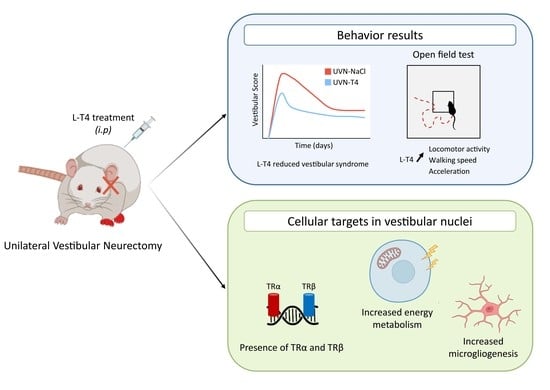
L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Unilateral Vestibular Neurectomy
2.3. Criteria for Exclusion
- -
- loss of body weight equal to more than 20% of the pre-operative value.
- -
- if the facial nerve had been sectioned.
- -
- abnormalities in behavioral scoring, i.e., inability of the animal to stand on all four paws after 5 days post-UVN, convulsions, hemiataxia, etc.
2.4. Study Design
2.5. Qualitative Assessment of the Vestibular Syndrome
- -
- Tail hanging behavior: Animals were picked up from the ground at the base of the tail and body rotation was scored from 0 point (no rotation) to 3 points (several rotations of 360°)
- -
- Landing reflex: After animals were picked up from the ground at the base of the tail, we scored the first 3 landings from 0 (presence of a landing reflex on the 3 landings) to 3 points (absence of landing reflex on the 3 landings). When lifted by the tail, control rats exhibit a landing reflex, consisting of forelimb extension, that allows them to land successfully (i.e., they land on all four legs). Rats with impaired vestibular function do not exhibit a forelimb extension, they spin or bend ventrally, sometimes “crawling” up toward their tails, causing them to miss their landings.
- -
- Rearing: the ability of the rat to rear was scored from 0 point (rearing is observed) to 1 point (rearing is absent)
- -
- Grooming: the ability of the rat to groom correctly were scored as follows: 0 point (correct grooming of full body) 1 point (grooming of the face, belly, and flanks but not the base of the tail), 2 points (grooming of the face and belly), 3 points (grooming of the face), 4 points (inability of the animal to groom itself)
- -
- Displacement: quality of the displacement of the rat was scored from 0 (displacement of the rat with no visible deficit) to 3 points (several deficits in the displacement of the rat)
- -
- Head tilt was scored by estimating the angle between the jaw plane and the horizontal with 0 points (absence of a head-tilt) to 3 points (for a 90° angle)
- -
- Barrel rolling was scored as follows: 0 points (absence of barrel rolling), 1 point (barrel rolling evoked by an acceleration in the vertical axis of the rat in our hand), 2 points (spontaneous barrel rolling)
- -
- Retropulsion characterizes backwards movements and was scored from 0 (absence of retropulsion) to 1 point (presence of retropulsion)
- -
- Circling was scored from 0 point (absence of circling behavior) to 1 point (presence of circling behavior)
- -
- Bobbing is related to rapid head tilts to the side and was scored from 0 point (absence of bobbing) to 1 point (presence of bobbing)
2.6. Weight Distribution
2.7. Open Field Test
2.8. Support Surface
2.9. Tissue Preparation
2.10. Immunohistochemistry
2.11. Cells Count and Statistical Analysis
2.12. Quantification of KCC2
2.13. Cytochrome Oxidase Histochemistry
2.14. Statistical Analysis
3. Results
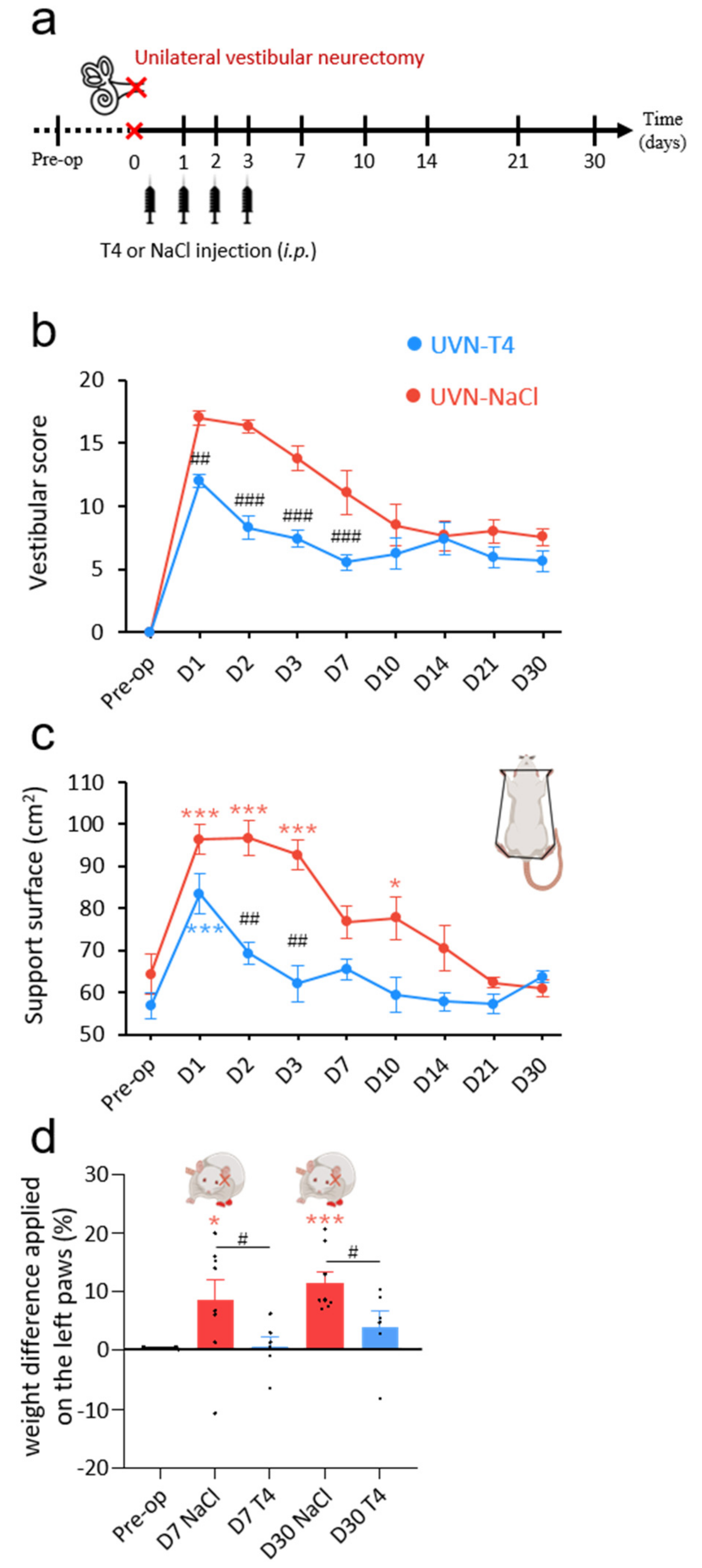
3.1. L-Thyroxine Treatment Reduced Postural Vestibular Deficits
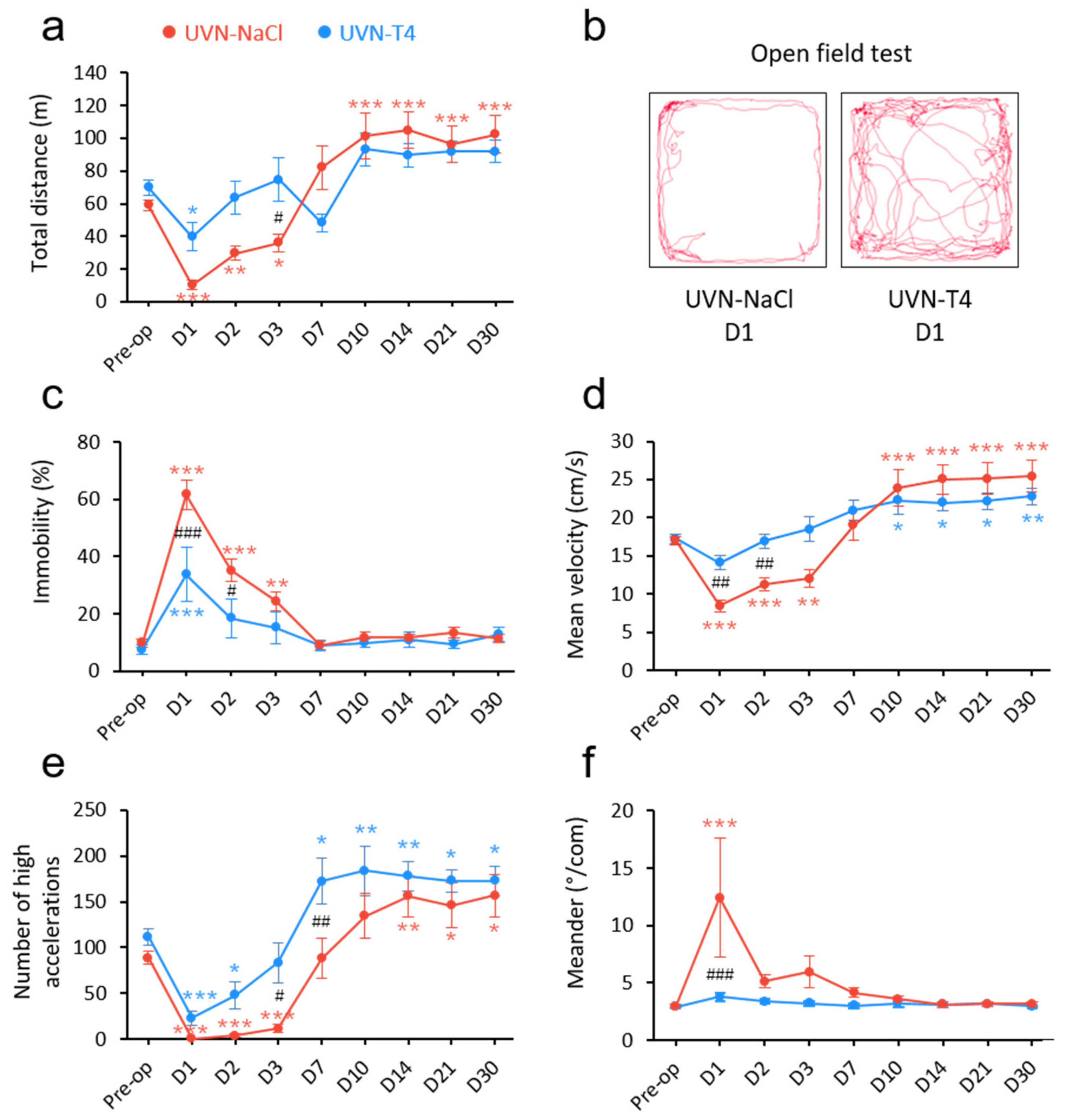
3.2. L-Thyroxine Treatment Improved Locomotor Recovery after UVN
