Dimerization of the Glucocorticoid Receptor and Its Importance in (Patho)physiology: A Primer
Abstract
:1. Introduction
1.1. Glucocorticoids
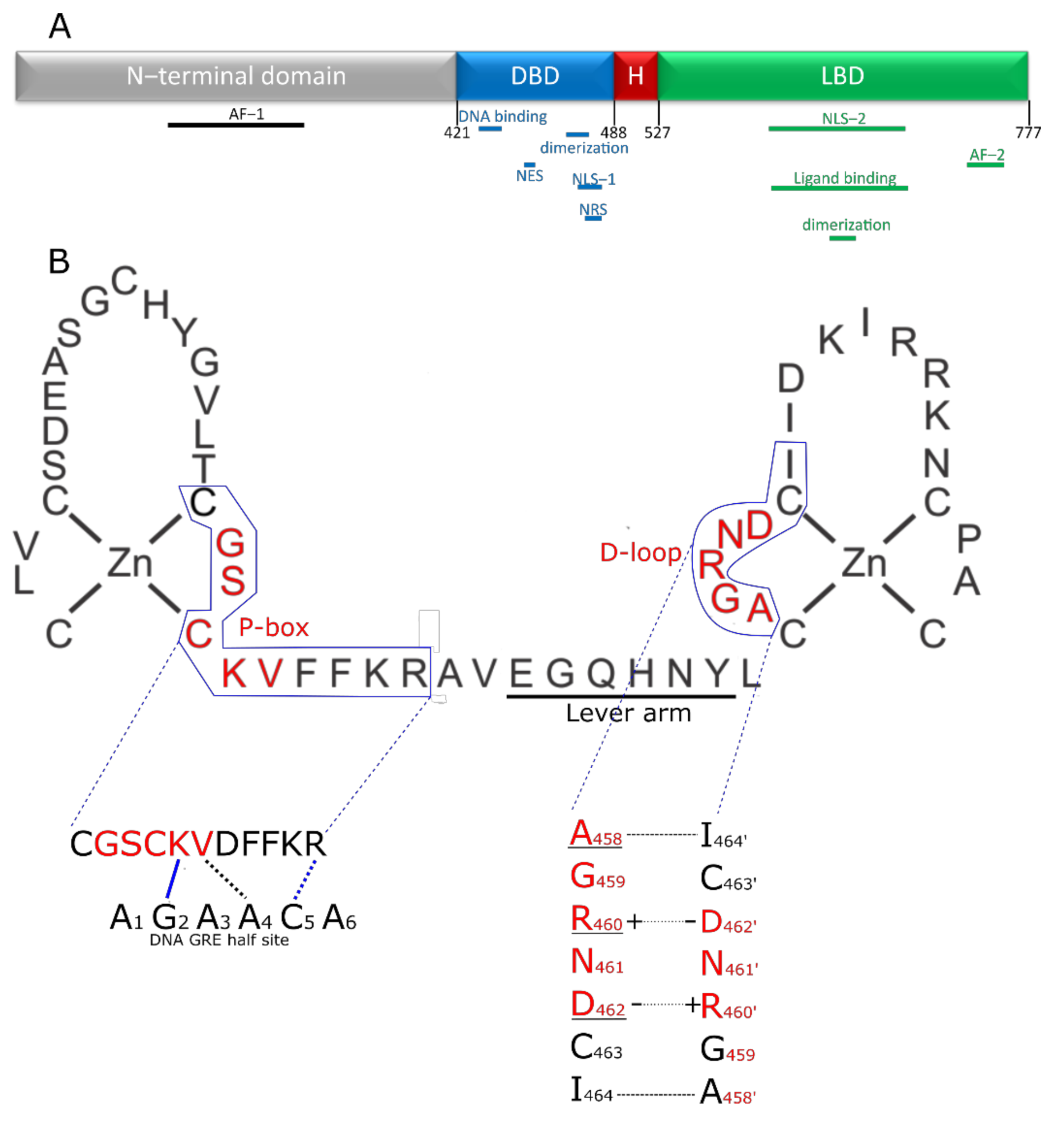
1.2. Glucocorticoid Receptor: Structure and Function
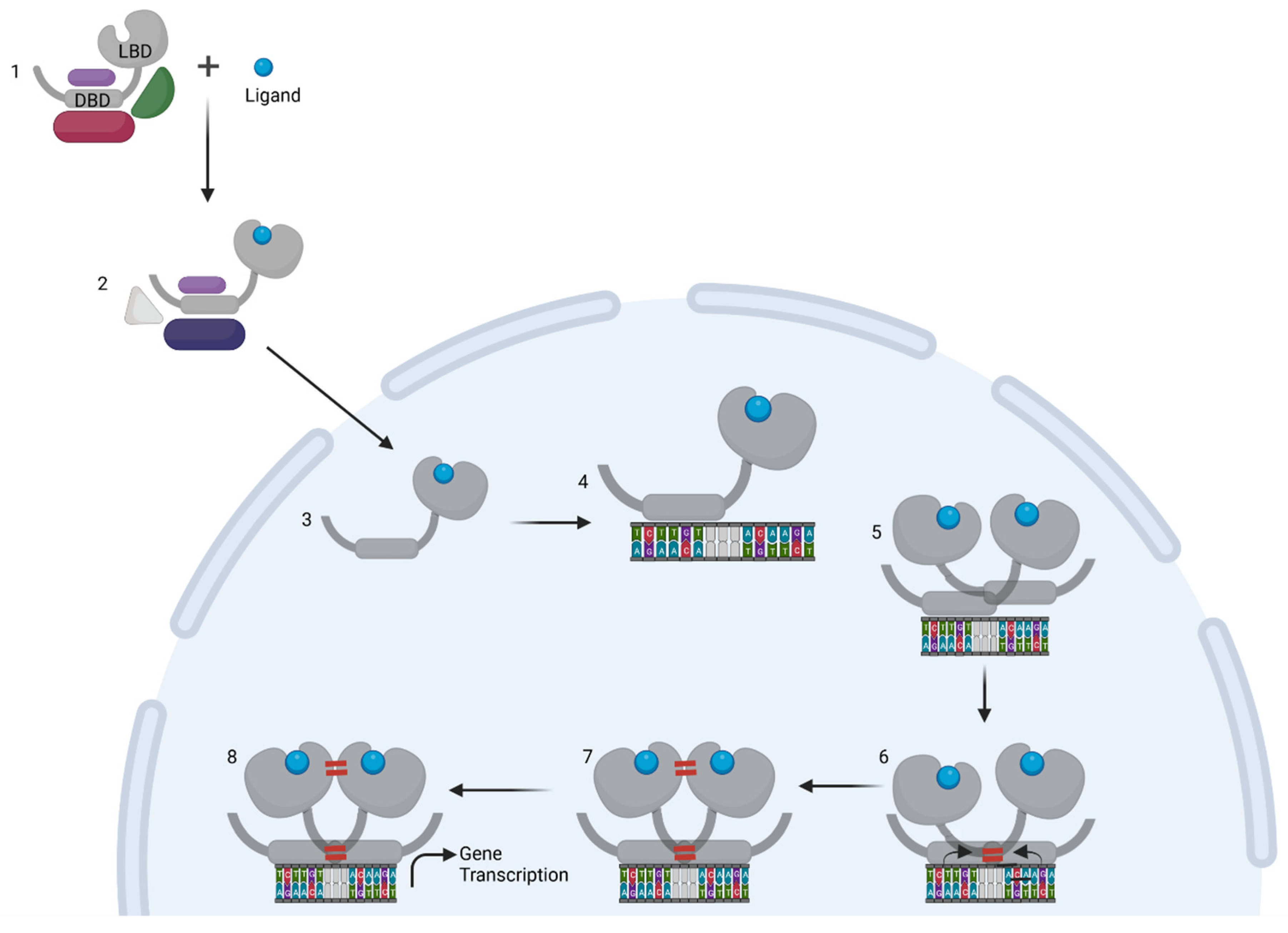
2. Ligand-Induced GR Homodimer Formation
2.1. The DBD Interface
2.2. The LBD Interface
3. GR Dimer Mutations
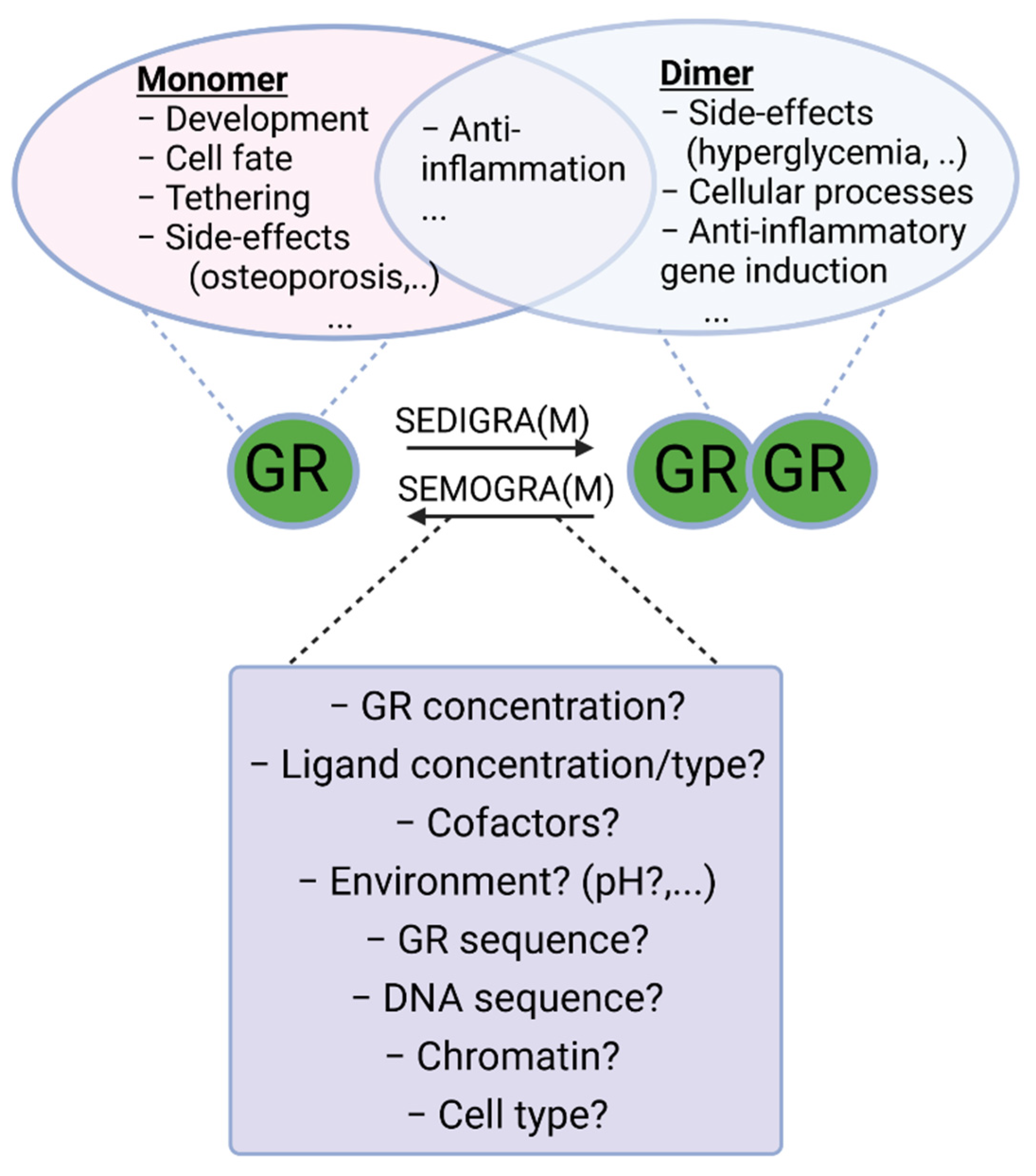
4. GR Dimer and Monomer Transcriptional Regulation
5. Alternative Partners for Dimer Formation
6. Role of GR Complex Formation in SIRS and Sepsis
6.1. SIRS and Sepsis
6.2. Anti-Inflammatory Genes Induced by GR Complex Formation
6.3. Pro-Inflammatory Genes Suppressed by GR Complex Formation
6.4. Hemodynamic and Metabolic Parameters Controlled by GR Complex Formation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiga, F.; Walker, J.J.; Terry, J.R.; Lightman, S.L. HPA axis-rhythms. Compr. Physiol. 2014, 4, 1273–1298. [Google Scholar]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Topete, D.; Cidlowski, J. One Hormone, Two Actions: Anti- and Pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-C.; Gray, N.E.; Kuo, T.; Harris, C.A. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2012, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Ten, S.; New, M.; Maclaren, N. Clinical review 130: Addison’s disease 2001. J. Clin. Endocrinol. Metab. 2001, 86, 2909–2922. [Google Scholar] [CrossRef] [Green Version]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef]
- Cutolo, M.; Seriolo, B.; Pizzorni, C.; Secchi, M.E.; Soldano, S.; Paolino, S.; Montagna, P.; Sulli, A. Use of glucocorticoids and risk of infections. Autoimmun. Rev. 2008, 8, 153–155. [Google Scholar] [CrossRef]
- Wilkinson, L.; Verhoog, N.J.D.; Louw, A. Disease- and treatment-associated acquired glucocorticoid resistance. Endocr. Connect. 2018, 7, R328–R349. [Google Scholar] [CrossRef] [Green Version]
- Souffriau, J.; Eggermont, M.; Van Ryckeghem, S.; Van Looveren, K.; Van Wyngene, L.; Van Hamme, E.; Vuylsteke, M.; Beyaert, R.; De Bosscher, K.; Libert, C. A screening assay for Selective Dimerizing Glucocorticoid Receptor Agonists and Modulators (SEDIGRAM) that are effective against acute inflammation. Sci. Rep. 2018, 8, 12894. [Google Scholar] [CrossRef]
- Van Moortel, L.; Gevaert, K.; De Bosscher, K. Improved Glucocorticoid Receptor Ligands: Fantastic Beasts, but How to Find Them? Front. Endocrinol. 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Mazaira, G.I.; Zgajnar, N.R.; Lotufo, C.M.; Daneri-Becerra, C.; Sivils, J.C.; Soto, O.B.; Cox, M.B.; Galigniana, A.M.D. The Nuclear Receptor Field: A Historical Overview and Future Challenges. Nucl. Recept. Res. 2018, 5, 101320. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Stromstedt, P.E.; Quinn, P.G.; Carlstedt-Duke, J.; Gustafsson, J.A.; Granner, D.K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 1990, 10, 4712–4719. [Google Scholar] [CrossRef]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of Glucose Homeostasis by Glucocorticoids. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2015; Volume 872, pp. 99–126. [Google Scholar]
- Escoter-Torres, L.; Greulich, F.; Quagliarini, F.; Wierer, M.; Uhlenhaut, N.H. Anti-inflammatory functions of the glucocorticoid receptor require DNA binding. Nucleic Acids Res. 2020, 48, 8393–8407. [Google Scholar] [CrossRef]
- Ivy, J.R.; Carter, R.N.; Zhao, J.; Buckley, C.; Urquijo, H.; Rog-Zielinska, E.A.; Panting, E.; Hrabalkova, L.; Nicholson, C.; Agnew, E.J.; et al. Glucocorticoids regulate mitochondrial fatty acid oxidation in fetal cardiomyocytes. J. Physiol. 2021, 599, 4901–4924. [Google Scholar] [CrossRef]
- Lee, M.-J.; Pramyothin, P.; Karastergiou, K.; Fried, S.K. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1842, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Præstholm, S.M.; Correia, C.M.; Grøntved, L. Multifaceted Control of GR Signaling and Its Impact on Hepatic Transcriptional Networks and Metabolism. Front. Endocrinol. 2020, 11, 572981. [Google Scholar] [CrossRef]
- Tronche, F.; Opherk, C.; Moriggl, R.; Kellendonk, C.; Reimann, A.; Schwake, L.; Reichardt, H.M.; Stangl, K.; Gau, D.; Hoeflich, A.; et al. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev. 2004, 18, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W. Corticosteroid receptors and the central nervous system. J. Steroid Biochem. Mol. Biol. 1994, 49, 381–384. [Google Scholar] [CrossRef]
- Lerch, J.K.; Madalena, K.M. Glucocorticoids and nervous system plasticity. Neural Regen. Res. 2016, 11, 37–41. [Google Scholar] [CrossRef]
- Lee, M.-J.; Fried, S.K. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. 2014, 38, 1228–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell-Price, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Simpson, S.L. Addison’s disease. Br. Med. J. 1950, 2, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The nuclear receptor superfamily: A structural perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef]
