Synergistic Cytokine Production by ATP and PGE2 via P2X4 and EP3 Receptors in Mouse Bone-Marrow-Derived Mast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Cell Culture
2.4. Measurement of Cytokine Secretion
2.5. Quantitative RT-PCR
2.6. Western Blotting
2.7. Statistics
3. Results
3.1. Effects of ATP Combined with PGE2 and Ag on Cytokine mRNA Expression and Secretion in BMMCs
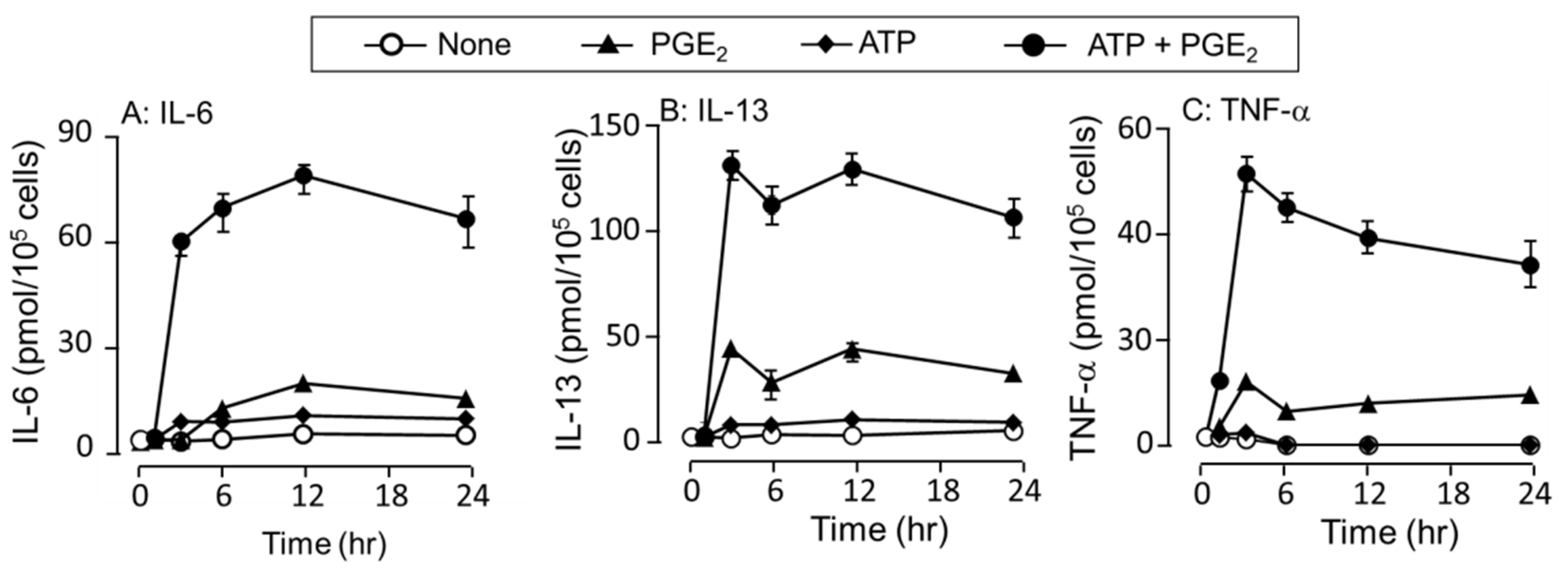
3.2. Time-Dependent Increase in IL-6 and TNF-α Secretion in Response to ATP, PGE2, and Their Co-Stimulation
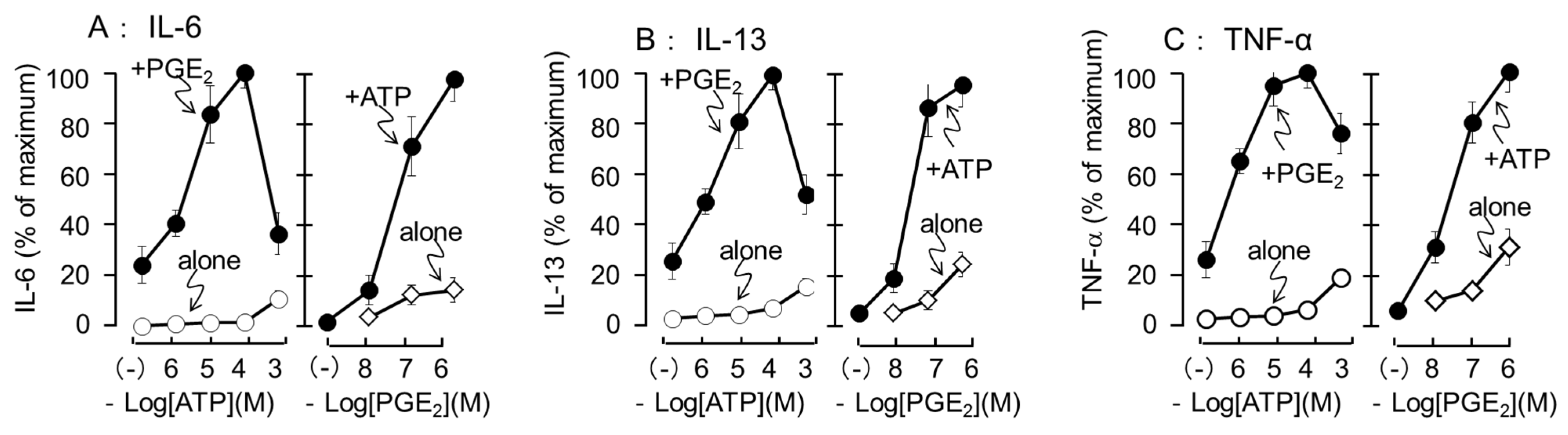
3.3. Effects of Different Concentrations of ATP and PGE2 on IL-6, IL-13, and TNF-α Secretion
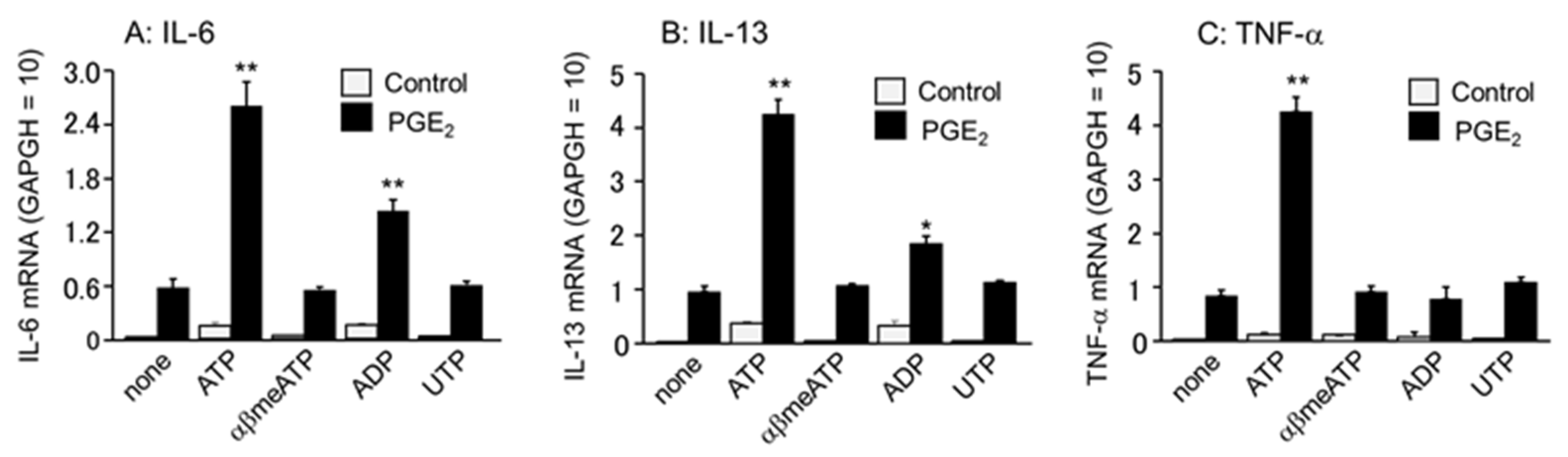
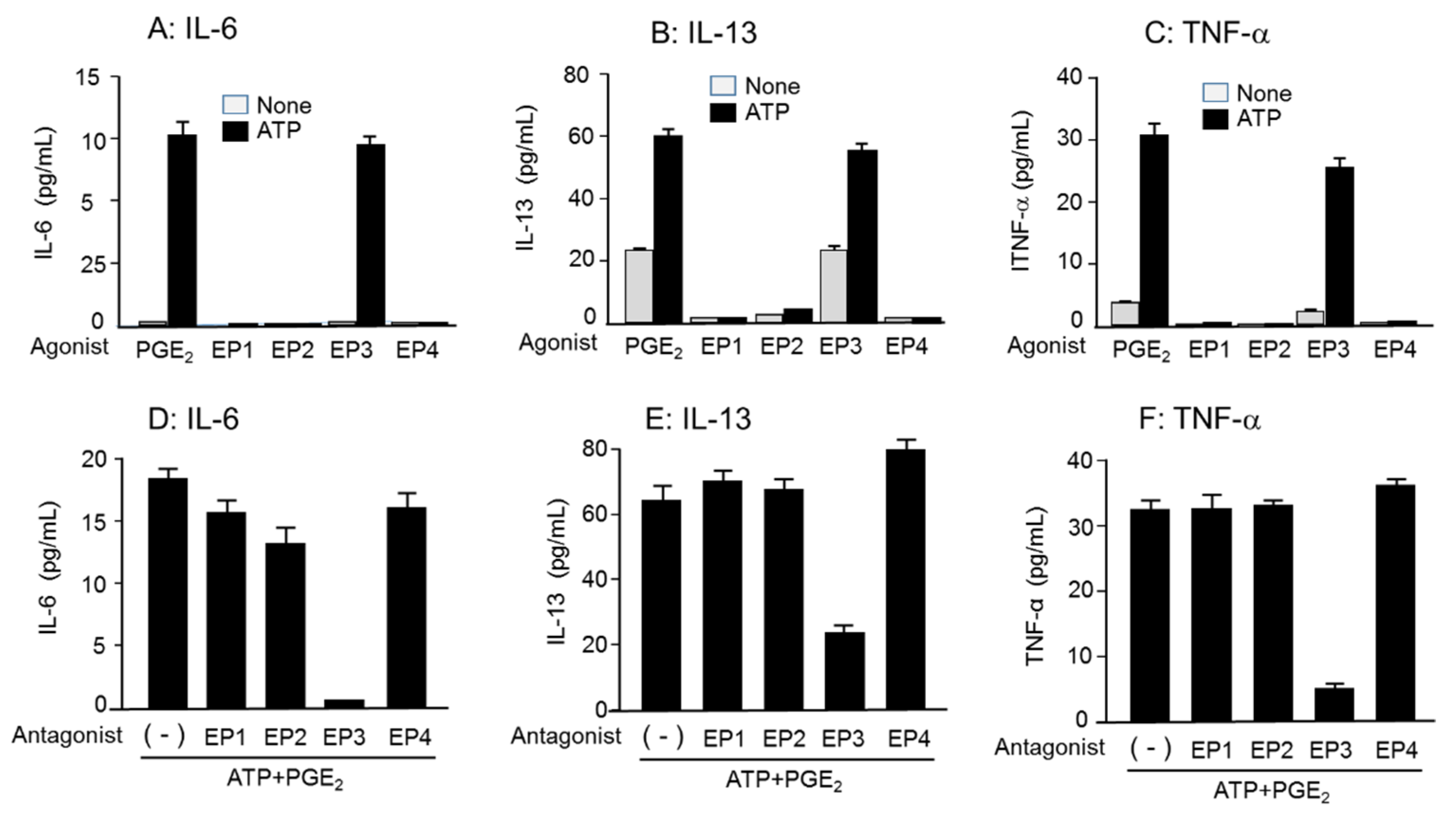
3.4. Effects of Different Purinergic Receptor Agonists Combined with PGE2 on IL-6, IL-13, and TNF-α mRNA Expression
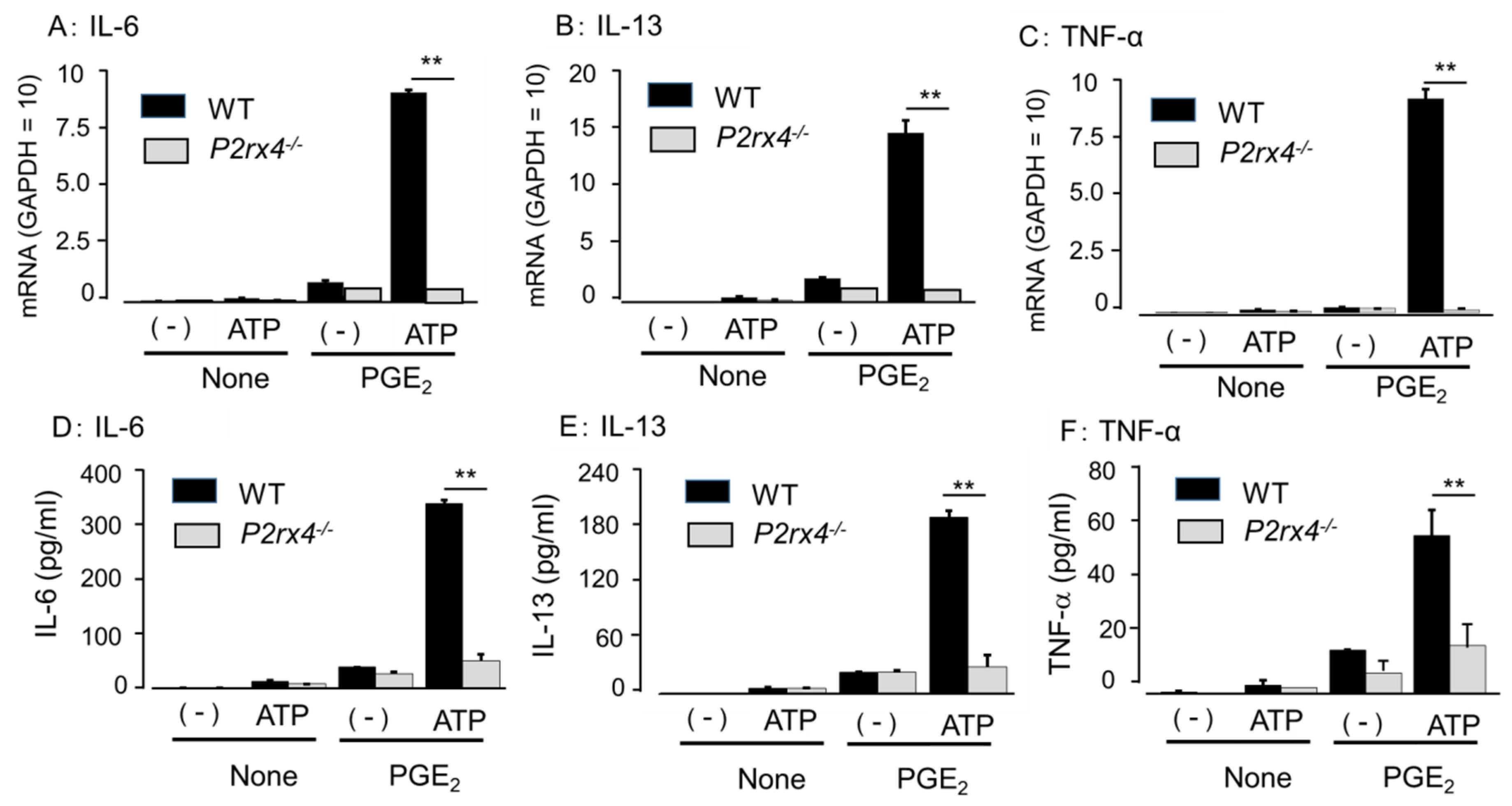
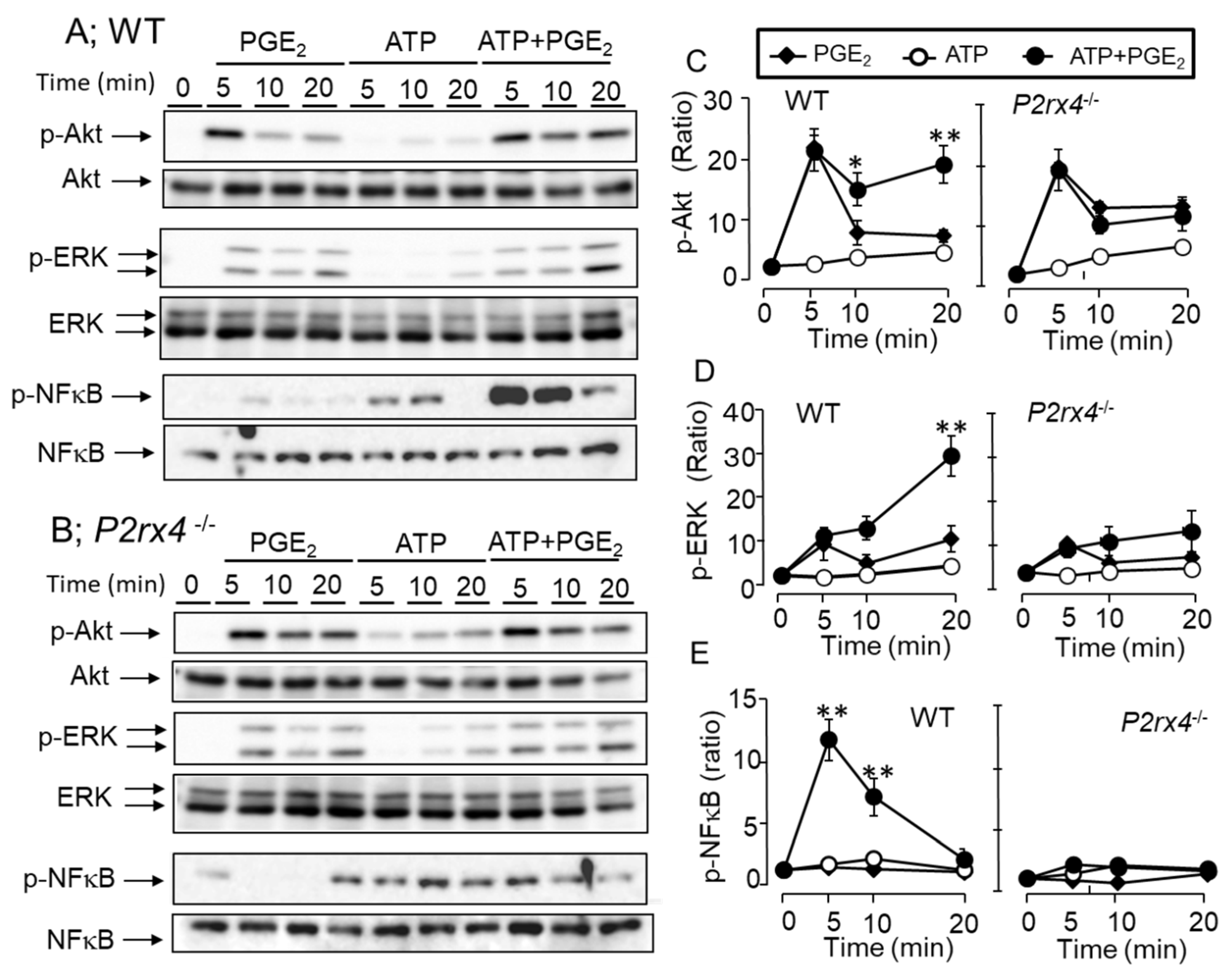
3.5. Role of P2X4 Receptor in Enhancing Cytokine mRNA Expression and Secretion by Co-Stimulation of ATP and PGE2
3.6. Role of EP3R in Enhancing Cytokine mRNA Expression and Secretion by Co-Stimulation of ATP and PGE2
3.7. Effects of ATP and PGE2 on ERK/Akt/NF-κB Signaling Pathway
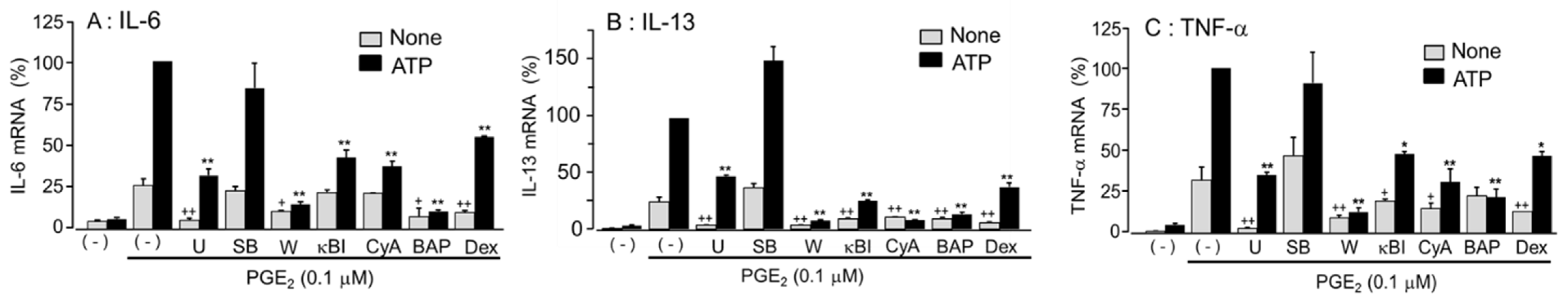
