Two Types of Liposomal Formulations Improve the Therapeutic Ratio of Prednisolone Phosphate in a Zebrafish Model for Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Lines and Maintenance
2.2. Liposome Preparation and PLP Encapsulation
2.3. Cryogenic Transmission Electron Microscopy for Liposome Imaging
2.4. Injection of Drugs
2.5. Microscopy
2.6. Laser Wounding
2.7. Regeneration Assay
2.8. Image Analysis
2.9. Statistical Analysis
3. Results
3.1. Macrophage-Targeting Liposomes (AmbiMACs) and PEGylated Liposomes Showed Different Biodistributions in Zebrafish Embryos
3.2. Liposomes Accumulated near the Wound after Laser Wounding
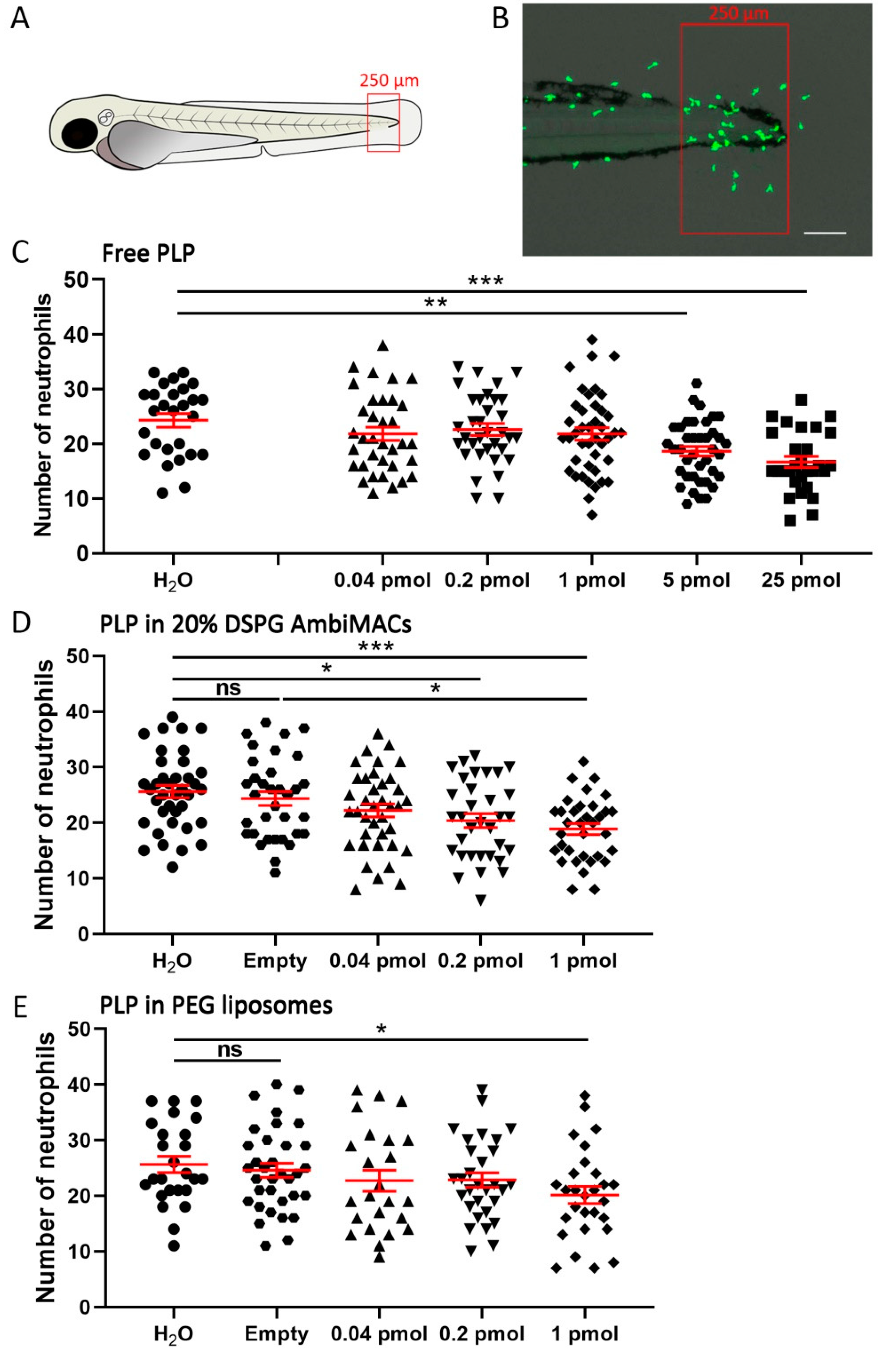
3.3. Encapsulated PLP Showed a More Potent Effect on Wounding-Induced Neutrophil Migration Than Free PLP
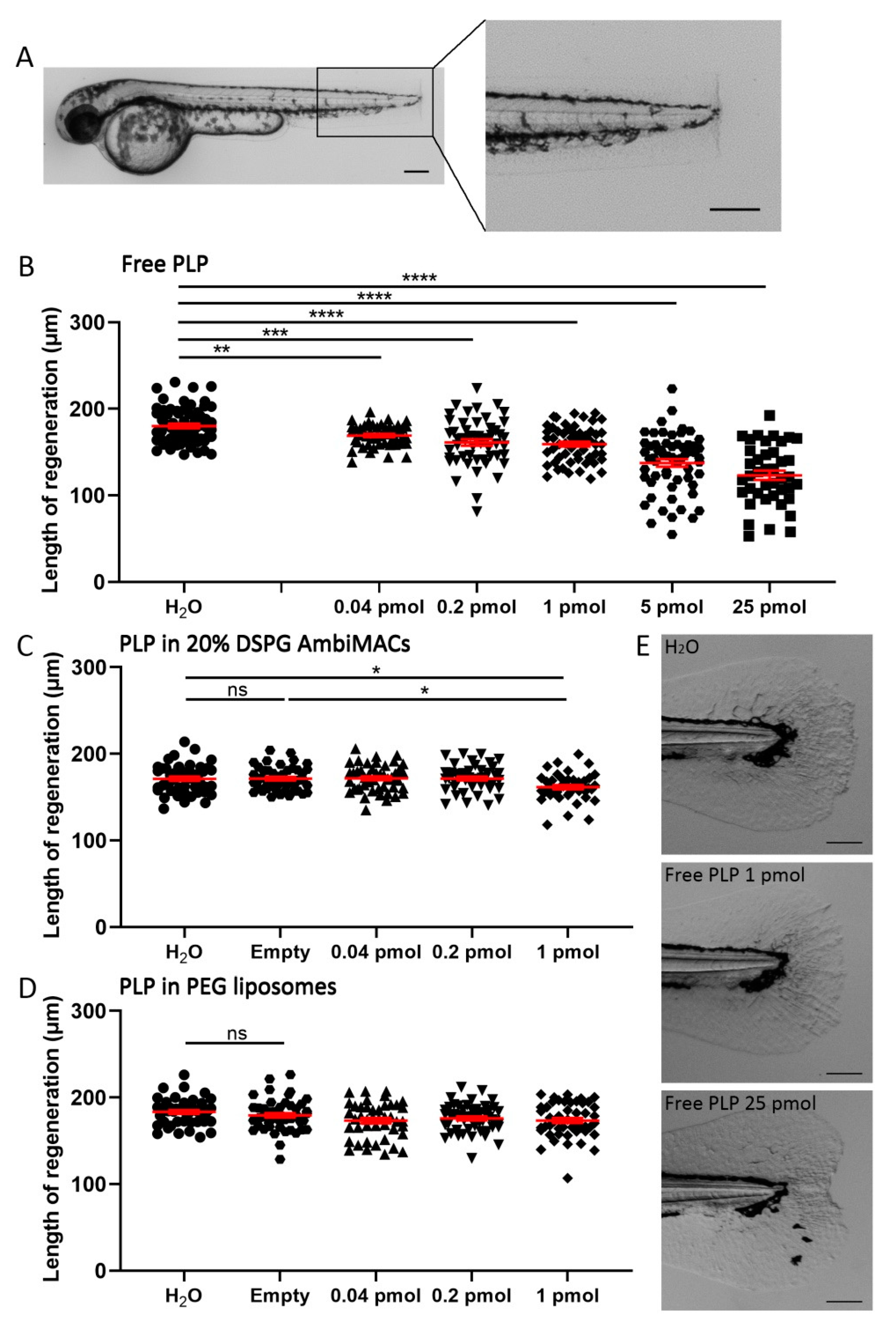
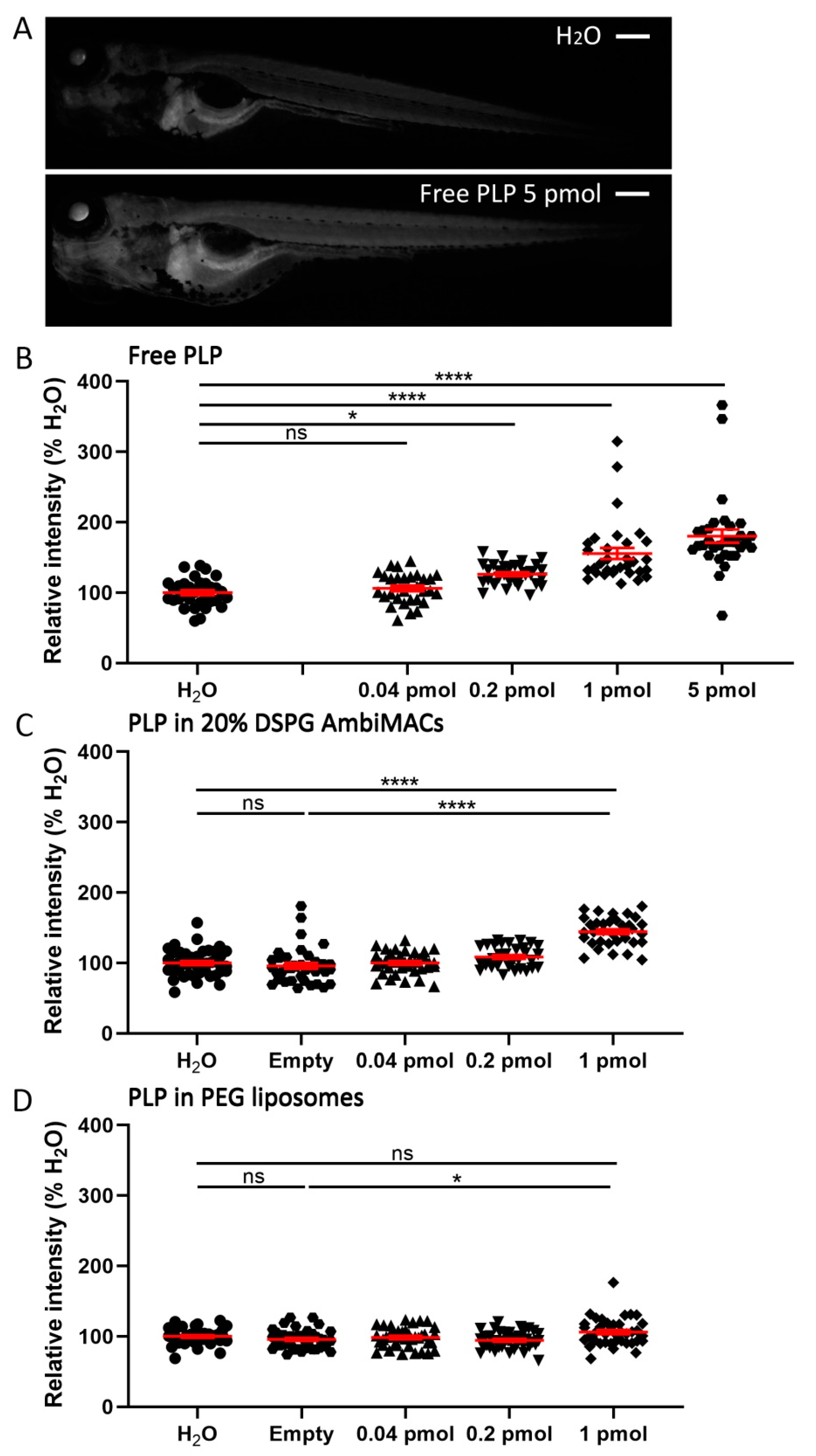
3.4. Encapsulated PLP Had a Smaller Effect on Tissue Regeneration and Transactivation Than Free PLP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2011, 163, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Goyal, A.; Bansal, P.; Sonthalia, S. Corticosteroid Adverse Effects; StatPearls Publishing: Treasure Island, Finland, 2021; NBK531462. [Google Scholar]
- Ulbrich, W.; Lamprecht, A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J. R. Soc. Interface 2010, 7, S55–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardania, H.; Tarvirdipour, S.; Dorkoosh, F. Liposome-targeted delivery for highly potent drugs. Artif. Cellsnanomedicineand Biotechnol. 2017, 45, 1478–1489. [Google Scholar] [CrossRef]
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 9, 2539. [Google Scholar]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C.W. A framework for designing delivery systems. Nat. Nanotechnol 2020, 15, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.-Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef]
- Taurin, S.; Nehoff, H.; Greish, K. Anticancer nanomedicine and tumor vascular permeability; where is the missing link? J. Control. Release 2012, 164, 265–275. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voinea, M.; Simionescu, M. Designing of ‘intelligent’liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002, 6, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]