- Kino, T. Single Nucleotide Variations of the Human GR Gene Manifested as Pathologic Mutations or Polymorphisms. Endocrinology 2018, 159, 2506–2519. [Google Scholar] [CrossRef]
- Mackeh, R.; Marr, A.K.; Dargham, S.; Syed, N.; Fakhro, K.A.; Kino, T. Single-Nucleotide Variations of the Human Nuclear Hormone Receptor Genes in 60,000 Individuals. J. Endocr. Soc. 2017, 2, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Thompson, E. Folding of the glucocorticoid receptor N-terminal transactivation function: Dynamics and regulation. Mol. Cell. Endocrinol. 2012, 348, 450–456. [Google Scholar] [CrossRef]
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, M.T.K.K.R. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 2017, 18, 159–174. [Google Scholar] [CrossRef]
- Lu, N.Z.; Cidlowski, J.A. Translational Regulatory Mechanisms Generate N-Terminal Glucocorticoid Receptor Isoforms with Unique Transcriptional Target Genes. Mol. Cell 2005, 18, 331–342. [Google Scholar] [CrossRef]
- Petta, I.; Dejager, L.; Ballegeer, M.; Lievens, S.; Tavernier, J.; De Bosscher, K.; Libert, C. The Interactome of the Glucocorticoid Receptor and Its Influence on the Actions of Glucocorticoids in Combatting Inflammatory and Infectious Diseases. Microbiol. Mol. Biol. Rev. 2016, 80, 495–522. [Google Scholar] [CrossRef] [Green Version]
- De Castro, M.; Elliot, S.; Kino, T.; Bamberger, C.; Karl, M.; Webster, E.; Chrousos, G.P. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): Tissue levels, mechanism of action, and potential physiologic role. Mol. Med. 1996, 2, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Oakley, R.H.; Sar, M.; Cidlowski, J.A. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 1996, 271, 9550–9559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruchter, O.; Kino, T.; Zoumakis, E.; Alesci, S.; De Martino, M.; Chrousos, G.; Hochberg, Z. The human glucocorticoid receptor (GR) isoform {beta} differentially suppresses GR{alpha}-induced transactivation stimulated by synthetic glucocorticoids. J. Clin. Endocrinol. Metab. 2005, 90, 3505–3509. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic 2011, 13, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Czar, M.J.; Renoir, J.M.; Pratt, W.B.; Lyons, R.H.; Welsh, M.J. Evidence that the FK506-binding immunophilin heat shock protein 56 is required for trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus. Mol. Endocrinol. 1995, 9, 1549–1560. [Google Scholar] [CrossRef] [Green Version]
- Davies, T.H.; Ning, Y.M.; Sanchez, E.R. A new first step in activation of steroid receptors-Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef] [Green Version]
- Freedman, N.D.; Yamamoto, K.R. Importin 7 and importin alpha/Importin beta are nuclear import receptors for the glucocorticoid receptor. Mol. Biol. Cell 2004, 15, 2276–2286. [Google Scholar] [CrossRef]
- Savory, J.G.A.; Preéfontaine, G.G.; Lamprecht, C.; Liao, M.; Walther, R.F.; Lefebvre, Y.A.; Hacheé, R.J.G. Glucocorticoid Receptor Homodimers and Glucocorticoid-Mineralocorticoid Receptor Heterodimers Form in the Cytoplasm through Alternative Dimerization Interfaces. Mol. Cell. Biol. 2001, 21, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Robblee, J.P.; Miura, M.T.; Bain, D.L. Glucocorticoid Receptor–Promoter Interactions: Energetic Dissection Suggests a Framework for the Specificity of Steroid Receptor-Mediated Gene Regulation. Biochemistry 2012, 51, 4463–4472. [Google Scholar] [CrossRef] [Green Version]
- Payvar, F.; DeFranco, D.; Firestone, G.L.; Edgar, B.; Wrange, Ö.; Okret, S.; Gustafsson, J.; Yamamoto, K.R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell 1983, 35, 381–392. [Google Scholar] [CrossRef]
- Wrange, O.; Eriksson, P.; Perlmann, T. The Purified Activated Glucocorticoid Receptor is a Homodimer. J. Biol. Chem. 1989, 264, 5253–5259. [Google Scholar] [CrossRef]
- Presman, D.M.; Hager, G.L. More than meets the dimer: What is the quaternary structure of the glucocorticoid receptor? Austin Transcr. 2017, 8, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paakinaho, V.; Johnson, T.A.; Presman, D.M.; Hager, G.L. Glucocorticoid receptor quaternary structure drives chromatin occupancy and transcriptional outcome. Genome Res. 2019, 29, 1223–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, A.; Prefontaine, K.E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, H.M.; Kaestner, K.H.; Tuckermann, J.; Kretz, O.; Wessely, O.; Bock, R.; Gass, P.; Schmid, W.; Herrlich, P.; Angel, P.; et al. DNA Binding of the Glucocorticoid Receptor Is Not Essential for Survival. Cell 1998, 93, 531–541. [Google Scholar] [CrossRef] [Green Version]