3.8. Effects of Signal Transduction Pathway Inhibitors on the Enhanced Cytokine mRNA Expression by Co-Stimulation with ATP and PGE2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnstock, G. Pathophysiology and Therapeutic Potential of Purinergic Signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef]
- North, R.A.; Surprenant, A. Pharmacology of Cloned P2X Receptors. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 563–580. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; Von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. J. Cereb. Blood Flow Metab. 2020, 177, 2413–2433. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, I.; Ohkubo, S. ATP- and adenosine-mediated signaling in the central nervous system: Adenosine receptor activation by ATP through rapid and localized generation of adenosine by ecto-nucleotidases. J. Pharmacol. Sci. 2004, 94, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Pinna, C.; Knigh, G.E.; Puglisi, L.; Burnstock, G. Neurogenic and non-neurogenic responses in the urinary bladder of hiber-nating hamster. Br. J. Pharmacol. 1998, 123, 1281–1287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoyle, C.H.; Knight, G.E.; Burnstock, G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. J. Cereb. Blood Flow Metab. 1990, 99, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Nakanish, H.; Takeda, H. The possibility that adenosine triphosphate is an excitatory transmitter in guinea-pig seminal vesicle. Jpn. J. Pharmacol. 1972, 22, 269–270. [Google Scholar] [CrossRef]