- Schmidt, J.; Metselaar, J.M.; Wauben, M.H.; Toyka, K.V.; Storm, G.; Gold, R. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain 2003, 126, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoletto, A.; Maso, K.; Mero, A.; Rosato, A.; Schiavon, O.; Pasut, G. Drug and protein delivery by polymer conjugation. J. Drug Deliv. Sci. Technol. 2016, 32, 132–141. [Google Scholar] [CrossRef]
- Yan, X.; Scherphof, G.L.; Kamps, J.A. Liposome opsonization. J. Liposome Res. 2005, 15, 109–139. [Google Scholar] [CrossRef]
- Metselaar, J.M.; Wauben, M.H.; Wagenaar-Hilbers, J.P.; Boerman, O.C.; Storm, G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 2059–2066. [Google Scholar] [CrossRef]
- Hofkens, W.; Grevers, L.C.; Walgreen, B.; de Vries, T.J.; Leenen, P.J.; Everts, V.; Storm, G.; van den Berg, W.B.; van Lent, P.L. Intravenously delivered glucocorticoid liposomes inhibit osteoclast activity and bone erosion in murine antigen-induced arthritis. J. Control. Release 2011, 152, 363–369. [Google Scholar] [CrossRef]
- Metselaar, J.V.; van den Berg, W.; Holthuysen, A.; Wauben, M.; Storm, G.v.; van Lent, P. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann. Rheum. Dis. 2004, 63, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Lobatto, M.E.; Fayad, Z.A.; Silvera, S.; Vucic, E.; Calcagno, C.; Mani, V.; Dickson, S.D.; Nicolay, K.; Banciu, M.; Schiffelers, R.M. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 2010, 7, 2020–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffelers, R.M.; Metselaar, J.M.; Fens, M.H.; Janssen, A.P.; Molema, G.; Storm, G. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia 2005, 7, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Banciu, M.; Metselaar, J.M.; Schiffelers, R.M.; Storm, G. Liposomal glucocorticoids as tumor-targeted anti-angiogenic nanomedicine in B16 melanoma-bearing mice. J. Steroid Biochem. Mol. Biol. 2008, 111, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hofkens, W.; van den Hoven, J.M.; Pesman, G.J.; Nabbe, K.C.; Sweep, F.C.; Storm, G.; van den Berg, W.B.; van Lent, P.L. Safety of glucocorticoids can be improved by lower yet still effective dosages of liposomal steroid formulations in murine antigen-induced arthritis: Comparison of prednisolone with budesonide. Int. J. Pharm. 2011, 416, 493–498. [Google Scholar] [CrossRef]