3.3. Presence of Thyroid Hormone Receptors and Thyroxine Deiodinase 2 in the Vestibular Nuclei
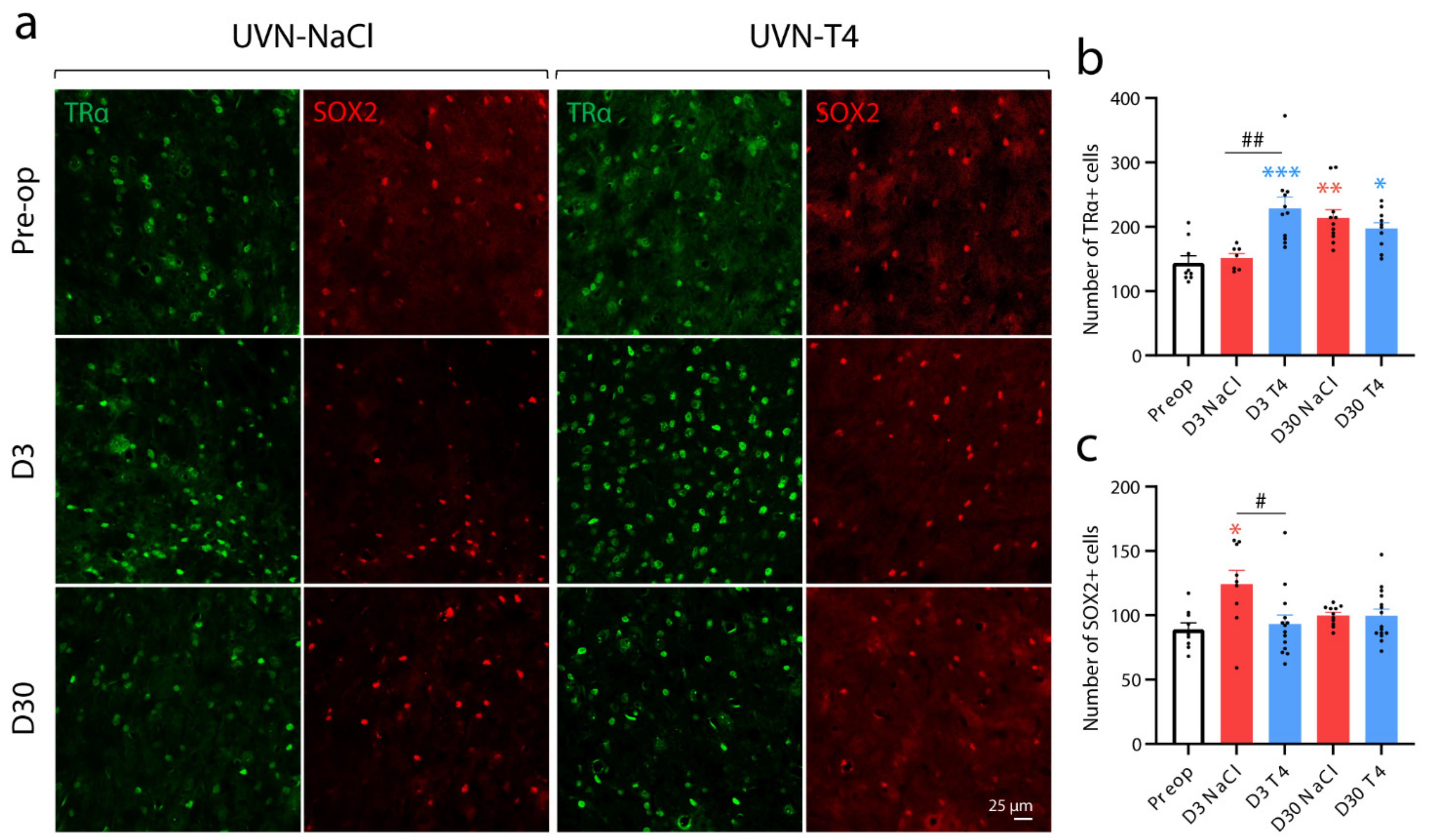
3.4. L-Thyroxine Up-Regulates TRα and Block the Up-Regulation of SOX2 in the Deafferented Vestibular Nuclei
3.5. L-Thyroxine Increases Cell Proliferation and Alters the Cellular Fate of Newly Generated Cells in the Deafferented Vestibular Nuclei
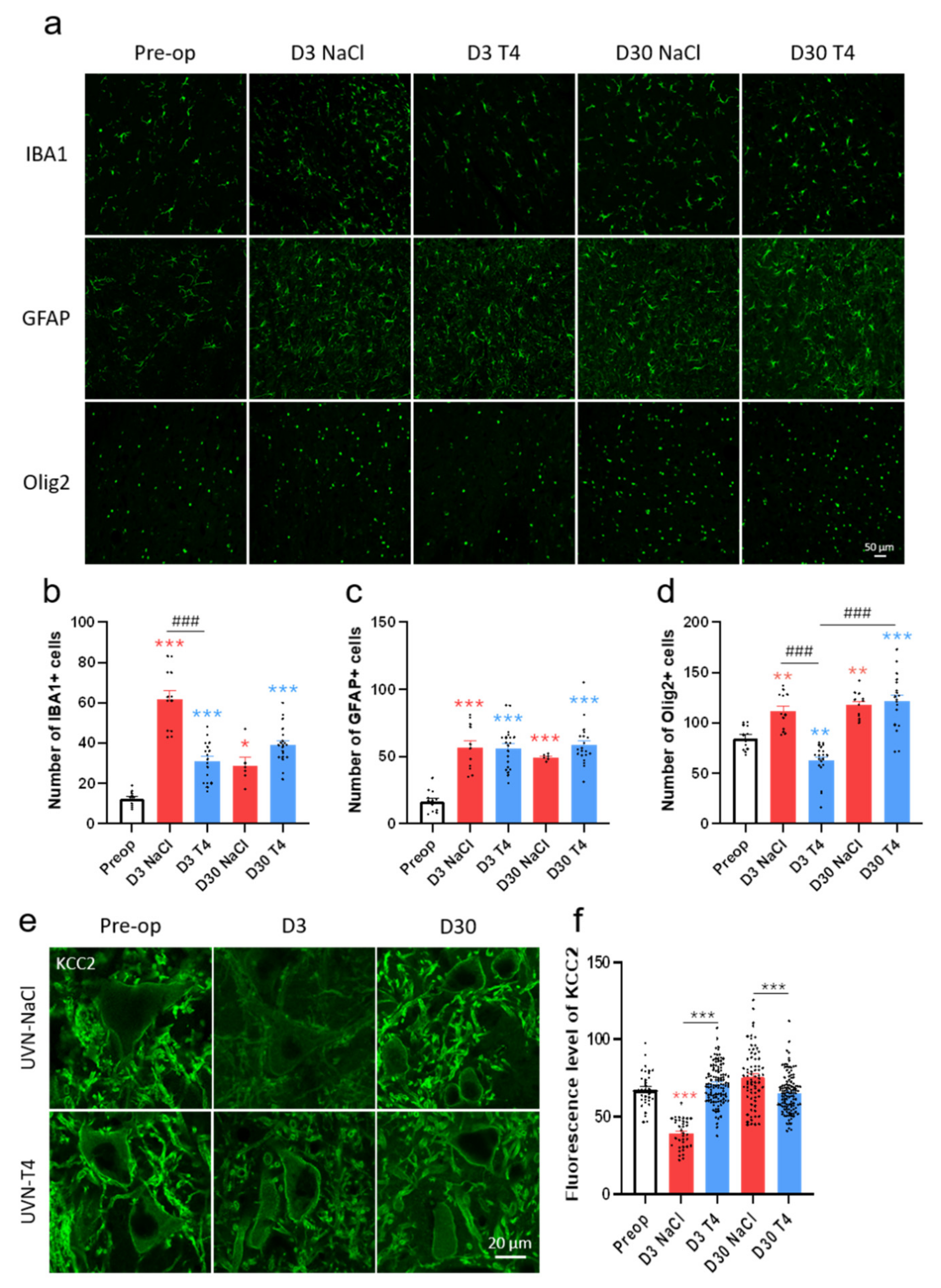
3.6. L-Thyroxine Modulates the Glial Reaction and Prevents Downregulation of KCC2 in the Deafferented Vestibular Nuclei
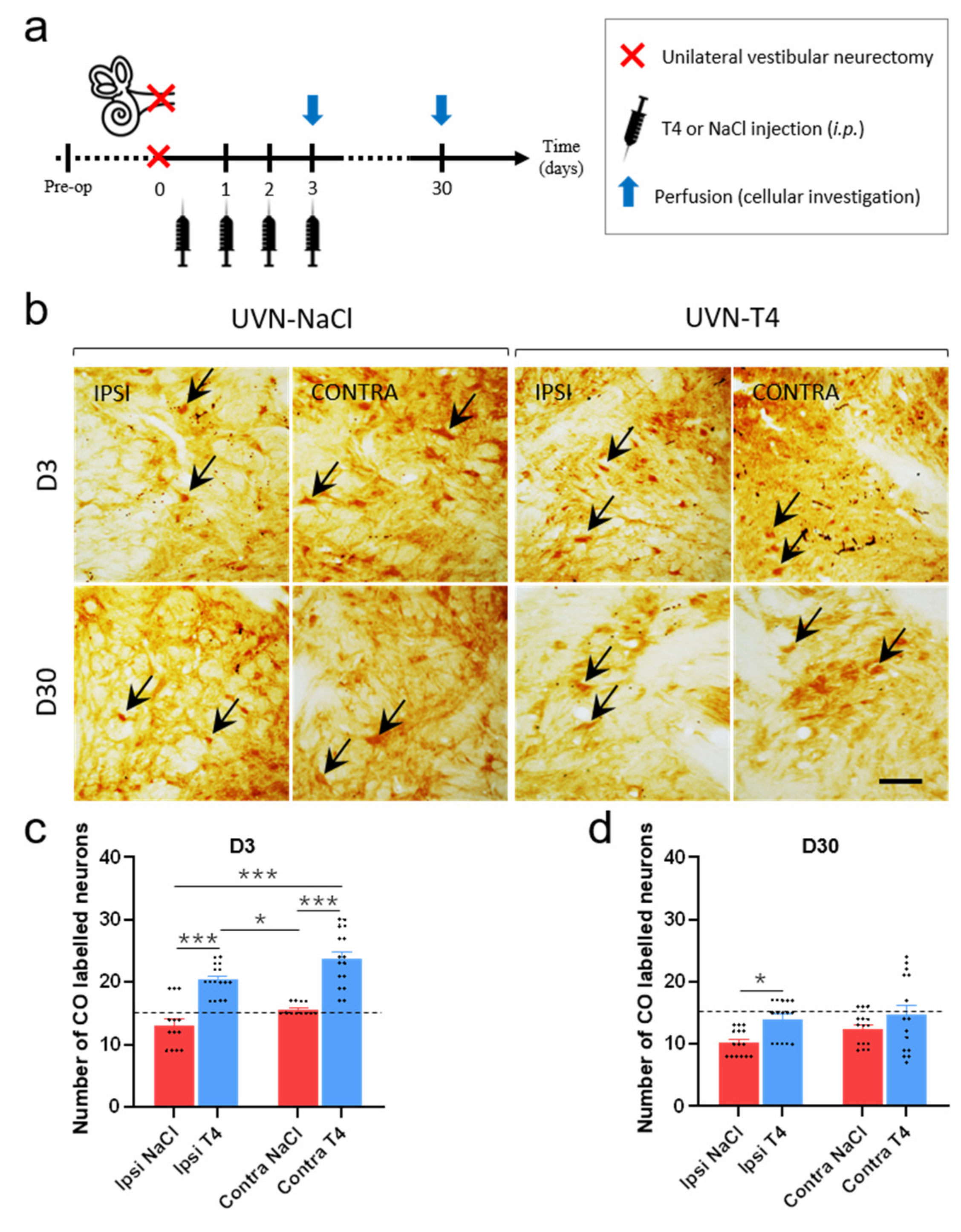
3.7. L-T4-Treated Rats Exhibit Enhanced Metabolic Activity in the Vestibular Nuclei Three Days after UVN
4. Discussion
Clinical Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curthoys, I.S. Vestibular Compensation and Substitution. Curr. Opin. Neurol. 2000, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Dutia, M.B. Mechanisms of Vestibular Compensation: Recent Advances. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Tighilet, B. Plastic Events in the Vestibular Nuclei during Vestibular Compensation: The Brain Orchestration of a "Deafferentation" Code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Leonard, J.; Mourre, C.; Chabbert, C. Apamin Treatment Accelerates Equilibrium Recovery and Gaze Stabilization in Unilateral Vestibular Neurectomized Cats: Cellular and Behavioral Aspects. Neuropharmacology 2019, 144, 133–142. [Google Scholar] [CrossRef]
- Dutheil, S.; Brezun, J.M.; Leonard, J.; Lacour, M.; Tighilet, B. Neurogenesis and Astrogenesis Contribution to Recovery of Vestibular Functions in the Adult Cat Following Unilateral Vestibular Neurectomy: Cellular and Behavioral Evidence. Neuroscience 2009, 164, 1444–1456. [Google Scholar] [CrossRef]
- Dutheil, S.; Lacour, M.; Tighilet, B. Neurogenic Potential of the Vestibular Nuclei and Behavioural Recovery Time Course in the Adult Cat Are Governed by the Nature of the Vestibular Damage. PLoS ONE 2011, 6, e22262. [Google Scholar] [CrossRef][Green Version]
- Dutheil, S.; Escoffier, G.; Gharbi, A.; Watabe, I.; Tighilet, B. GABAA Receptor Agonist and Antagonist Alter Vestibular Compensation and Different Steps of Reactive Neurogenesis in Deafferented Vestibular Nuclei of Adult Cats. J. Neurosci. 2013, 33, 15555–15566. [Google Scholar] [CrossRef]
- Dutheil, S.; Watabe, I.; Sadlaoud, K.; Tonetto, A.; Tighilet, B. BDNF Signaling Promotes Vestibular Compensation by Increasing Neurogenesis and Remodeling the Expression of Potassium-Chloride Cotransporter KCC2 and GABAA Receptor in the Vestibular Nuclei. J. Neurosci. 2016, 36, 6199–6212. [Google Scholar] [CrossRef]
- Tighilet, B.; Brezun, J.M.; Dit Duflo Sylvie, G.; Gaubert, C.; Lacour, M. New Neurons in the Vestibular Nuclei Complex after Unilateral Vestibular Neurectomy in the Adult Cat: Reactive Neurogenesis in Adult Vestibular Lesioned Cats. Eur. J. Neurosci. 2007, 25, 47–58. [Google Scholar] [CrossRef]
- Rastoldo, G.; El Mahmoudi, N.; Marouane, E.; Pericat, D.; Watabe, I.; Toneto, A.; López-Juárez, A.; Chabbert, C.; Tighilet, B. Adult and Endemic Neurogenesis in the Vestibular Nuclei after Unilateral Vestibular Neurectomy. Prog. Neurobiol. 2021, 196, 101899. [Google Scholar] [CrossRef]
