systemic signs. |
| I | One tumor confined to the dermis, without regional lymph node involvement. a. Without systemic signs. b. With systemic signs. |
| II | One tumor confined to the dermis, with regional lymph node involvement. a. Without systemic signs. b. With systemic signs. |
| III | Multiple dermal tumors; large, infiltrating tumors with or without regional lymph node involvement. a. Without systemic signs. b. With systemic signs. |
| IV | Any tumor with distant metastasis, including blood or bone marrow involvement. |
| Stage | Tumor | Lymph Node | Metastasis |
|---|---|---|---|
| I | Single nodule, <3 cm, well circumscribed. | − | − |
| II | +1 nodule, <3 cm, intralesional distance > 10 cm, well circumscribed. | − | − |
| III | 1 or + nodule, >3 cm, intralesional distance < 10 cm, poorly circumscribed or ulcerated. | − | − |
| IV | Any type of lesion. | + | − |
| V | Any type of lesion. | + or − | + |
| Stage | Description |
|---|---|
| I | Single tumor, without regional lymph node involvement. |
| II | Multiple tumors (≥ 3), without regional lymph node involvement. |
| III | Single tumor, with regional lymph node involvement. |
| IV | Large and infiltrative tumors, without delimitation, or multiple tumors (≥ 3), with regional lymph node involvement. |
| V | Any tumor with distant metastasis, including bone marrow invasion and the presence of mast cells in the peripheral blood. |
| Prognostic Factor | Positive | Negative | Reference |
|---|---|---|---|
| Histopathological Grading | Grade 1–low Grade | Grade 3–high Grade | Patnaik et al. [47,48]; Kiupel et al. [80] |
| History of Tumor Recurrence | First clinical presentation | Recurrent tumor | Horta et al. [11] |
| Tumor Size | Higher diameter < 3 cm | Higher diameter > 3 cm | Hahn et al. [134] |
| Metastasis (regional and/or distance) | Absent | Present | Hume et al. [51]; Book et al. [121]; Warland et al. [7,122] |
| Surgical Margins | Free margins | Contaminated margins | Ozaki et al. [133] |
| Mitotic Count | <5 or <7 | >5 or >7 | Romansik et al. [92]; Elston et al. [93] |
| KIT Pattern | KIT 1 | KIT 2 and 3 | Kiupel et al. [19] |
| c-KIT Mutationc-KIT Mutation | Absent | Present | Webster et al. [32,35,36] |
| Ki67 Index | <23 | >23 | Webster et al. [32,139] |
| Chemotherapeutic Protocol | n | Histopathological Grading | Overall Survival Median |
|---|---|---|---|
| Prednisolone and Vinblastine [168,169] | 61 (a, b) | a. Grade 2/3 b. Grade 3 | a. 1374 days b. 800 days |
| Prednisone and Vinblastine [179,180] | 41 | Grade III and Grade 2/High grade | 904 days |
| Prednisolone, Vinblastine and Lomustine [149,150] | 21 | Grade 2 | 1359 days |
| Lomustine and Vinblastine [180,181] | 20 | Grade 2/3 | 336 days |
| Chemotherapeutic Protocol | n | RR (C + P) |
|---|---|---|
| Prednisone/Prednisolone [216] | 60 | 63% |
| Prednisone [195,196] | 49 | 70% |
| Prednisone [215,216] | 16 | 63% |
| Prednisone and Vinblastine [225,226] | 41 | 47% |
| Prednisolone, Vinblastine, and Cyclophosphamide [226,227] | 35 | 63% |
| Prednisone, Vinblastine, and Lomustine [197,198] | 35 | 65% |
| Prednisone, Vinblastine, and Lomustine [213,214] | 56 | 57% |
| Lomustine [227,228] | 19 | 40% |
| Chlorambucil and Prednisolone [196,197] | 21 | 38% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Nardi, A.B.; dos Santos Horta, R.; Fonseca-Alves, C.E.; de Paiva, F.N.; Linhares, L.C.M.; Firmo, B.F.; Ruiz Sueiro, F.A.; de Oliveira, K.D.; Lourenço, S.V.; De Francisco Strefezzi, R.; et al. Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors. Cells 2022, 11, 618. https://doi.org/10.3390/cells11040618
de Nardi AB, dos Santos Horta R, Fonseca-Alves CE, de Paiva FN, Linhares LCM, Firmo BF, Ruiz Sueiro FA, de Oliveira KD, Lourenço SV, De Francisco Strefezzi R, et al. Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors. Cells. 2022; 11(4):618. https://doi.org/10.3390/cells11040618
Chicago/Turabian Stylede Nardi, Andrigo Barboza, Rodrigo dos Santos Horta, Carlos Eduardo Fonseca-Alves, Felipe Noleto de Paiva, Laís Calazans Menescal Linhares, Bruna Fernanda Firmo, Felipe Augusto Ruiz Sueiro, Krishna Duro de Oliveira, Silvia Vanessa Lourenço, Ricardo De Francisco Strefezzi, and et al. 2022. "Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors" Cells 11, no. 4: 618. https://doi.org/10.3390/cells11040618
APA Stylede Nardi, A. B., dos Santos Horta, R., Fonseca-Alves, C. E., de Paiva, F. N., Linhares, L. C. M., Firmo, B. F., Ruiz Sueiro, F. A., de Oliveira, K. D., Lourenço, S. V., De Francisco Strefezzi, R., Brunner, C. H. M., Rangel, M. M. M., Jark, P. C., Castro, J. L. C., Ubukata, R., Batschinski, K., Sobral, R. A., da Cruz, N. O., Nishiya, A. T., ... Dagli, M. L. Z. (2022). Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors. Cells, 11(4), 618. https://doi.org/10.3390/cells11040618