- De Bosscher, K.; Beck, I.M.; Ratman, D.; Berghe, W.V.; Libert, C. Activation of the Glucocorticoid Receptor in Acute Inflammation: The SEDIGRAM Concept. Trends Pharmacol. Sci. 2016, 37, 4–16. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Tuckermann, J.; Libert, C. New Insights into the Anti-inflammatory Mechanisms of Glucocorticoids: An Emerging Role for Glucocorticoid-Receptor-Mediated Transactivation. Endocrinology 2013, 154, 993–1007. [Google Scholar] [CrossRef] [Green Version]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A—A selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef] [Green Version]
- Baschant, U.; Frappart, L.; Rauchhaus, U.; Bruns, L.; Reichardt, H.M.; Kamradt, T.; Bräuer, R.; Tuckermann, J.P. Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19317–19322. [Google Scholar] [CrossRef] [Green Version]
- Koenen, M.; Culemann, S.; Vettorazzi, S.; Caratti, G.; Frappart, L.; Baum, W.; Krönke, G.; Baschant, U.; Tuckermann, J.P. Glucocorticoid receptor in stromal cells is essential for glucocorticoid-mediated suppression of inflammation in arthritis. Ann. Rheum. Dis. 2018, 77, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Tuckermann, J.P.; Kleiman, A.; Moriggl, R.; Spanbroek, R.; Neumann, A.; Illing, A.; Clausen, B.; Stride, B.; Förster, I.; Habenicht, A.; et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Investig. 2007, 117, 1381–1390. [Google Scholar] [CrossRef]
- Tuckermann, J.P.; Reichardt, H.M.; Arribas, R.; Richter, K.H.; Schütz, G.; Angel, P. The DNA Binding-Independent Function of the Glucocorticoid Receptor Mediates Repression of Ap-1–Dependent Genes in Skin. J. Cell Biol. 1999, 147, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, H.M.; Tuckermann, J.P.; Göttlicher, M.; Vujic, M.; Weih, F.; Angel, P.; Herrlich, P.; Schütz, G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001, 20, 7168–7173. [Google Scholar] [CrossRef] [Green Version]
- Schweingruber, N.; Fischer, H.J.; Fischer, L.; Brandt, J.V.D.; Karabinskaya, A.; Labi, V.; Villunger, A.; Kretzschmar, B.; Huppke, P.; Simons, M.; et al. Chemokine-mediated redirection of T cells constitutes a critical mechanism of glucocorticoid therapy in autoimmune CNS responses. Acta Neuropathol. 2014, 127, 713–729. [Google Scholar] [CrossRef] [Green Version]
- Klaßen, C.; Karabinskaya, A.; Dejager, L.; Vettorazzi, S.; Van Moorleghem, J.; Lühder, F.; Meijsing, S.H.; Tuckermann, J.P.; Bohnenberger, H.; Libert, C.; et al. Airway Epithelial Cells Are Crucial Targets of Glucocorticoids in a Mouse Model of Allergic Asthma. J. Immunol. 2017, 199, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Baake, T.; Jörß, K.; Suennemann, J.; Roßmann, L.; Bohnenberger, H.; Tuckermann, J.P.; Reichardt, H.M.; Fischer, H.J.; Reichardt, S.D. The glucocorticoid receptor in recipient cells keeps cytokine secretion in acute graft-versus-host disease at bay. Oncotarget 2018, 9, 15437. [Google Scholar] [CrossRef] [Green Version]
- Vandevyver, S.; Dejager, L.; Van Bogaert, T.; Kleyman, A.; Liu, Y.; Tuckermann, J.; Libert, C. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J. Clin. Investig. 2012, 122, 2130–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballegeer, M.; Van Looveren, K.; Timmermans, S.; Eggermont, M.; Vandevyver, S.; Thery, F.; Dendoncker, K.; Souffriau, J.; Vandewalle, J.; Van Wyngene, L.; et al. Glucocorticoid receptor dimers control intestinal STAT1 and TNF-induced inflammation in mice. J. Clin. Investig. 2018, 128, 3265–3279. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, S.; Bode, C.; Dejager, L.; Frappart, L.; Shelest, E.; Klaßen, C.; Tasdogan, A.; Reichardt, H.M.; Libert, C.; Schneider, M.; et al. Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat. Commun. 2015, 6, 7796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Looveren, K.; Timmermans, S.; Vanderhaeghen, T.; Wallaeys, C.; Ballegeer, M.; Souffriau, J.; Eggermont, M.; Vandewalle, J.; Van Wyngene, L.; De Bosscher, K.; et al. Glucocorticoids limit lipopolysaccharide-induced lethal inflammation by a double control system. EMBO Rep. 2020, 21, e49762. [Google Scholar] [CrossRef]