- Fanibunda, S.E.; Desouza, L.A.; Kapoor, R.; Vaidya, R.A.; Vaidya, V.A. Thyroid Hormone Regulation of Adult Neurogenesis. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2018; Volume 106, pp. 211–251. ISBN 978-0-12-814116-8. [Google Scholar]
- Gothié, J.-D.; Demeneix, B.; Remaud, S. Comparative Approaches to Understanding Thyroid Hormone Regulation of Neurogenesis. Mol. Cell. Endocrinol. 2017, 459, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Fanibunda, S.E.; Desouza, L.A.; Guha, S.K.; Vaidya, V.A. Perspectives on Thyroid Hormone Action in Adult Neurogenesis. J. Neurochem. 2015, 133, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Remaud, S.; Gothié, J.-D.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid Hormone Signaling and Adult Neurogenesis in Mammals. Front. Endocrinol. 2014, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Impellizzeri, D.; Ahmad, A.; Cornelius, C.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Post-Ischaemic Thyroid Hormone Treatment in a Rat Model of Acute Stroke. Brain Res. 2013, 1513, 92–102. [Google Scholar] [CrossRef]
- Mokhtari, T.; Akbari, M.; Malek, F.; Kashani, I.R.; Rastegar, T.; Noorbakhsh, F.; Ghazi-Khansari, M.; Attari, F.; Hassanzadeh, G. Improvement of Memory and Learning by Intracerebroventricular Microinjection of T3 in Rat Model of Ischemic Brain Stroke Mediated by Upregulation of BDNF and GDNF in CA1 Hippocampal Region. DARU J. Pharm. Sci. 2017, 25, 1–11. [Google Scholar] [CrossRef]
- Bavarsad, K.; Hadjzadeh, M.-A.-R.; Hosseini, M.; Pakdel, R.; Beheshti, F.; Bafadam, S.; Ashaari, Z. Effects of Levothyroxine on Learning and Memory Deficits in a Rat Model of Alzheimer’s Disease: The Role of BDNF and Oxidative Stress. Drug Chem. Toxicol. 2018, 43, 57–63. [Google Scholar] [CrossRef]
- Shulga, A.; Blaesse, A.; Kysenius, K.; Huttunen, H.J.; Tanhuanpää, K.; Saarma, M.; Rivera, C. Thyroxin Regulates BDNF Expression to Promote Survival of Injured Neurons. Mol. Cell. Neurosci. 2009, 42, 408–418. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Brent, G.A. The Role of Thyroid Hormone in Neuronal Protection. Compr. Physiol. 2021, 11, 2075–2095. [Google Scholar] [CrossRef]
- Talhada, D.; Santos, C.R.A.; Gonçalves, I.; Ruscher, K. Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms After Stroke. Front. Neurol. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular Aspects of Thyroid Hormone Actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Vaitkus, J.A.; Farrar, J.S.; Celi, F.S. Thyroid Hormone Mediated Modulation of Energy Expenditure. Int. J. Mol. Sci. 2015, 16, 16158–16175. [Google Scholar] [CrossRef] [PubMed]
- Péricat, D.; Farina, A.; Agavnian-Couquiaud, E.; Chabbert, C.; Tighilet, B. Complete and Irreversible Unilateral Vestibular Loss: A Novel Rat Model of Vestibular Pathology. J. Neurosci. Methods 2017, 283, 83–91. [Google Scholar] [CrossRef]
