Central Nervous System Complications in Cystinosis: The Role of Neuroimaging
Abstract
:1. Introduction
2. Clinical Presentation
2.1. Clinical Data
2.2. Risk Factors for the Development of Central Nervous System Complications
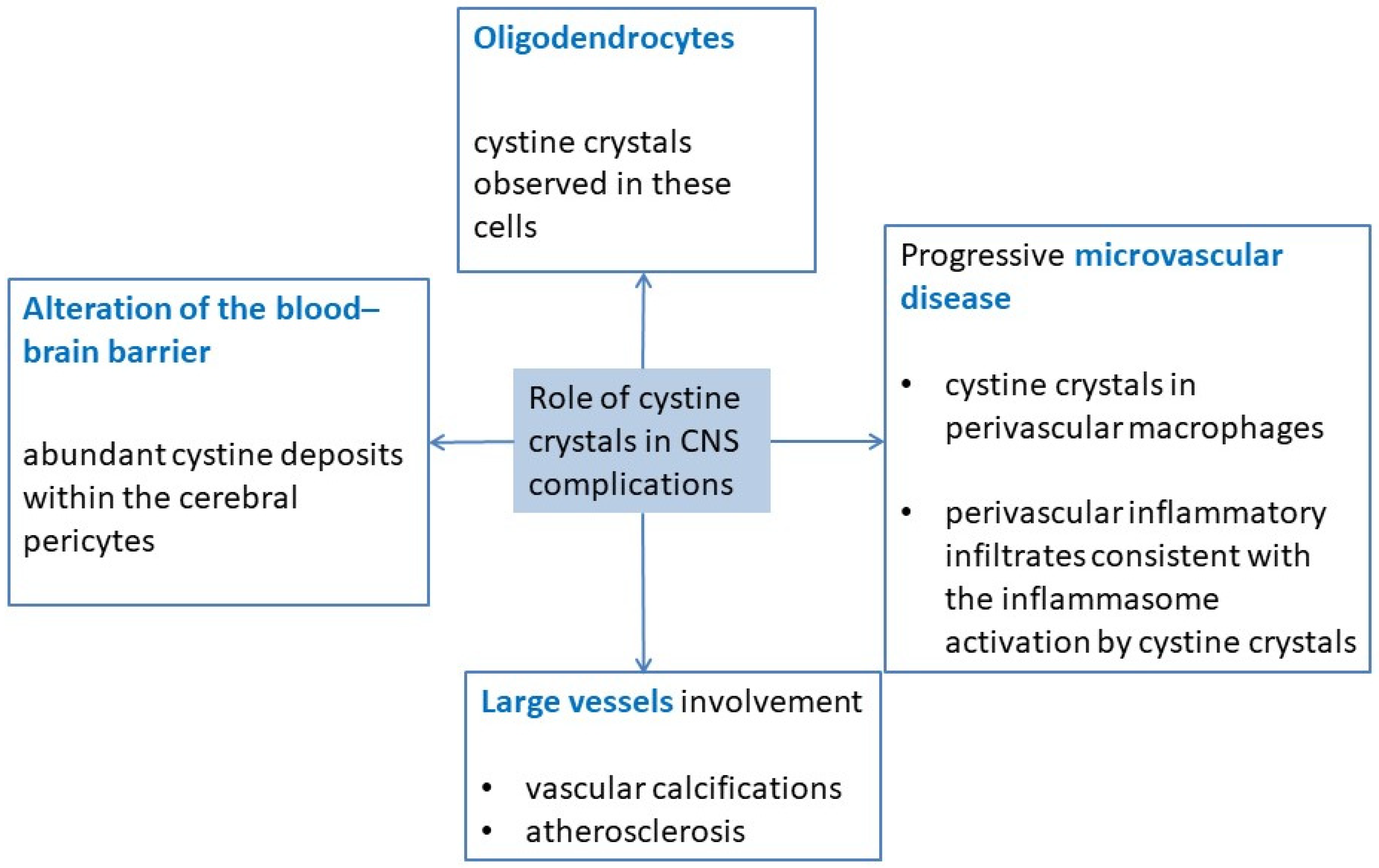
3. Pathophysiology
4. Neurocognitive Impairments
5. Radiological Data
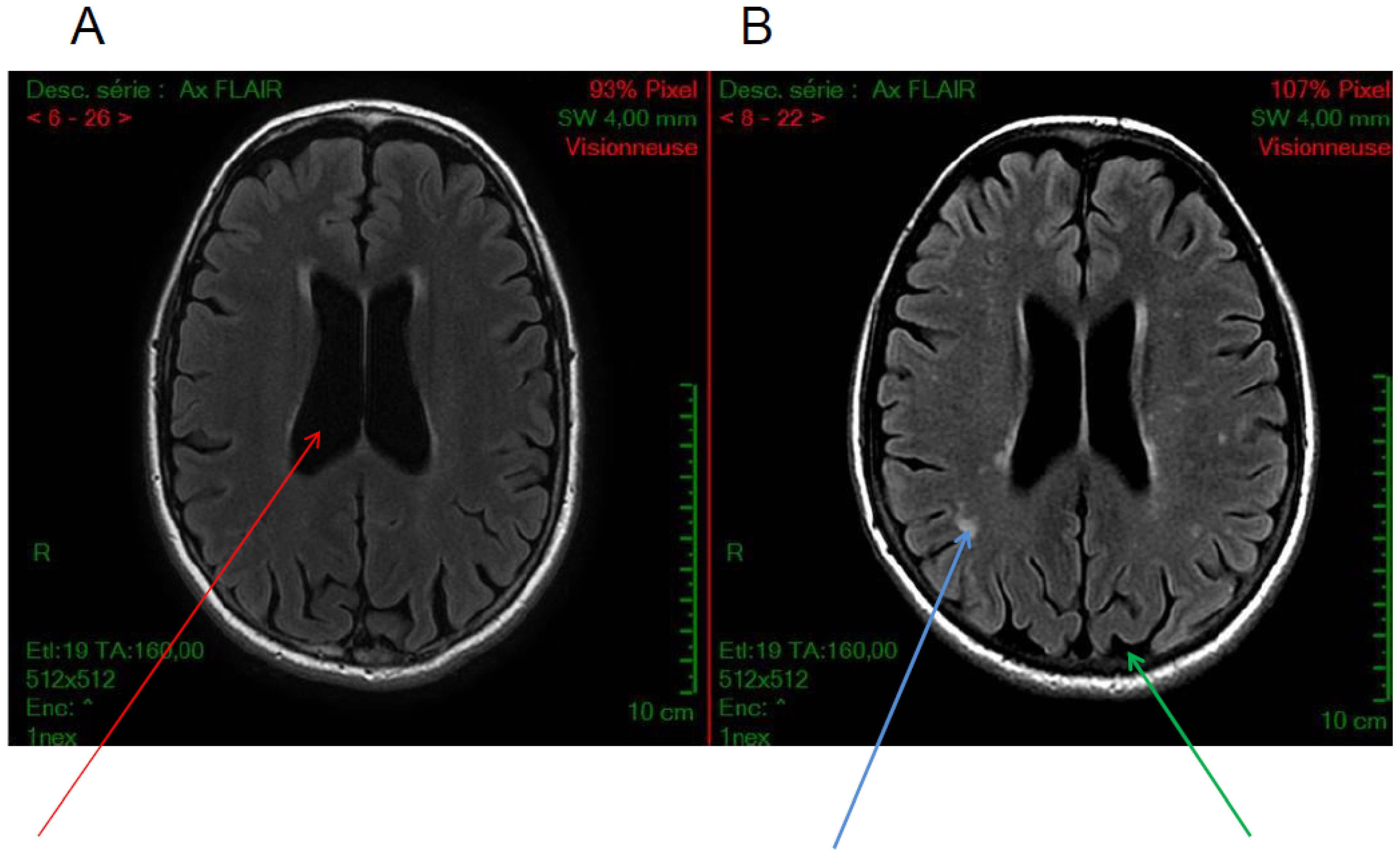
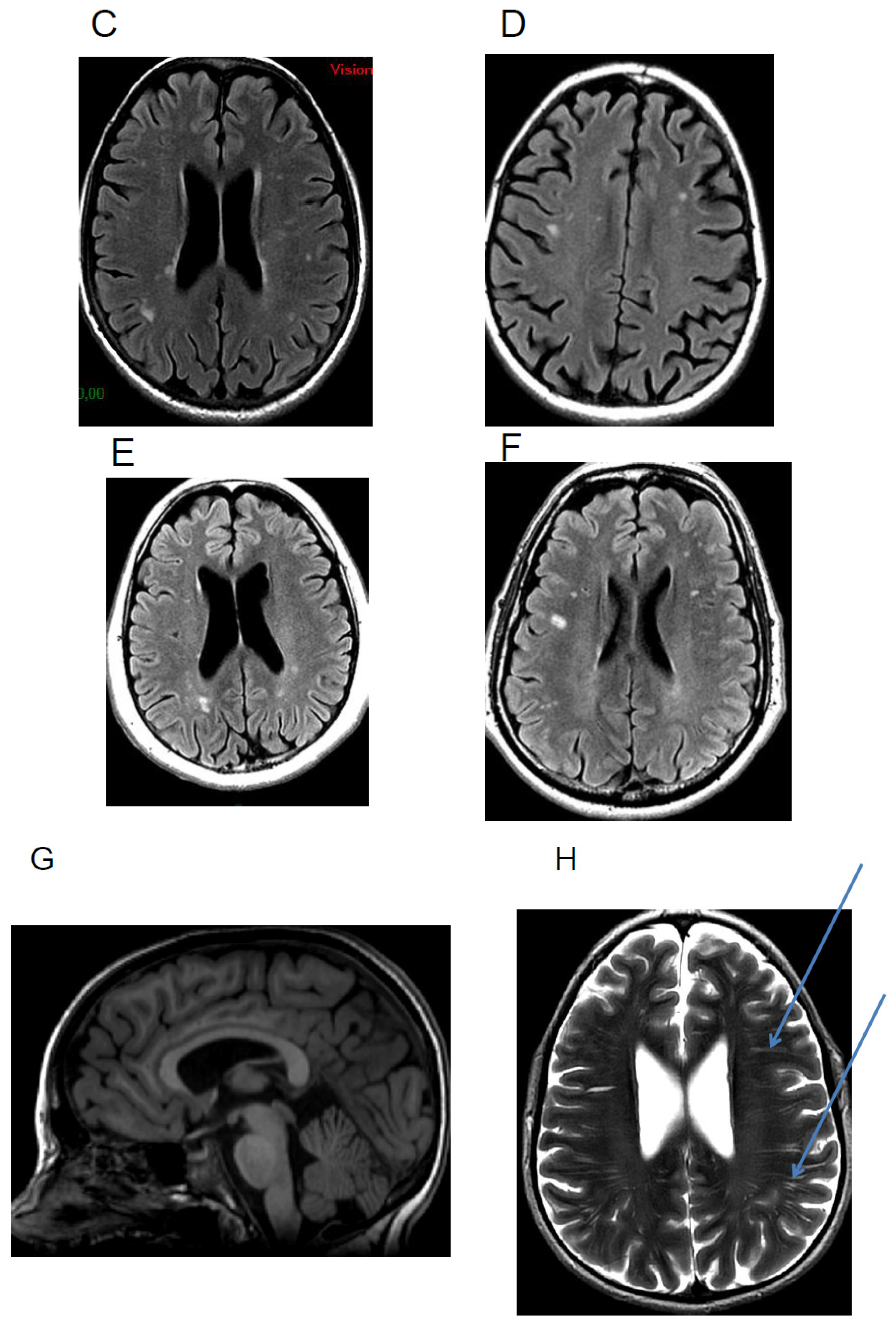
5.1. Calcifications
5.2. Cortical Atrophy
5.3. White Matter Hyperintensities
5.4. Others
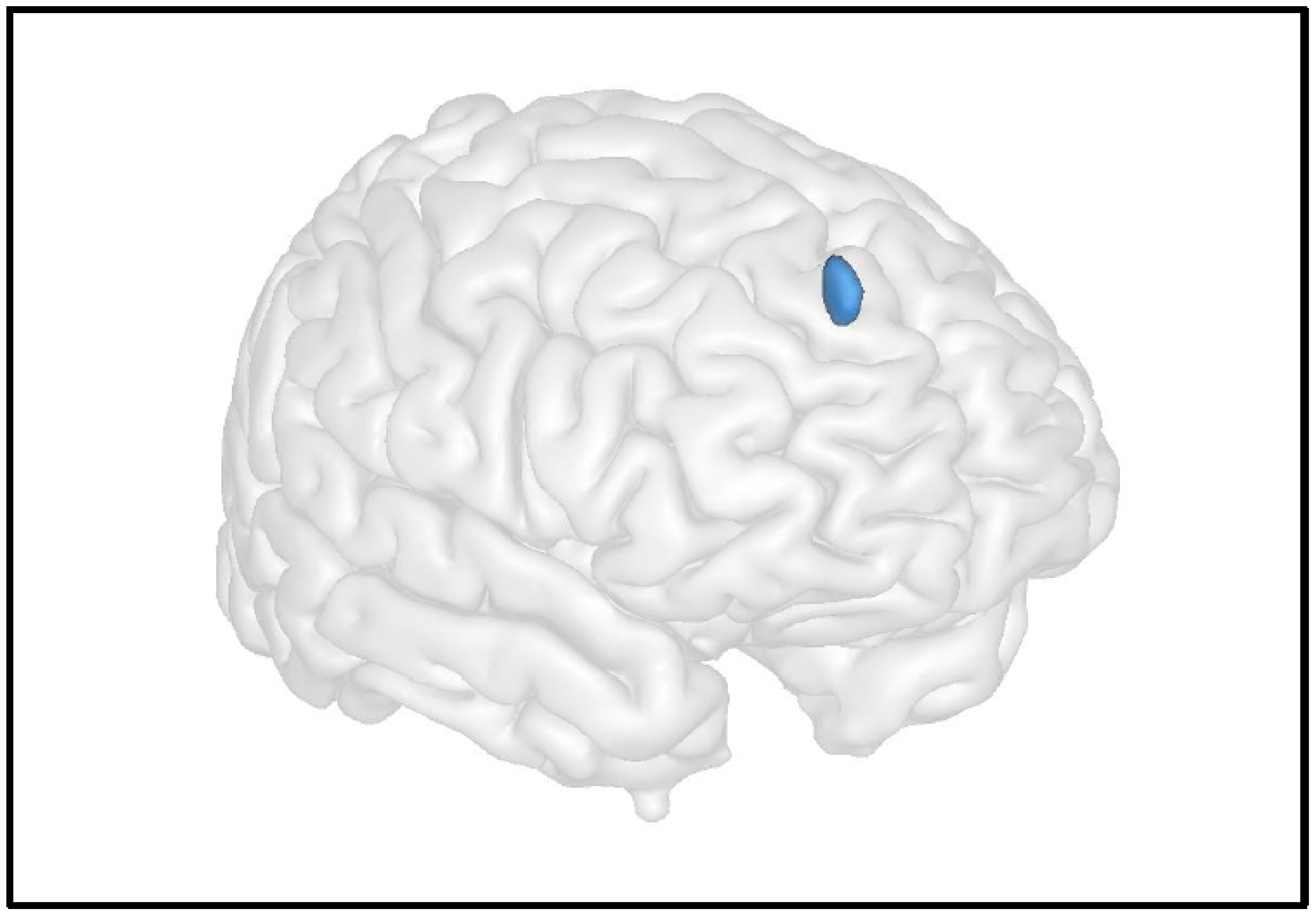
6. Neuroimaging Investigations of Anatomo-Functional CNS Abnormalities
6.1. Grey Matter
6.2. White Matter
6.3. Resting Brain Function
7. Electrophysiological Activity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kalatzis, V.; Cherqui, S.; Antignac, C.; Gasnier, B. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lyso-somal cystine transporter. EMBO J. 2001, 20, 5940–5949. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.; Morinière, V.; Grünfeld, J.-P.; Noël, L.-H.; Goujon, J.-M.; Chadefaux-Vekemans, B.; Antignac, C. Late-Onset Nephropathic Cystinosis: Clinical Presentation, Outcome, and Genotyping. Clin. J. Am. Soc. Nephrol. 2008, 3, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Gahl, W.A.; Kaiser-Kupfer, M.I. Complications of nephropathic cystinosis after renal failure. Pediatr. Nephrol. 1987, 1, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Schneider, J.A.; Thoene, J.G.; Chesney, R. Course of nephropathic cystinosis after age 10 years. J. Pediatr. 1986, 109, 605–608. [Google Scholar] [CrossRef]