- Wepler, M.; Preuss, J.M.; Merz, T.; Hartmann, C.; Wachter, U.; McCook, O.; Vogt, J.; Kress, S.; Gröger, M.; Fink, M.; et al. Impaired Glucocorticoid Receptor Dimerization Aggravates LPS-Induced Circulatory and Pulmonary Dysfunction. Front. Immunol. 2020, 10, 3152. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Mukhopadhyay, P.; Belyavskaya, E.; Tonelli, L.H.; Revenis, B.D.; Doran, J.H.; Ballard, B.E.; Tam, J.; Pacher, P.; Sternberg, E.M. Glucocorticoid receptor dimerization is required for proper recovery of LPS-induced inflammation, sickness behavior and metabolism in mice. Mol. Psychiatry 2012, 18, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiman, A.; Hübner, S.; Parkitna, J.M.R.; Neumann, A.; Hofer, S.; Weigand, M.A.; Bauer, M.; Schmid, W.; Schütz, G.; Libert, C.; et al. Glucocorticoid receptor dimerization is required for survival in septic shock via suppression of interleukin-1 in macrophages. FASEB J. 2011, 26, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Timmermans, S.; Paakinaho, V.; Vancraeynest, L.; Dewyse, L.; Vanderhaeghen, T.; Wallaeys, C.; Van Wyngene, L.; Van Looveren, K.; Nuyttens, L.; et al. Combined glucocorticoid resistance and hyperlactatemia contributes to lethal shock in sepsis. Cell Metab. 2021, 33, 1763–1776.e5. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, S.D.; Föller, M.; Rexhepaj, R.; Pathare, G.; Minnich, K.; Tuckermann, J.P.; Lang, F.; Reichardt, H.M. Glucocorticoids Enhance Intestinal Glucose Uptake Via the Dimerized Glucocorticoid Receptor in Enterocytes. Endocrinology 2012, 153, 1783–1794. [Google Scholar] [CrossRef] [Green Version]
- Frijters, R.; Fleuren, W.; Toonen, E.J.; Tuckermann, J.P.; Reichardt, H.M.; van der Maaden, H.; van Elsas, A.; van Lierop, M.-J.; Dokter, W.; de Vlieg, J.; et al. Prednisolone-induced differential gene expression in mouse liver carrying wild type or a dimerization-defective glucocorticoid receptor. BMC Genom. 2010, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandic, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids Suppress Bone Formation by Attenuating Osteoblast Differentiation via the Monomeric Glucocorticoid Receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Conaway, H.H.; Henning, P.; Lie, A.; Tuckermann, J.; Lerner, U.H. Activation of dimeric glucocorticoid receptors in osteoclast progenitors potentiates RANKL induced mature osteoclast bone resorbing activity. Bone 2016, 93, 43–54. [Google Scholar] [CrossRef]
- Hachemi, Y.; Rapp, A.E.; Picke, A.-K.; Weidinger, G.; Ignatius, A.; Tuckermann, J. Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J. Mol. Endocrinol. 2018, 61, R75–R90. [Google Scholar] [CrossRef]
- Waddell, D.; Baehr, L.M.; Brandt, J.V.D.; Johnsen, S.; Reichardt, H.M.; Furlow, J.D.; Bodine, S.C. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am. J. Physiol. Metab. 2008, 295, E785–E797. [Google Scholar] [CrossRef]
- Grose, R.; Werner, S.; Kessler, D.; Tuckermann, J.; Huggel, K.; Durka, S.; Reichardt, H.M.; Werner, S. A role for endogenous glucocorticoids in wound repair. EMBO Rep. 2002, 3, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, S.D.; Weinhage, T.; Rotte, A.; Föller, M.; Oppermann, M.; Lühder, F.; Tuckermann, J.P.; Lang, F.; Brandt, J.V.D.; Reichardt, H.M. Glucocorticoids Induce Gastroparesis in Mice Through Depletion of l-Arginine. Endocrinology 2014, 155, 3899–3908. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.C.; Millar, J.C.; Clark, A.F. Glucocorticoid Receptor Transactivation Is Required for Glucocorticoid-Induced Ocular Hypertension and Glaucoma. Investig. Opthalmol. Vis. Sci. 2019, 60, 1967–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glantschnig, C.; Koenen, M.; Gil-Lozano, M.; Karbiener, M.; Pickrahn, I.; Williams-Dautovich, J.; Patel, R.; Cummins, C.L.; Giroud, M.; Hartleben, G.; et al. A miR-29a-driven negative feedback loop regulates peripheral glucocorticoid receptor signaling. FASEB J. 2019, 33, 5924–5941. [Google Scholar] [CrossRef]
- Asada, M.; Rauch, A.; Shimizu, H.; Maruyama, H.; Miyaki, S.; Shibamori, M.; Kawasome, H.; Ishiyama, H.; Tuckermann, J.; Asahara, H. DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab. Investig. 2010, 91, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Jewell, C.M.; Scoltock, A.B.; Hamel, B.L.; Yudt, M.R.; Cidlowski, J.A. Complex Human Glucocorticoid Receptor dim Mutations Define Glucocorticoid Induced Apoptotic Resistance in Bone Cells. Mol. Endocrinol. 2012, 26, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Oitzl, M.S.; Reichardt, H.M.; Joels, M.; de Kloet, R. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. USA 2001, 98, 12790–12795. [Google Scholar] [CrossRef] [Green Version]
- Van Looveren, K.; Van Boxelaere, M.; Callaerts-Vegh, Z.; Libert, C. Cognitive dysfunction in mice lacking proper glucocorticoid receptor dimerization. PLoS ONE 2019, 14, e0226753. [Google Scholar] [CrossRef] [Green Version]
- Van Wyngene, L.; Vanderhaeghen, T.; Petta, I.; Timmermans, S.; Corbeels, K.; Van der Schueren, B.; Vandewalle, J.; Van Looveren, K.; Wallaeys, C.; Eggermont, M.; et al. ZBTB32 performs crosstalk with the glucocorticoid receptor and is crucial in glucocorticoid responses to starvation. iScience 2021, 24, 102790. [Google Scholar] [CrossRef]
- Vanderhaeghen, T.; Timmermans, S.; Watts, D.; Paakinaho, V.; Eggermont, M.; Vandewalle, J.; Wallaeys, C.; Van Wyngene, L.; Van Looveren, K.; Nuyttens, L.; et al. Reprogramming of glucocorticoid receptor function by hypoxia. EMBO Rep. 2021, 23, e53083. [Google Scholar] [CrossRef]