- Boadas-Vaello, P.; Riera, J.; Llorens, J. Behavioral and Pathological Effects in the Rat Define Two Groups of Neurotoxic Nitriles. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 88, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Hardisty-Hughes, R.E.; Parker, A.; Brown, S.D.M. A Hearing and Vestibular Phenotyping Pipeline to Identify Mouse Mutants with Hearing Impairment. Nat. Protoc. 2010, 5, 177–190. [Google Scholar] [CrossRef]
- Liberge, M.; Manrique, C.; Bernard-Demanze, L.; Lacour, M. Changes in TNFa, NF B and MnSOD Protein in the Vestibular Nuclei after Unilateral Vestibular Deafferentation. J. Neroinflamm. 2010, 7, 91. [Google Scholar] [CrossRef]
- Tighilet, B.; Péricat, D.; Frelat, A.; Cazals, Y.; Rastoldo, G.; Boyer, F.; Dumas, O.; Chabbert, C. Adjustment of the Dynamic Weight Distribution as a Sensitive Parameter for Diagnosis of Postural Alteration in a Rodent Model of Vestibular Deficit. PLoS ONE 2017, 12, e0187472. [Google Scholar] [CrossRef]
- Rastoldo, G.; Marouane, E.; El Mahmoudi, N.; Péricat, D.; Bourdet, A.; Timon-David, E.; Dumas, O.; Chabbert, C.; Tighilet, B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Front. Neurol. 2020, 11, 505. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-0-12-391949-6. [Google Scholar]
- Tighilet, B.; Dutheil, S.; Siponen, M.I.; Noreña, A.J. Reactive Neurogenesis and Down-Regulation of the Potassium-Chloride Cotransporter KCC2 in the Cochlear Nuclei after Cochlear Deafferentation. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Kaila, K.; Price, T.J.; Payne, J.A.; Puskarjov, M.; Voipio, J. Cation-Chloride Cotransporters in Neuronal Development, Plasticity and Disease. Nat. Rev. Neurosci. 2014, 15, 637–654. [Google Scholar] [CrossRef]
- Marouane, E.; Rastoldo, G.; El Mahmoudi, N.; Péricat, D.; Chabbert, C.; Artzner, V.; Tighilet, B. Identification of New Biomarkers of Posturo-Locomotor Instability in a Rodent Model of Vestibular Pathology. Front. Neurol. 2020, 11, 470. [Google Scholar] [CrossRef]
- Tighilet, B.; Léonard, J.; Watabe, I.; Bernard-Demanze, L.; Lacour, M. Betahistine Treatment in a Cat Model of Vestibular Pathology: Pharmacokinetic and Pharmacodynamic Approaches. Front. Neurol. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Flamant, F.; Cheng, S.-Y.; Hollenberg, A.N.; Moeller, L.C.; Samarut, J.; Wondisford, F.E.; Yen, P.M.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomenclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the Local Control of Thyroid Hormone Action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Guadano-Ferraz, A.; Obregon, M.J.; Germain, D.L.S.; Bernal, J. The Type 2 Iodothyronine Deiodinase Is Expressed Primarily in Glial Cells in the Neonatal Rat Brain. Proc. Natl. Acad. Sci. USA 1997, 94, 10391–10396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Brent, G.A. Thyroid Hormone and the Brain: Mechanisms of Action in Development and Role in Protection and Promotion of Recovery after Brain Injury. Pharmacol. Ther. 2018, 186, 176–185. [Google Scholar] [CrossRef]
- Mohácsik, P.; Zeöld, A.; Bianco, A.C.; Gereben, B. Thyroid Hormone and the Neuroglia: Both Source and Target. J. Thyroid Res. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- López-Juárez, A.; Remaud, S.; Hassani, Z.; Jolivet, P.; Pierre Simons, J.; Sontag, T.; Yoshikawa, K.; Price, J.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid Hormone Signaling Acts as a Neurogenic Switch by Repressing Sox2 in the Adult Neural Stem Cell Niche. Cell Stem Cell 2012, 10, 531–543. [Google Scholar] [CrossRef]