- van den Hoven, J.M.; Hofkens, W.; Wauben, M.H.; Wagenaar-Hilbers, J.P.; Beijnen, J.H.; Nuijen, B.; Metselaar, J.M.; Storm, G. Optimizing the therapeutic index of liposomal glucocorticoids in experimental arthritis. Int. J. Pharm. 2011, 416, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.R.; Venkatesha, S.H.; Acharya, B.; Moudgil, K.D. Peptide-targeted liposomal delivery of dexamethasone for arthritis therapy. Nanomedicine 2019, 14, 1455–1469. [Google Scholar] [CrossRef]
- Verma, A.; Jain, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Agrawal, G.; Jain, S.K. Folate conjugated double liposomes bearing prednisolone and methotrexate for targeting rheumatoid arthritis. Pharm. Res. 2019, 36, 123. [Google Scholar] [CrossRef]
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1033–1043. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Appeldoorn, C.C.; Rip, J.; Dorland, R.; van der Pol, S.M.; Kooij, G.; de Vries, H.E.; Reijerkerk, A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J. Control. Release 2012, 164, 364–369. [Google Scholar] [CrossRef]
- Sylvester, B.; Porfire, A.; Muntean, D.-M.; Vlase, L.; Lupuţ, L.; Licarete, E.; Sesarman, A.; Alupei, M.C.; Banciu, M.; Achim, M. Optimization of prednisolone-loaded long-circulating liposomes via application of Quality by Design (QbD) approach. J. Liposome Res. 2018, 28, 49–61. [Google Scholar] [CrossRef]
- Lobatto, M.E.; Calcagno, C.; Otten, M.J.; Millon, A.; Ramachandran, S.; Paridaans, M.P.; van der Valk, F.M.; Storm, G.; Stroes, E.S.; Fayad, Z.A. Pharmaceutical development and preclinical evaluation of a GMP-grade anti-inflammatory nanotherapy. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1133–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patras, L.; Sylvester, B.; Luput, L.; Sesarman, A.; Licarete, E.; Porfire, A.; Muntean, D.; Drotar, D.M.; Rusu, A.D.; Nagy, A.-L. Liposomal prednisolone phosphate potentiates the antitumor activity of liposomal 5-fluorouracil in C26 murine colon carcinoma in vivo. Cancer Biol. Ther. 2017, 18, 616–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, E.A.W.; Soetekouw, J.A.; Pieters, E.H.E.; Smits, C.J.P.; de Wijs-Rot, N.; Vromans, H. The availability of drug by liposomal drug delivery: Individual kinetics and tissue distribution of encapsulated and released drug in mice after administration of PEGylated liposomal prednisolone phosphate. Investig. New Drugs 2019, 37, 890–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Valk, F.M.; van Wijk, D.F.; Lobatto, M.E.; Verberne, H.J.; Storm, G.; Willems, M.C.; Legemate, D.A.; Nederveen, A.J.; Calcagno, C.; Mani, V. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Turk, M.J.; Waters, D.J.; Low, P.S. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004, 213, 165–172. [Google Scholar] [CrossRef]
- Tie, Y.; Zheng, H.; He, Z.; Yang, J.; Shao, B.; Liu, L.; Luo, M.; Yuan, X.; Liu, Y.; Zhang, X. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal. Transduct. Target. Ther. 2020, 5, 6. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake characteristics of liposomes by rat alveolar macrophages: Influence of particle size and surface mannose modification. J. Pharm. Pharmacol. 2007, 59, 75–80. [Google Scholar] [CrossRef]
- Düffels, A.; Green, L.G.; Ley, S.V.; Miller, A.D. Synthesis of high-mannose type neoglycolipids: Active targeting of liposomes to macrophages in gene therapy. Chem.-A Eur. J. 2000, 6, 1416–1430. [Google Scholar] [CrossRef]
- Andersen, M.N.; Etzerodt, A.; Graversen, J.H.; Holthof, L.C.; Moestrup, S.K.; Hokland, M.; Møller, H.J. STAT3 inhibition specifically in human monocytes and macrophages by CD163-targeted corosolic acid-containing liposomes. Cancer Immunol. Immunother. 2019, 68, 489–502. [Google Scholar] [CrossRef]
- Tentillier, N.; Etzerodt, A.; Olesen, M.N.; Rizalar, F.S.; Jacobsen, J.; Bender, D.; Moestrup, S.K.; Romero-Ramos, M. Anti-inflammatory modulation of microglia via CD163-targeted glucocorticoids protects dopaminergic neurons in the 6-OHDA Parkinson’s disease model. J. Neurosci. 2016, 36, 9375–9390. [Google Scholar] [CrossRef] [Green Version]
- Montes-Cobos, E.; Ring, S.; Fischer, H.J.; Heck, J.; Strauß, J.; Schwaninger, M.; Reichardt, S.D.; Feldmann, C.; Lühder, F.; Reichardt, H.M. Targeted delivery of glucocorticoids to macrophages in a mouse model of multiple sclerosis using inorganic-organic hybrid nanoparticles. J. Control. Release 2017, 245, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Rev. 2019, 151-152, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthew, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; del Cid, N.; Traver, D. Perspectives on antigen presenting cells in zebrafish. Dev. Comp. Immunol. 2014, 46, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef] [Green Version]