- Hachemi, Y.; Rapp, A.E.; Lee, S.; Dorn, A.K.; Krüger, B.T.; Kaiser, K.; Ignatius, A.; Tuckermann, J. Intact Glucocorticoid Receptor Dimerization Is Deleterious in Trauma-Induced Impaired Fracture Healing. Front. Immunol. 2021, 11, 3913. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-W.; Uhlenhaut, N.H.; Rauch, A.; Weiner, J.; Hübner, S.; Hübner, N.; Won, K.-J.; Lazar, M.A.; Tuckermann, J.; Steger, D.J. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 2015, 25, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truss, M.; Chalepakis, G.; Slater, E.P.; Mader, S.; Beato, M. Functional interaction of hybrid response elements with wild-type and mutant steroid hormone receptors. Mol. Cell. Biol. 1991, 11, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Drouin, J.; Sun, Y.L.; Tremblay, S.; Lavender, P.; Schmidt, T.J.; De Léan, A.; Nemer, M. Homodimer formation is rate-limiting for high affinity DNA binding by glucocorticoid receptor. Mol. Endocrinol. 1992, 6, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segard-Maurel, I.; Rajkowski, K.; Jibard, N.; Schweizer-Groyer, G.; Baulieu, A.E.-E.; Cadepond, F. Glucocorticosteroid Receptor Dimerization Investigated by Analysis of Receptor Binding to Glucocorticosteroid Responsive Elements Using a Monomer−Dimer Equilibrium Model. Biochemistry 1996, 35, 1634–1642. [Google Scholar] [CrossRef]
- Oakley, R.H.; Jewell, C.M.; Yudt, M.R.; Bofetiado, D.M.; Cidlowski, J.A. The dominant negative activity of the human glucocorticoid receptor beta isoform-Specificity and mechanisms of action. J. Biol. Chem. 1999, 274, 27857–27866. [Google Scholar] [CrossRef] [Green Version]
- Chalepakis, G.; Schauer, M.; Cao, X.; Beato, M. Efficient Binding of Glucocorticoid Receptor to Its Responsive Element Requires a Dimer and DNA Flanking Sequences. DNA Cell Biol. 1990, 9, 355–368. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucl. Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Luisi, B.F.; Xu, W.X.; Otwinowski, Z.; Freedman, L.P.; Yamamoto, K.R.; Sigler, P.B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 1991, 352, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Claessens, F.; Gewirth, D.T. DNA recognition by nuclear receptors. Essays Biochem. 2004, 40, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Zilliacus, J.; Wright, A.; Carlstedt-Duke, J.; Gustafsson, J. Structural determinants of DNA-binding specificity by steroid receptors. Mol. Endocrinol. 1995, 9, 389–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louw, A. GR Dimerization and the Impact of GR Dimerization on GR Protein Stability and Half-Life. Front. Immunol. 2019, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.C.; Kuchenbecker, K.M.; Schiller, B.J.; Gross, J.D.; Pufall, M.A.; Yamamoto, K.R. The glucocorticoid receptor dimer interface allosterically transmits sequence-specific DNA signals. Nat. Struct. Mol. Biol. 2013, 20, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.; Meijer, O.C.; Wang, J.; Bhargava, A.; Pearce, D. Homodimerization of the Glucocorticoid Receptor Is Not Essential for Response Element Binding: Activation of the Phenylethanolamine N-Methyltransferase Gene by Dimerization-Defective Mutants. Mol. Endocrinol. 2003, 17, 2583–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas-Chollier, M.; Watson, L.C.; Cooper, S.B.; Pufall, M.; Liu, J.S.; Borzym, K.; Vingron, M.; Yamamoto, K.R.; Meijsing, S.H. A naturally occurring insertion of a single amino acid rewires transcriptional regulation by glucocorticoid receptor isoforms. Proc. Natl. Acad. Sci. USA 2013, 110, 17826–17831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlman-Wright, K.; Wright, A.; Gustafsson, J.; Carlstedt-Duke, J. Interaction of the glucocorticoid receptor DNA-binding domain with DNA as a dimer is mediated by a short segment of five amino acids. J. Biol. Chem. 1991, 266, 3107–3112. [Google Scholar] [CrossRef]
- Meijsing, S.H.; Pufall, M.A.; So, A.Y.; Bates, D.L.; Chen, L.; Yamamoto, K.R. DNA Binding Site Sequence Directs Glucocorticoid Receptor Structure and Activity. Science 2009, 324, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bledsoe, R.K.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; McKee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal Structure of the Glucocorticoid Receptor Ligand Binding Domain Reveals a Novel Mode of Receptor Dimerization and Coactivator Recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Bianchetti, L.; Wassmer, B.; Defosset, A.; Smertina, A.; Tiberti, M.L.; Stote, R.H.; Dejaegere, A. Alternative dimerization interfaces in the glucocorticoid receptor-α ligand binding domain. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1810–1825. [Google Scholar] [CrossRef]