- Li, H.; Godfrey, D.A.; Rubin, A.M. Astrocyte Reaction in the Rat Vestibular Nuclei after Unilateral Removal of Scarpa’s Ganglion. Ann. Otol. Rhinol. Laryngol. 1999, 108, 181–188. [Google Scholar] [CrossRef]
- Remaud, S.; Ortiz, F.C.; Perret-Jeanneret, M.; Aigrot, M.-S.; Gothié, J.-D.; Fekete, C.; Kvárta-Papp, Z.; Gereben, B.; Langui, D.; Lubetzki, C.; et al. Transient Hypothyroidism Favors Oligodendrocyte Generation Providing Functional Remyelination in the Adult Mouse Brain. eLife 2017, 6, e29996. [Google Scholar] [CrossRef]
- Hartley, M.D.; Banerji, T.; Tagge, I.J.; Kirkemo, L.L.; Chaudhary, P.; Calkins, E.; Galipeau, D.; Shokat, M.D.; DeBell, M.J.; Van Leuven, S.; et al. Myelin Repair Stimulated by CNS-Selective Thyroid Hormone Action. JCI Insight 2019, 4, e126329. [Google Scholar] [CrossRef] [PubMed]
- Gothié, J.D.; Sébillot, A.; Luongo, C.; Legendre, M.; Nguyen Van, C.; Le Blay, K.; Perret-Jeanneret, M.; Remaud, S.; Demeneix, B.A. Adult Neural Stem Cell Fate Is Determined by Thyroid Hormone Activation of Mitochondrial Metabolism. Mol. Metab. 2017, 6, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; De Koninck, Y. Microglia Control Neuronal Network Excitability via BDNF Signalling. Neural Plast. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-Induced TrkB Activation down-Regulates the K+–Cl− Cotransporter KCC2 and Impairs Neuronal Cl−Extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Thyroid Hormone Action in Mitochondria. J. Mol. Endocrinol. 2001, 26, 67–77. [Google Scholar] [CrossRef]
- McCall, A.A.; Miller, D.M.; Yates, B.J. Descending Influences on Vestibulospinal and Vestibulosympathetic Reflexes. Front. Neurol. 2017, 8, 112. [Google Scholar] [CrossRef]
- Murray, A.J.; Croce, K.; Belton, T.; Akay, T.; Jessell, T.M. Balance Control Mediated by Vestibular Circuits Directing Limb Extension or Antagonist Muscle Co-Activation. Cell Rep. 2018, 22, 1325–1338. [Google Scholar] [CrossRef]
- Alsalaheen, B.A.; Mucha, A.; Morris, L.O.; Whitney, S.L.; Furman, J.M.; Camiolo-Reddy, C.E.; Collins, M.W.; Lovell, M.R.; Sparto, P.J. Vestibular Rehabilitation for Dizziness and Balance Disorders after Concussion. J. Neurol. Phys. Ther. JNPT 2010, 34, 87–93. [Google Scholar] [CrossRef]
- Meldrum, D.; Herdman, S.; Vance, R.; Murray, D.; Malone, K.; Duffy, D.; Glennon, A.; McConn-Walsh, R. Effectiveness of Conventional Versus Virtual Reality–Based Balance Exercises in Vestibular Rehabilitation for Unilateral Peripheral Vestibular Loss: Results of a Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 1319–1328. [Google Scholar] [CrossRef]
- Verdecchia, D.H.; Monzón, A.M.; Urbina Jaimes, V.; Oliveira, F.R.; Paiva, L.d.S.; de Carvalho, T.D. Patient-Reported and Performance Outcomes Significantly Improved in Elderly Patients with Vestibular Impairment Following Rehabilitation: A Retrospective Study. J. Aging Res. 2018, 2018, 5093501. [Google Scholar] [CrossRef] [PubMed]