- Theodoropoulos, D.S.; Krasnewich, D.; Kaiser-Kupfer, M.I.; Gahl, W.A. Classic nephropathic cystinosis as an adult disease. JAMA J. Am. Med. Assoc. 1993, 270, 2200–2204. [Google Scholar] [CrossRef]
- Broyer, M.; Tete, M.J.; Guest, G.; Bertheleme, J.P.; Labrousse, F.; Poisson, M. Clinical polymorphism of cystinosis encephalopathy. Results of treatment with cysteamine. J. Inherit. Metab. Dis. 1996, 19, 65–75. [Google Scholar] [CrossRef]
- Rossi, A.; Biancheri, R. Magnetic Resonance Spectroscopy in Metabolic Disorders. Neuroimaging Clin. N. Am. 2013, 23, 425–448. [Google Scholar] [CrossRef]
- Fink, J.K.; Brouwers, P.; Barton, N.; Mohammed, H.M.; Sato, S.; Hill, S.; Cohen, W.E.; Fivush, B.; Gahl, W.A. Neurologic Complications in Long-standing Nephropathic Cystinosis. Arch. Neurol. 1989, 46, 543–548. [Google Scholar] [CrossRef]
- Trauner, D.A.; Chase, C.; Scheller, J.; Katz, B.; Schneider, J.A. Neurologic and cognitive deficits in children with cystinosis. J. Pediatr. 1988, 112, 912–914. [Google Scholar] [CrossRef]
- Trauner, D.A.; Williams, J.; Ballantyne, A.O.; Spilkin, A.M.; Crowhurst, J.; Hesselink, J. Neurological impairment in nephropathic cystinosis: Motor coordination deficits. Pediatr. Nephrol. 2010, 25, 2061–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viltz, L.; Trauner, D.A. Effect of Age at Treatment on Cognitive Performance in Patients with Cystinosis. J. Pediatr. 2013, 163, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogulu, C.F.; Tsilou, E.; Rubin, B.; FitzGibbon, E.J.; Kaiser-Kupper, M.I.; Rennert, O.M.; Gahl, W.A. Idiopathic intracranial hypertension in cystinosis. J. Pediatr. 2004, 145, 673–678. [Google Scholar] [CrossRef]