- Biggadike, K.; Bledsoe, R.K.; Coe, D.M.; Cooper, T.W.J.; House, D.; Iannone, M.A.; Macdonald, S.J.F.; Madauss, K.P.; McLay, I.M.; Shipley, T.J.; et al. Design and X-ray crystal structures of high-potency nonsteroidal glucocorticoid agonists exploiting a novel binding site on the receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 18114–18119. [Google Scholar] [CrossRef] [Green Version]
- Biggadike, K.; Bledsoe, R.K.; Hassell, A.M.; Kirk, B.E.; McLay, I.M.; Shewchuk, L.M.; Stewart, E.L. X-ray Crystal Structure of the Novel Enhanced-Affinity Glucocorticoid Agonist Fluticasone Furoate in the Glucocorticoid Receptor−Ligand Binding Domain. J. Med. Chem. 2008, 51, 3349–3352. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yi, W.; Suino-Powell, K.; Zhou, X.E.; Tolbert, W.; Tang, X.; Yang, J.; Yang, H.; Shi, J.; Hou, L.; et al. Structures and mechanism for the design of highly potent glucocorticoids. Cell Res. 2014, 24, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppi, B.; Jakob, C.; Farnegardh, M.; Yang, J.; Ahola, H.; Alarcon, M.; Calles, K.; Engstrom, O.; Harlan, J.; Muchmore, S.; et al. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain-RU-486 induces a transconformation that leads to active antagonism. J. Biol. Chem. 2003, 278, 22748–22754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madauss, K.P.; Bledsoe, R.K.; Mclay, I.; Stewart, E.L.; Uings, I.J.; Weingarten, G.; Williams, S.P. The first X-ray crystal structure of the glucocorticoid receptor bound to a non-steroidal agonist. Bioorg. Med. Chem. Lett. 2008, 18, 6097–6099. [Google Scholar] [CrossRef]
- Beck, I.M.; De Bosscher, K.; Haegeman, G. Glucocorticoid receptor mutants: Man-made tools for functional research. Trends Endocrinol. Metab. 2011, 22, 295–310. [Google Scholar] [CrossRef]
- Heck, S.; Kullmann, M.; Gast, A.; Ponta, H.; Rahmsdorf, H.J.; Herrlich, P.; Cato, A.C. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994, 13, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Sauter, N.K.; Pearce, D. Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 12480–12484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presman, D.; Ogara, M.F.; Stortz, M.; Alvarez, L.D.; Pooley, J.R.; Schiltz, R.L.; Grøntved, L.; Johnson, T.A.; Mittelstadt, P.R.; Ashwell, J.D.; et al. Live Cell Imaging Unveils Multiple Domain Requirements for In Vivo Dimerization of the Glucocorticoid Receptor. PLoS Biol. 2014, 12, e1001813. [Google Scholar] [CrossRef] [Green Version]
- Presman, D.M.; Ganguly, S.; Schiltz, R.L.; Johnson, T.A.; Karpova, T.; Hager, G.L. DNA binding triggers tetramerization of the glucocorticoid receptor in live cells. Proc. Natl. Acad. Sci. USA 2016, 113, 8236–8241. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, S.; Verhoog, N.J.; Van Looveren, K.; Dewaele, S.; Hochepied, T.; Eggermont, M.; Gilbert, B.; Munck, A.B.-D.; Vanderhaeghen, T.; Berghe, J.V.; et al. Point mutation I634A in the glucocorticoid receptor causes embryonic lethality by reduced ligand binding. J. Biol. Chem. 2022, 298, 101574. [Google Scholar] [CrossRef]
- Tiwari, M.; Oasa, S.; Yamamoto, J.; Mikuni, S.; Kinjo, M. A Quantitative Study of Internal and External Interactions of Homodimeric Glucocorticoid Receptor Using Fluorescence Cross-Correlation Spectroscopy in a Live Cell. Sci. Rep. 2017, 7, 4336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhardt, J.C.M.; Suter, D.; Roy, R.; Zhao, Z.W.; Chapman, A.R.; Basu, S.; Maniatis, T.; Xie, X.S. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 2013, 10, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheschowitsch, K.; Leite, J.A.; Assreuy, J. New Insights in Glucocorticoid Receptor Signaling—More Than Just a Ligand-Binding Receptor. Front. Endocrinol. 2017, 8, 16. [Google Scholar] [CrossRef]
- Pooley, J.R.; Rivers, C.A.; Kilcooley, M.T.; Paul, S.N.; Cavga, A.D.; Kershaw, Y.M.; Muratcioglu, S.; Gursoy, A.; Keskin, O.; Lightman, S.L. Beyond the heterodimer model for mineralocorticoid and glucocorticoid receptor interactions in nuclei and at DNA. PLoS ONE 2020, 15, e0227520. [Google Scholar] [CrossRef]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Surjit, M.; Ganti, K.P.; Mukherji, A.; Ye, T.; Hua, G.; Metzger, D.; Li, M.; Chambon, P. Widespread Negative Response Elements Mediate Direct Repression by Agonist- Liganded Glucocorticoid Receptor. Cell 2011, 145, 224–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Schiller, B.J.; Chodankar, R.; Watson, L.C.; Stallcup, M.R.; Yamamoto, K.R. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol. 2014, 15, 418. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Paakinaho, V.; Kim, S.; Hager, G.L.; Presman, D.M. Genome-wide binding potential and regulatory activity of the glucocorticoid receptor’s monomeric and dimeric forms. Nat. Commun. 2021, 12, 1987. [Google Scholar] [CrossRef]