- Elliott, M.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovitch, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, M.; Song, J.; Sorokin, L.; Isfort, K.; Schwerdtle, T.; Leipziger, J.; Robaye, B.; Conley, P.B.; Kim, H.-C.; Sargin, S.; et al. Autocrine Purinergic Receptor Signaling Is Essential for Macrophage Chemotaxis. Sci. Signal. 2010, 3, ra55. [Google Scholar] [CrossRef]
- Yip, L.; Woehrle, T.; Corriden, R.; Hirsh, M.; Chen, Y.; Inoue, Y.; Ferrari, V.; Insel, P.A.; Junger, W.G. Autocrine regulation of T-cell activation by ATP release and P2X 7 receptors. FASEB J. 2009, 23, 1685–1693. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflam-masome-dependent IL-1beta secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef]
- Bulanova, E.; Bulfone-Paus, S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2009, 6, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Weber, F.C.; Esser, P.; Müller, T.; Ganesan, J.; Pellegatti, P.; Simon, M.M.; Zeiser, R.; Idzko, M.; Jakob, T.; Martin, S.F. Lack of the purinergic receptor P2X7 results in resistance to contact hypersensitivity. J. Exp. Med. 2010, 207, 2609–2619. [Google Scholar] [CrossRef] [PubMed]