- Ross, D.L.; Strife, C.F.; Towbin, R.; Bove, K.E. Nonabsorptive hydrocephalus associated with nephropathic cystinosis. Neurology 1982, 32, 1330. [Google Scholar] [CrossRef] [PubMed]
- Brodin-Sartorius, A.; Tête, M.; Niaudet, P.; Antignac, C.; Guest, G.; Ottolenghi, C.; Charbit, M.; Moyse, D.; Legendre, C.; Lesavre, P.; et al. Progression of Nephropathic Cystinosis in Late Adolescents and Adults: The Impact of Cysteamine Therapy. Kidney Int. 2012, 81, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahl, W.A.; Balog, J.Z.; Kleta, R. Nephropathic Cystinosis in Adults: Natural History and Effects of Oral Cysteamine Therapy. Ann. Intern. Med. 2007, 147, 242–250. [Google Scholar] [CrossRef]
- Servais, A.; Saitovitch, A.; Hummel, A.; Boisgontier, J.; Scemla, A.; Sberro-Soussan, R.; Snanoudj, R.; Lemaitre, H.; Legendre, C.; Pontoizeau, C.; et al. Central nervous system complications in adult cystinosis patients. J. Inherit. Metab. Dis. 2020, 43, 348–356. [Google Scholar] [CrossRef]
- Ueda, M.; O’brien, K.; Rosing, D.R.; Ling, A.; Kleta, R.; McAreavey, D.; Bernardini, I.; Gahl, W.A. Coronary Artery and Other Vascular Calcifications in Patients with Cystinosis after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2006, 1, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Borrell-Pagès, M.; Canals, J.M.; Cordelières, F.P.; Parker, J.A.; Pineda, J.R.; Grange, G.; Bryson, E.A.; Guillermier, M.; Hirsch, E.; Hantraye, P.; et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J. Clin. Investig. 2006, 116, 1410–1424. [Google Scholar] [CrossRef] [Green Version]