- Greulich, F.; Bielefeld, K.A.; Scheundel, R.; Mechtidou, A.; Strickland, B.; Uhlenhaut, N.H. Enhancer RNA Expression in Response to Glucocorticoid Treatment in Murine Macrophages. Cells 2021, 11, 28. [Google Scholar] [CrossRef]
- McDowell, I.C.; Barrera, A.; D’Ippolito, A.M.; Vockley, C.M.; Hong, L.K.; Leichter, S.M.; Bartelt, L.C.; Majoros, W.H.; Song, L.; Safi, A.; et al. Glucocorticoid receptor recruits to enhancers and drives activation by motif-directed binding. Genome Res. 2018, 28, 1272–1284. [Google Scholar] [CrossRef] [Green Version]
- Drouin, J.; Sun, Y.L.; Chamberland, M.; Gauthier, Y.; De Léan, A.; Nemer, M.; Schmidt, T.J. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 1993, 12, 145–156. [Google Scholar] [CrossRef]
- Hudson, W.; Youn, C.; Ortlund, E. The structural basis of direct glucocorticoid-mediated transrepression. Nat. Struct. Mol. Biol. 2012, 20, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Hua, G.Q.; Paulen, L.; Chambon, P. GR SUMOylation and formation of an SUMO-SMRT/NCoR1-HDAC3 repressing complex is mandatory for GC-induced IR nGRE-mediated transrepression. Proc. Natl. Acad. Sci. USA 2016, 113, E626–E634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasse, S.K.; Kadiyala, V.; Danhorn, T.; Panettieri, R.A.; Phang, T.L.; Gerber, A.N. Glucocorticoid Receptor ChIP-Seq Identifies PLCD1 as a KLF15 Target that Represses Airway Smooth Muscle Hypertrophy. Am. J. Resp. Cell Mol. 2017, 57, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Puimege, L.; Van Hauwermeiren, F.; Steeland, S.; Van Ryckeghem, S.; Vandewalle, J.; Lodens, S.; Dejager, L.; Vandevyver, S.; Staelens, J.; Timmermans, S.; et al. Glucocorticoid-induced microRNA-511 protects against TNF by down-regulating TNFR1. EMBO Mol. Med. 2015, 7, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Polman, J.A.E.; Welten, J.E.; Bosch, D.S.; de Jonge, R.T.; Balog, J.; van der Maarel, S.M.; de Kloet, E.R.; Datson, N.A. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci. 2012, 13, 118. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.R.; Belvisi, M.G. Maps and legends: The quest for dissociated ligands of the glucocorticoid receptor. Pharmacol. Ther. 2012, 134, 54–67. [Google Scholar] [CrossRef]
- Funder, J.W.; Pearce, P.T.; Smith, R.; Smith, A.I. Mineralocorticoid Action: Target Tissue Specificity Is Enzyme, Not Receptor, Mediated. Science 1988, 242, 583–585. [Google Scholar] [CrossRef]
- Karssen, A.M.; de Kloet, E.R. Synthetic Glucocorticoids. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: New York, NY, USA, 2007; pp. 704–708. [Google Scholar]
- Trapp, T.; Rupprecht, R.; Castrén, M.; Reul, J.M.; Holsboer, F. Heterodimerization between mineralocorticoid and glucocorticoid receptor: A new principle of glucocorticoid action in the CNS. Neuron 1994, 13, 1457–1462. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Yu, G.; Liu, W.; Pearce, D. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J. Biol. Chem. 1997, 272, 14087–14092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bosscher, K.; Desmet, S.J.; Clarisse, D.; Estebanez-Perpina, E.; Brunsveld, L. Nuclear receptor crosstalk—Defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 2020, 16, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Claessens, F.; Joniau, S.; Helsen, C. Comparing the rules of engagement of androgen and glucocorticoid receptors. Cell. Mol. Life Sci. 2017, 74, 2217–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moehren, U.; Denayer, S.; Podvinec, M.; Verrijdt, G.; Claessens, F. Identification of androgen-selective androgen-response elements in the human aquaporin-5 and Rad9 genes. Biochem. J. 2008, 411, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Spaanderman, D.C.; Nixon, M.; Buurstede, J.C.; Sips, H.H.C.M.; Schilperoort, M.; Kuipers, E.N.; Backer, E.A.; Kooijman, S.; Rensen, P.C.N.; Homer, N.Z.; et al. Androgens modulate glucocorticoid receptor activity in adipose tissue and liver. J. Endocrinol. 2019, 240, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougarne, N.; Paumelle, R.; Caron, S.; Hennuyer, N.; Mansouri, R.; Gervois, P.; Staels, B.; Haegeman, G.; De Bosscher, K. PPAR alpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappa B. Proc. Natl. Acad. Sci. USA 2009, 106, 7397–7402. [Google Scholar] [CrossRef] [Green Version]