- Wrisley, D.M.; Walker, M.L.; Echternach, J.L.; Strasnick, B. Reliability of the Dynamic Gait Index in People with Vestibular Disorders. Arch. Phys. Med. Rehabil. 2003, 84, 1528–1533. [Google Scholar] [CrossRef]
- Kristiansen, L.; Magnussen, L.H.; Wilhelmsen, K.T.; Mæland, S.; Nordahl, S.H.G.; Clendaniel, R.; Hovland, A.; Juul-Kristensen, B. Efficacy of Intergrating Vestibular Rehabilitation and Cognitive Behaviour Therapy in Persons with Persistent Dizziness in Primary Care- a Study Protocol for a Randomised Controlled Trial. Trials 2019, 20, 575. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.L.; Alghwiri, A.A.; Alghadir, A. An Overview of Vestibular Rehabilitation. Handb. Clin. Neurol. 2016, 137, 187–205. [Google Scholar] [CrossRef]
- Hwang, G.; Saadi, R.; Patel, V.A.; Liaw, J.; Isildak, H. Thyroid Dysfunction in Ménière’s Disease: A Comprehensive Review. ORL J. Oto-Rhino-Laryngol. Its Relat. Spec. 2021, 83, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-L.; Chen, C.-Y.; Hsu, T.-Y.; Chen, W.-K.; Lin, C.-L.; Chen, H.-C. Hypothyroidism Is an Independent Risk Factor for Menière’s Disease: A Population-Based Cohort Study. Medicine 2019, 98, e15166. [Google Scholar] [CrossRef]
- Miśkiewicz-Orczyk, K.A.; Lisowska, G.; Kajdaniuk, D.; Wojtulek, M. Can Hashimoto’s Thyroiditis Cause Vertigo? Endokrynol. Pol. 2020, 70, 76–86. [Google Scholar] [CrossRef]
- Santosh, U.P.; Rao, M.S.S. Incidence of Hypothyroidism in Meniere’s Disease. J. Clin. Diagn. Res. JCDR 2016, 10, MC01–MC03. [Google Scholar] [CrossRef]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic Actions of Thyroid Hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.-Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin AVβ3 Contains a Cell Surface Receptor Site for Thyroid Hormone That Is Linked to Activation of Mitogen-Activated Protein Kinase and Induction of Angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Darlington, C.L.; Smith, P.F. Molecular Mechanisms of Recovery from Vestibular Damage in Mammals: Recent Advances. Prog. Neurobiol. 2000, 62, 313–325. [Google Scholar] [CrossRef]
- Smith, P.F.; Curthoys, I.S. Mechanisms of Recovery Following Unilateral Labyrinthectomy: A Review. Brain Res. Rev. 1989, 14, 155–180. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Halmagyi, G.M. Vestibular Compensation: A Review of the Oculomotor, Neural, and Clinical Consequences of Unilateral Vestibular Loss. J. Vestib. Res. Equilib. Orientat. 1995, 5, 67–107. [Google Scholar] [CrossRef]
- Ris, L.; de Waele, C.; Serafin, M.; Vidal, P.P.; Godaux, E. Neuronal Activity in the Ipsilateral Vestibular Nucleus Following Unilateral Labyrinthectomy in the Alert Guinea Pig. J. Neurophysiol. 1995, 74, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.H.; Welcker, J.; Gaston, A.J.; Hatch, S.A.; Palace, V.; Hare, J.F.; Speakman, J.R.; Anderson, W.G. Thyroid Hormones Correlate with Resting Metabolic Rate, Not Daily Energy Expenditure, in Two Charadriiform Seabirds. Biol. Open 2013, 2, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, B. Thyroid Hormone as a Determinant of Energy Expenditure and the Basal Metabolic Rate. Thyroid Off. J. Am. Thyroid Assoc. 2008, 18, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.V.; Williams, D.B.; Fitzgerald, R.M.; Im, H.K.; Vonvoigtlander, P.F. Thyroid Hormonal Modulation of the Binding and Activity of the GABAA Receptor Complex of Brain. Neuroscience 1996, 73, 705–713. [Google Scholar] [CrossRef]