- Ariceta, G.; Lara, E.; Camacho, J.A.; Oppenheimer, F.; Vara, J.; Santos, F.; Muñoz, M.A.; Cantarell, C.; Calvo, M.G.; Romero, R.; et al. Cysteamine (Cystagon(R)) adherence in patients with cystinosis in Spain: Successful in children and a challenge in adolescents and adults. Nephrol. Dial. Transplant. 2015, 30, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Maurice, T.; Hippert, C.; Serratrice, N.; Dubois, G.; Jacquet, C.; Antigna, C.; Kremer, E.J.; Kalatzis, V. Cystine accumulation in the CNS results in severe age-related memory deficits. Neurobiol. Aging 2009, 30, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.G.; Malekzadeh, M.H.; Cornford, M.E.; Schneider, J.A.; Shields, W.D.; Vinters, H.V. Central nervous system involvement in nephropathic cystinosis. J. Neuropathol. Exp. Neurol. 1990, 49, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, G.; Gahl, W. Nephropathic cystinosis: Late complications of a multisystemic disease. Pediatr. Nephrol. 2007, 23, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R.; Dillon, D.A.; Young, B.A.; Goldstein, S.J.; Nelson, P. Cystinosis of the brain and spinal cord with associated vasculopathy. J. Neurol. Sci. 2009, 284, 182–185. [Google Scholar] [CrossRef]
- Prencipe, G.; Caiello, I.; Cherqui, S.; Whisenant, T.; Petrini, S.; Emma, F.; De Benedetti, F. Inflammasome Activation by Cystine Crystals: Implications for the Pathogenesis of Cystinosis. J. Am. Soc. Nephrol. 2014, 25, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Strayer, D.S. Cystinosis and a Dissecting Aortic Aneurysm in a 7-Year-Old Boy. Arch. Pediatr. Adolesc. Med. 1979, 133, 436–438. [Google Scholar] [CrossRef]
- Jonas, A.J.; Conley, S.B.; Marshall, R.; Johnson, R.A.; Marks, M.; Rosenberg, H. Nephropathic cystinosis with central nervous system involvement. Am. J. Med. 1987, 83, 966–970. [Google Scholar] [CrossRef]