- Laffargue, M.; Calvez, R.; Finan, P.; Trifilieff, A.; Barbier, M.; Altruda, F.; Hirsch, E.; Wymann, M.P. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity 2002, 16, 441–451. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Ding, Y.; Jacobson, K.A. P2Y13 receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol. Res. 2010, 62, 500–505. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Wei, Q.; Jayasekara, M.P.S.; Jacobson, K.A. The role of P2Y14 and other P2Y receptors in degranulation of human LAD2 mast cells. Purinergic Signal. 2012, 9, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ito, M.-A.; Sato, N.; Obayashi, K.; Yamamoto, K.; Koizumi, S.; Tanaka, S.; Furuta, K.; Matsuoka, I. Extracellular ATP Augments Antigen-Induced Murine Mast Cell Degranulation and Allergic Responses via P2X4 Receptor Activation. J. Immunol. 2020, 204, 3077–3085. [Google Scholar] [CrossRef]
- Yoshida, K.; Tajima, M.; Nagano, T.; Obayashi, K.; Ito, M.; Yamamoto, K.; Matsuoka, I. Ito Co-Stimulation of Purinergic P2X4 and Prostanoid EP3 Receptors Triggers Synergistic Degranulation in Murine Mast Cells. Int. J. Mol. Sci. 2019, 20, 5157. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sokabe, T.; Matsumoto, T.; Yoshimura, K.; Shibata, M.; Ohura, N.; Fukuda, T.; Sato, T.; Sekine, K.; Kato, S.; et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat. Med. 2006, 12, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ito, M.; Matsuoka, I. Divergent regulatory roles of extracellular ATP in the degranulation response of mouse bone marrow-derived mast cells. Int. Immunopharmacol. 2017, 43, 99–107. [Google Scholar] [CrossRef]
- Ito, M.; Matsuoka, I. Inhibition of P2Y6 receptor-mediated phospholipase C activation and Ca2+ signalling by prostaglandin E2 in J774 murine macrophages. Eur. J. Pharmacol. 2015, 749, 124–132. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yamashita, T.; Sasaki, A.; Nakata, E.; Kohno, K.; Masuda, T.; Tozaki-Saitoh, H.; Imai, T.; Kuraishi, Y.; Tsuda, M.; et al. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci. Rep. 2016, 6, 32461. [Google Scholar] [CrossRef]
- Kane, L.P.; Mollenauer, M.N.; Xu, Z.; Turck, C.W.; Weiss, A. Akt-dependent phosphorylation specifically regulates Cot in-duction of NF-kappa B-dependent transcription. Mol. Cell Biol. 2002, 22, 5962–5974. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Rivera, J. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 2009, 228, 149–169. [Google Scholar] [CrossRef]
- Furumoto, Y.; Nunomura, S.; Terada, T.; Rivera, J.; Ra, C. The FcεRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IkappaB kinase phosphorylation and mast cell cytokine production. J. Biol. Chem. 2004, 279, 49177–49187. [Google Scholar] [CrossRef]