- Schaaf, M.J.; Champagne, D.; van Laanen, I.H.; van Wijk, D.C.; Meijer, A.H.; Meijer, O.C.; Spaink, H.P.; Richardson, M.K. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology 2008, 149, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Models Mech. 2019, 12, dmm037887. [Google Scholar] [CrossRef] [Green Version]
- Chatzopoulou, A.; Heijmans, J.P.; Burgerhout, E.; Oskam, N.; Spaink, H.P.; Meijer, A.H.; Schaaf, M.J. Glucocorticoid-induced attenuation of the inflammatory response in zebrafish. Endocrinology 2016, 157, 2772–2784. [Google Scholar] [CrossRef] [Green Version]
- Arias-Alpizar, G.; Kong, L.; Vlieg, R.C.; Rabe, A.; Papadopoulou, P.; Meijer, M.S.; Bonnet, S.; Vogel, S.; van Noort, J.; Kros, A.; et al. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat. Commun 2020, 11, 3638. [Google Scholar] [CrossRef]
- Campbell, F.; Bos, F.L.; Sieber, S.; Arias-Alpizar, G.; Koch, B.E.; Huwyler, J.r.; Kros, A.; Bussmann, J. Directing nanoparticle biodistribution through evasion and exploitation of Stab2-dependent nanoparticle uptake. Acs Nano 2018, 12, 2138–2150. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Uhl, P.; Detampel, P.; Mier, W.; Witzigmann, D.; Huwyler, J. Zebrafish as a predictive screening model to assess macrophage clearance of liposomes in vivo. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Arias-Alpizar, G.; Koch, B.; Hamelmann, N.M.; Neustrup, M.A.; Paulusse, J.M.J.; Jiskoot, W.; Kros, A.; Bussmann, J. Stabilin-1 is required for the endothelial clearance of small anionic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102395. [Google Scholar] [CrossRef]
- Kong, L.; Chen, Q.; Campbell, F.; Snaar-Jagalska, E.; Kros, A. Light-Triggered Cancer Cell Specific Targeting and Liposomal Drug Delivery in a Zebrafish Xenograft Model. Adv. Healthc. Mater. 2020, 9, 1901489. [Google Scholar] [CrossRef] [Green Version]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.; Elworthy, S.; Ingham, P.W.; Whyte, M.K. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef] [PubMed]