- Neutel, D.; Geraldes, R.; Pereira, P.; Da Costa, A.G.; Pimentel, J.; Melo, T.P.E. Recurrent Ischemic Stroke in an Adult with Cystinosis: A Clinical–Pathological Case. J. Stroke Cerebrovasc. Dis. 2013, 22, e674–e675. [Google Scholar] [CrossRef]
- Mitsnefes, M.M. Cardiovascular Disease in Children with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2012, 23, 578–585. [Google Scholar] [CrossRef]
- Scarvie, K.M.; Ballantyne, A.O.; Trauner, D.A. Visuomotor performance in children with infantile nephropathic cystinosis. Percept. Mot. Skills 1996, 82, 67–75. [Google Scholar] [CrossRef]
- Ballantyne, A.O.; Spilkin, A.M.; Trauner, D.A. Executive Function in Nephropathic Cystinosis. Cogn. Behav. Neurol. 2013, 26, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, R.; Makar, S.; El Bakri, A.; Soliman, N.A. Neurocognitive functions and behavioral profiles in children with nephropathic cystinosis. Saudi. J. Kidney Dis. Transpl. 2014, 25, 1224–1231. [Google Scholar] [PubMed]
- Besouw, M.T.P.; Hulstijn-Dirkmaat, G.M.; van der Rijken, R.E.A.; Cornelissen, E.A.M.; van Dael, C.M.; Walle, J.V.; Lilien, M.R. Neurocognitive functioning in school-aged cystinosis patients. J. Inherit. Metab. Dis. 2010, 33, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Sathappan, A.; Trauner, D. Hierarchical processing of visual stimuli in nephropathic cystinosis. J. Inherit. Metab. Dis. 2019, 42, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Curie, A.; Touil, N.; Gaillard, S.; Galanaud, D.; Leboucq, N.; Deschênes, G.; Morin, D.; Abad, F.; Luauté, J.; Bodenan, E.; et al. Neuropsychological