- Puia, G.; Losi, G. Thyroid Hormones Modulate GABAA Receptor-Mediated Currents in Hippocampal Neurons. Neuropharmacology 2011, 60, 1254–1261. [Google Scholar] [CrossRef]
- Paterson, J.M.; Short, D.; Flatman, P.W.; Seckl, J.R.; Aitken, A.; Dutia, M.B. Changes in Protein Expression in the Rat Medial Vestibular Nuclei during Vestibular Compensation. J. Physiol. 2006, 575, 777–788. [Google Scholar] [CrossRef]
- Blomstrand, C.; Hallén, O.; Hamberger, A.; Jarlstedt, J. Quantitative Cytochemical Aspects on the Mechanism of Central Compensation after Unilateral Vestibular Neurotomy. Acta Otolaryngol. 1966, 61, 113–120. [Google Scholar] [CrossRef]
- Shulga, A.; Thomas-Crusells, J.; Sigl, T.; Blaesse, A.; Mestres, P.; Meyer, M.; Yan, Q.; Kaila, K.; Saarma, M.; Rivera, C.; et al. Posttraumatic GABAA-Mediated [Ca2+]i Increase Is Essential for the Induction of Brain-Derived Neurotrophic Factor-Dependent Survival of Mature Central Neurons. J. Neurosci. 2008, 28, 6996–7005. [Google Scholar] [CrossRef]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-Regulation of the Potassium-Chloride Cotransporter KCC2 Contributes to Spasticity after Spinal Cord Injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Thomas-Crusells, J.; Li, H.; Emri, Z.; Sipilä, S.; Payne, J.A.; Minichiello, L.; Saarma, M.; Kaila, K. Mechanism of Activity-Dependent Downregulation of the Neuron-Specific K-Cl Cotransporter KCC2. J. Neurosci. 2004, 24, 4683–4691. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.; Rivera, C. Interplay between Thyroxin, BDNF and GABA in Injured Neurons. Neuroscience 2013, 239, 241–252. [Google Scholar] [CrossRef]
- Nygård, M.; Wahlström, G.M.; Gustafsson, M.V.; Tokumoto, Y.M.; Bondesson, M. Hormone-Dependent Repression of the E2F-1 Gene by Thyroid Hormone Receptors. Mol. Endocrinol. 2003, 17, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Capen, C.C. Mechanistic Data and Risk Assessment of Selected Toxic End Points of the Thyroid Gland. Toxicol. Pathol. 1997, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Marouane, E.; El Mahmoudi, N.; Rastoldo, G.; Péricat, D.; Watabe, I.; Lapôtre, A.; Tonetto, A.; Xavier, F.; Dumas, O.; Chabbert, C.; et al. Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei. Cells 2021, 10, 3377. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.R.; Gervais, A.; Colin, C.; Izembart, M.; Neto, V.M.; Mallat, M. Regulation of Microglial Development: A Novel Role for Thyroid Hormone. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2028–2038. [Google Scholar] [CrossRef]
- Grosch, M.; Lindner, M.; Bartenstein, P.; Brandt, T.; Dieterich, M.; Ziegler, S.; Zwergal, A. Dynamic Whole-Brain Metabolic Connectivity during Vestibular Compensation in the Rat. NeuroImage 2020, 226, 117588. [Google Scholar] [CrossRef]