- Benato, F.; Colletti, E.; Skobo, T.; Moro, E.; Colombo, L.; Argenton, F.; Valle, L.D. A living biosensor model to dynamically trace glucocorticoid transcriptional activity during development and adult life in zebrafish. Mol. Cell. Endocrinol. 2014, 392, 60–72. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. Predicting hydrophilic drug encapsulation inside unilamellar liposomes. Int. J. Pharm. 2012, 423, 410–418. [Google Scholar] [CrossRef]
- Sciolla, F.; Truzzolillo, D.; Chauveau, E.; Trabalzini, S.; di Marzio, L.; Carafa, M.; Marianecci, C.; Sarra, A.; Bordi, F.; Sennato, S. Influence of drug/lipid interaction on the entrapment efficiency of isoniazid in liposomes for antitubercular therapy: A multi-faced investigation. Colloids Surf. B Biointerfaces 2021, 208, 112054. [Google Scholar] [CrossRef]
- Redd, M.J.; Kelly, G.; Dunn, G.; Way, M.; Martin, P. Imaging macrophage chemotaxis in vivo: Studies of microtubule function in zebrafish wound inflammation. Cell Motil. Cytoskelet. 2006, 63, 415–422. [Google Scholar] [CrossRef]
- Mathew, L.K.; Sengupta, S.; Kawakami, A.; Andreasen, E.A.; Löhr, C.V.; Loynes, C.A.; Renshaw, S.A.; Peterson, R.T.; Tanguay, R.L. Unraveling tissue regeneration pathways using chemical genetics. J. Biol. Chem. 2007, 282, 35202–35210. [Google Scholar] [CrossRef] [Green Version]
- Ringdén, O.; Meunier, F.; Tollemar, J.; Ricci, P.; Tura, S.; Kuse, E.; Viviani, M.A.; Gorin, N.C.; Klastersky, J.; Fenaux, P.; et al. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J. Antimicrob Chemother 1991, 28, 73–82. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Bresnik, M.; Ebrahimi, R.; Ullmann, A.J.; Bouza, E.; Heussel, C.P.; Lortholary, O.; Rieger, C.; Boehme, A. Liposomal amphotericin b as initial therapy for invasive mold infection: A randomized trial comparing a high–loading dose regimen with standard dosing (AmBiLoad Trial). Clin. Infect. Dis. 2007, 44, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Detampel, P.; Siegfried, S.; Witzigmann, D.; Huwyler, J. Zebrafish as an early stage screening tool to study the systemic circulation of nanoparticulate drug delivery systems in vivo. J. Control. Release 2017, 264, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Fenaroli, F.; Westmoreland, D.; Benjaminsen, J.; Kolstad, T.; Skjeldal, F.M.; Meijer, A.H.; van der Vaart, M.; Ulanova, L.; Roos, N.; Nystrom, B. Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: Direct visualization and treatment. Acs Nano 2014, 8, 7014–7026. [Google Scholar] [CrossRef] [PubMed]