- Ratman, D.; Mylka, V.; Bougarne, N.; Pawlak, M.; Caron, S.; Hennuyer, N.; Paumelle, R.; De Cauwer, L.; Thommis, J.; Rider, M.H.; et al. Chromatin recruitment of activated AMPK drives fasting response genes co-controlled by GR and PPAR alpha. Nucl. Acids Res. 2016, 44, 10539–10553. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, L.; Mazzon, E.; Bruscoli, S.; Esposito, E.; Crisafulli, C.; Di Paola, R.; Caminiti, R.; Riccardi, C.; Cuzzocrea, S. peroxisome Proliferator-activated receptor-α modulates the anti-inflammatory effect of glucocorticoids in a model of inflammatory bowel disease in mice. Shock 2009, 31, 308–316. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Manu, S.-H.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock {(Sepsis-3)}. JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Bertini, R.; Bianchi, M.; Ghezzi, P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J. Exp. Med. 1988, 167, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Van Bladel, S.; Brouckaert, P.; Fiers, W. The Influence of Modulating Substances on Tumor Necrosis Factor and Interleukin-6 Levels after Injection of Murine Tumor Necrosis Factor or Lipopolysaccharide in Mice. J. Immunother. 1991, 10, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Van Bogaert, T.; Vandevyver, S.; Dejager, L.; Van Hauwermeiren, F.; Pinheiro, I.; Petta, I.; Engblom, D.; Kleyman, A.; Schutz, G.; Tuckermann, J.; et al. Tumor necrosis factor inhibits glucocorticoid receptor function in mice: A strong signal toward lethal shock. J. Biol. Chem. 2011, 286, 26555–26567. [Google Scholar] [CrossRef] [Green Version]
- Ballegeer, M.; Vandewalle, J.; Eggermont, M.; Van Isterdael, G.; Dejager, L.; De Bus, L.; Decruyenaere, J.; Vandenbroucke, R.E.; Libert, C. Overexpression of Gilz Protects Mice Against Lethal Septic Peritonitis. Shock 2019, 52, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Diesel, B.; Eifler, L.K.; Schmid, T.; Brüne, B.; Kiemer, A.K. Glucocorticoid-induced leucine zipper is downregulated in human alveolar macrophages upon Toll-like receptor activation. Eur. J. Immunol. 2012, 42, 1282–1293. [Google Scholar] [CrossRef]
- Ellouze, M.; Vigouroux, L.; Tcherakian, C.; Woerther, P.; Guguin, A.; Robert, O.; Surenaud, M.; Tran, T.; Calmette, J.; Barbin, T.; et al. Overexpression of GILZ in macrophages limits systemic inflammation while increasing bacterial clearance in sepsis in mice. Eur. J. Immunol. 2019, 50, 589–602. [Google Scholar] [CrossRef]
- Wepler, M.; Preuss, J.M.; Merz, T.; McCook, O.; Radermacher, P.; Tuckermann, J.P.; Vettorazzi, S. Impact of downstream effects of glucocorticoid receptor dysfunction on organ function in critical illness-associated systemic inflammation. Intensiv. Care Med. Exp. 2020, 8, 37. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 306–311. [Google Scholar] [CrossRef] [Green Version]


| Process | Effect in GRdim/dim Mutant | References |
|---|---|---|
| Resolution of inflammation | ||
| Antigen- and G6PI-induced arthritis | DEX protection lost | [50] |
| Serum transfer-induced arthritis | DEX protection lost | [51] |
| Contact hypersensitivity | DEX protection lost | [52] |
| PMA-induced irritative skin inflammation | DEX protection intact | [53,54] |
| Experimental autoimmune encephalomyelitis | DEX protection intact | [55] |
| Allergic airway inflammation | DEX protection lost | [56] |
| Graft- vs host disease | Increased mortality | [57] |
| TNF-induced SIRS | Increased mortality + DEX protection lost | [58,59] |
| LPS-induced SIRS | Increased mortality + DEX protection lost | [60,61,62,63] |
| CLP-induced septic shock | Increased mortality | [64,65] |
| Side effects | ||
| Hyperglycemia | Pred effect reduced | [66,67] |
| Osteoporosis | Pred/DEX effect intact | [68,69,70] |
| Skeletal muscle atrophy | DEX effect intact | [71] |
| Wound repair | Wound repair reduced | [72] |
| Gastroparesis and gastric acid secretion | DEX effect lost | [73] |
| Ocular hypertension leading to glaucoma | DEX effect lost | [74] |
| Glucocorticoid resistance | DEX effect lost | [75] |
| Cellular processes | ||
| Adipogenesis | No adipogenesis | [76] |
| Apoptosis | DEX effect lost | [46,77] |
| Proliferation | Proliferation reduced | [46] |
| Spatial memory | Spatial memory reduced | [78] |
| Cognitive function under stress condition | CORT effect reduced | [79] |
| Weight control | Body weight increased | [80] |
| Activation HPA axis in 6% hypoxia | Activation of HPA axis reduced | [81] |
| Trauma-induced fracture healing | Protected | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timmermans, S.; Vandewalle, J.; Libert, C. Dimerization of the Glucocorticoid Receptor and Its Importance in (Patho)physiology: A Primer. Cells 2022, 11, 683. https://doi.org/10.3390/cells11040683
Timmermans S, Vandewalle J, Libert C. Dimerization of the Glucocorticoid Receptor and Its Importance in (Patho)physiology: A Primer. Cells. 2022; 11(4):683. https://doi.org/10.3390/cells11040683
Chicago/Turabian StyleTimmermans, Steven, Jolien Vandewalle, and Claude Libert. 2022. "Dimerization of the Glucocorticoid Receptor and Its Importance in (Patho)physiology: A Primer" Cells 11, no. 4: 683. https://doi.org/10.3390/cells11040683
APA StyleTimmermans, S., Vandewalle, J., & Libert, C. (2022). Dimerization of the Glucocorticoid Receptor and Its Importance in (Patho)physiology: A Primer. Cells, 11(4), 683. https://doi.org/10.3390/cells11040683