and neuroanatomical phenotype in 17 patients with cystinosis. Orphanet J. Rare Dis. 2020, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, S.L.; Press, G.A.; Schneider, J.A.; Trauner, D.A. Cortical atrophy and cognitive performance in infantile nephropathic cystinosis. Pediatr. Neurol. 1990, 6, 379–381. [Google Scholar] [CrossRef]
- Levine, S.; Paparo, G. Brain lesions in a case of cystinosis. Acta Neuropathol. 1982, 57, 217–220. [Google Scholar] [CrossRef]
- Cochat, P.; Drachman, R.; Gagnadoux, M.F.; Pariente, D.; Broyer, M. Cerebral atrophy and nephropathic cystinosis. Arch. Dis. Child. 1986, 61, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Ehrich, J.; Stoeppler, L.; Offner, G.; Brodehl, J. Evidence for Cerebral Involvement in Nephropathic Cystinosis. Neuropediatrics 1979, 10, 128–137. [Google Scholar] [CrossRef]
- Marquardt, L.; Kuramatsu, J.B.; Roesch, J.; Engelhorn, T.; Huttner, H.B. Posterior reversible encephalopathy syndrome in cystinosis. Clin. Neurol. Neurosurg. 2013, 115, 644–645. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Tsai, H.H.; Lai, Y.J.; Tseng, H.Y.; Wu, Y.; Peng, Y.; Chiu, C.; Chuang, Y. Cognitive impairment in patients with end-stage renal disease: Accelerated brain aging? J. Formos. Med. Assoc. 2019, 118, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Koo, B.-B.; Bhadelia, R.; Weiner, D.E.; Duncan, S.; La Garza, M.M.-D.; Gupta, A.; Tighiouart, H.; Scott, T.; Sarnak, M.J. White matter damage in maintenance hemodialysis patients: A diffusion tensor imaging study. BMC Nephrol. 