- Hofkens, W.; Schelbergen, R.; Storm, G.; van den Berg, W.B.; van Lent, P.L. Liposomal targeting of prednisolone phosphate to synovial lining macrophages during experimental arthritis inhibits M1 activation but does not favor M2 differentiation. PLoS ONE 2013, 8, e54016. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids inhibit wound healing: Novel mechanism of action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Nguyen-Chi, M.; Laplace-Builhé, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef]
- He, M.; Halima, M.; Xie, Y.; Schaaf, M.J.; Meijer, A.H.; Wang, M. Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells 2020, 9, 1107. [Google Scholar] [CrossRef]



| Treatment | ||||
| Free PLP | 0.901 | (9.8/10.9) | 0.177 | (9.8/55.6) |
| PLP in AmbiMACs (20% DSPG) | 4.707 | (26.8/5.7) | 0.603 | (26.8/44.5) |
| PLP in PEGylated liposome | 4.040 | (21.4/5.3) | 3.453 | (21.4/6.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Papadopoulou, P.; de Wit, B.; d’Engelbronner, J.C.; van Hage, P.; Kros, A.; Schaaf, M.J.M. Two Types of Liposomal Formulations Improve the Therapeutic Ratio of Prednisolone Phosphate in a Zebrafish Model for Inflammation. Cells 2022, 11, 671. https://doi.org/10.3390/cells11040671
Xie Y, Papadopoulou P, de Wit B, d’Engelbronner JC, van Hage P, Kros A, Schaaf MJM. Two Types of Liposomal Formulations Improve the Therapeutic Ratio of Prednisolone Phosphate in a Zebrafish Model for Inflammation. Cells. 2022; 11(4):671. https://doi.org/10.3390/cells11040671
Chicago/Turabian StyleXie, Yufei, Panagiota Papadopoulou, Björn de Wit, Jan C. d’Engelbronner, Patrick van Hage, Alexander Kros, and Marcel J. M. Schaaf. 2022. "Two Types of Liposomal Formulations Improve the Therapeutic Ratio of Prednisolone Phosphate in a Zebrafish Model for Inflammation" Cells 11, no. 4: 671. https://doi.org/10.3390/cells11040671
APA StyleXie, Y., Papadopoulou, P., de Wit, B., d’Engelbronner, J. C., van Hage, P., Kros, A., & Schaaf, M. J. M. (2022). Two Types of Liposomal Formulations Improve the Therapeutic Ratio of Prednisolone Phosphate in a Zebrafish Model for Inflammation. Cells, 11(4), 671. https://doi.org/10.3390/cells11040671