- Ito, M.; Kojima, E.; Yanagihara, Y.; Yoshida, K.; Matsuoka, I. Differential effects of Gq protein-coupled uridine receptor stim-ulation on IL-8 production in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2022, in press. [Google Scholar]
- Suurväli, J.; Boudinot, P.; Kanellopoulos, J.; Rüütel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Wareham, K.; Vial, C.; Wykes, R.; Bradding, P.; Seward, E. Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. J. Cereb. Blood Flow Metab. 2009, 157, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Fitzgerald, S.M.; Huang, S.K.; Li, C.; Chi, D.S.; Milhorn, D.M.; Krishnaswamy, G. Molecular regulation of interleu-kin-13 and monocyte chemoattractant protein-1 expression in human mast cells by interleukin-1β. Am. J. Respir. Cell Mol. Biol. 2004, 31, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. Series introduction: The transcription factor NF-kappaB and human disease. J Clin Invest. 2001, 107, 3–6. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Prefontaine, K.E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 752–756. [Google Scholar] [CrossRef]
- Jordan, P.M.; Andreas, N.; Groth, M.; Wegner, P.; Weber, F.; Jager, U.; Kuchler, C.; Werz, O.; Serfling, E.; Kamradt, T.; et al. ATP/IL-33-triggered hyperactivation of mast cells results in an amplified production of pro-inflammatory cyto-kines and eicosanoids. Immunology 2021, 164, 541–554. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obayashi, K.; Yoshida, K.; Ito, M.-a.; Mori, T.; Yamamoto, K.; Imai, T.; Matsuoka, I. Synergistic Cytokine Production by ATP and PGE2 via P2X4 and EP3 Receptors in Mouse Bone-Marrow-Derived Mast Cells. Cells 2022, 11, 616. https://doi.org/10.3390/cells11040616
Obayashi K, Yoshida K, Ito M-a, Mori T, Yamamoto K, Imai T, Matsuoka I. Synergistic Cytokine Production by ATP and PGE2 via P2X4 and EP3 Receptors in Mouse Bone-Marrow-Derived Mast Cells. Cells. 2022; 11(4):616. https://doi.org/10.3390/cells11040616
Chicago/Turabian StyleObayashi, Kosuke, Kazuki Yoshida, Masa-aki Ito, Tetsuya Mori, Kimiko Yamamoto, Toshiyashu Imai, and Isao Matsuoka. 2022. "Synergistic Cytokine Production by ATP and PGE2 via P2X4 and EP3 Receptors in Mouse Bone-Marrow-Derived Mast Cells" Cells 11, no. 4: 616. https://doi.org/10.3390/cells11040616
APA StyleObayashi, K., Yoshida, K., Ito, M.-a., Mori, T., Yamamoto, K., Imai, T., & Matsuoka, I. (2022). Synergistic Cytokine Production by ATP and PGE2 via P2X4 and EP3 Receptors in Mouse Bone-Marrow-Derived Mast Cells. Cells, 11(4), 616. https://doi.org/10.3390/cells11040616