2017, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bava, S.; Theilmann, R.J.; Sach, M.; May, S.J.; Frank, L.R.; Hesselink, J.R.; Vu, D.; Trauner, D.A. Developmental changes in cerebral white matter microstructure in a disorder of lysosomal storage. Cortex 2010, 46, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.I.; Hesselink, J.; Trauner, D.A.; Chiari, I. Malformation in Nephropathic Cystinosis. J. Pediatr. 2015, 167, 1126–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazals, X.; Lauvin, M.A.; Favelle, O.; Domengie, F.; Nivet, H.; Cottier, J.P. Cystinosis encephalopathy: MRI perivascular enhancement with micronodular T2* hypointensity. Diagn. Interv. Imaging 2013, 94, 653–655. [Google Scholar] [CrossRef] [Green Version]
- Basser, P.J.; Pierpaoli, C. A simplified method to measure the diffusion tensor from seven MR images. Magn. Reson. Med. 1998, 39, 928–934. [Google Scholar] [CrossRef]
- Doron, K.W.; Gazzaniga, M.S. Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex 2008, 44, 1023–1029. [Google Scholar] [CrossRef]
- Goldman, J.G.; Bledsoe, I.O.; Merkitch, D.; Dinh, V.; Bernard, B.; Stebbins, G.T. Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease. Neurology 2017, 88, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-C.; Jiang, S.-F.; Yang, S.-C.; Lien, S.-H. Pseudocontinuous arterial spin labeling perfusion magnetic resonance imaging—A normative study of reproducibility in the human brain. NeuroImage 2011, 56, 1244–1250. [Google Scholar] [CrossRef]
- Francisco, A.A.; Foxe, J.J.; Horsthuis, D.J.; Molholm, S. Impaired auditory sensory memory in Cystinosis despite typical sensory processing: A high-density electrical mapping study of the mismatch negativity (MMN). Neuroimage Clin. 2020, 25, 102170. [Google Scholar] [CrossRef]
- Ethier, A.-A.; Lippé, S.; Merouani, A.; Lassonde, M.; Saint-Amour, D. Reversible Visual Evoked Potential Abnormalities in Uremic Children. Pediatr. Neurol. 2012, 46, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Picton, T. The N1 Wave of the Human Electric and Magnetic Response to Sound: A Review and an Analysis of the Component Structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef] [PubMed]
- Giard, M.; Perrin, F.; Echallier, J.; Thévenet, M.; Froment, J.; Pernier, J. Dissociation of temporal and frontal components in the human auditory N1 wave: A scalp current density and dipole model analysis. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1994, 92, 238–252. [Google Scholar] [CrossRef]
- Francisco, A.A.; Berruti, A.S.; Kaskel, F.J.; Foxe, J.J.; Molholm, S. Assessing the integrity of auditory processing and sensory memory in adults with cystinosis (CTNS gene mutations). Orphanet J. Rare Dis. 2021, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servais, A.; Boisgontier, J.; Saitovitch, A.; Hummel, A.; Boddaert, N. Central Nervous System Complications in Cystinosis: The Role of Neuroimaging. Cells 2022, 11, 682. https://doi.org/10.3390/cells11040682
Servais A, Boisgontier J, Saitovitch A, Hummel A, Boddaert N. Central Nervous System Complications in Cystinosis: The Role of Neuroimaging. Cells. 2022; 11(4):682. https://doi.org/10.3390/cells11040682
Chicago/Turabian StyleServais, Aude, Jennifer Boisgontier, Ana Saitovitch, Aurélie Hummel, and Nathalie Boddaert. 2022. "Central Nervous System Complications in Cystinosis: The Role of Neuroimaging" Cells 11, no. 4: 682. https://doi.org/10.3390/cells11040682