- Zwergal, A.; Schlichtiger, J.; Xiong, G.; Beck, R.; Günther, L.; Schniepp, R.; Schöberl, F.; Jahn, K.; Brandt, T.; Strupp, M.; et al. Sequential [18F]FDG ΜPET Whole-Brain Imaging of Central Vestibular Compensation: A Model of Deafferentation-Induced Brain Plasticity. Brain Struct. Funct. 2016, 221, 159–170. [Google Scholar] [CrossRef]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of Thyroid Hormone in Skeletal Muscle Physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef] [PubMed]
- Simonides, W.S.; van Hardeveld, C. Thyroid Hormone as a Determinant of Metabolic and Contractile Phenotype of Skeletal Muscle. Thyroid Off. J. Am. Thyroid Assoc. 2008, 18, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zennou-Azogui, Y.; Borel, L.; Lacour, M.; Ez-Zaher, L.; Ouaknine, M. Recovery of Head Postural Control Following Unilateral Vestibular Neurectomy in the Cat: Neck Muscle Activity and Neuronal Correlates in Deiters’ Nuclei. Acta Otolaryngol. 1993, 113, 5–19. [Google Scholar] [CrossRef]
- Simon, F.; Pericat, D.; Djian, C.; Fricker, D.; Denoyelle, F.; Beraneck, M. Surgical Techniques and Functional Evaluation for Vestibular Lesions in the Mouse: Unilateral Labyrinthectomy (UL) and Unilateral Vestibular Neurectomy (UVN). J. Neurol. 2020, 267, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Teufert, K.B.; Doherty, J. Endolymphatic Sac Shunt, Labyrinthectomy, and Vestibular Nerve Section in Meniere’s Disease. Otolaryngol. Clin. N. Am. 2010, 43, 1091–1111. [Google Scholar] [CrossRef]
- Magnan, J.; Bremond, G.; Chays, A.; Gignac, D.; Florence, A. Vestibular Neurotomy by Retrosigmoid Approach: Technique, Indications, and Results. Am. J. Otol. 1991, 12, 101–104. [Google Scholar]
- Vignaux, G.; Chabbert, C.; Gaboyard-Niay, S.; Travo, C.; Machado, M.L.; Denise, P.; Comoz, F.; Hitier, M.; Landemore, G.; Philoxène, B.; et al. Evaluation of the Chemical Model of Vestibular Lesions Induced by Arsanilate in Rats. Toxicol. Appl. Pharmacol. 2012, 258, 61–71. [Google Scholar] [CrossRef]
- Callejo, A.; Sedó-Cabezón, L.; Juan, I.D.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Cassel, R.; Bordiga, P.; Carcaud, J.; Simon, F.; Beraneck, M.; Le Gall, A.; Benoit, A.; Bouet, V.; Philoxene, B.; Besnard, S.; et al. Morphological and Functional Correlates of Vestibular Synaptic Deafferentation and Repair in a Mouse Model of Acute-Onset Vertigo. Dis. Model. Mech. 2019, 12, dmm039115. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastoldo, G.; Marouane, E.; El-Mahmoudi, N.; Péricat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; El-Ahmadi, A.; Caron, P.; et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells 2022, 11, 684. https://doi.org/10.3390/cells11040684
Rastoldo G, Marouane E, El-Mahmoudi N, Péricat D, Watabe I, Lapotre A, Tonetto A, López-Juárez A, El-Ahmadi A, Caron P, et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells. 2022; 11(4):684. https://doi.org/10.3390/cells11040684
Chicago/Turabian StyleRastoldo, Guillaume, Emna Marouane, Nada El-Mahmoudi, David Péricat, Isabelle Watabe, Agnes Lapotre, Alain Tonetto, Alejandra López-Juárez, Abdessadek El-Ahmadi, Philippe Caron, and et al. 2022. "L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects" Cells 11, no. 4: 684. https://doi.org/10.3390/cells11040684
APA StyleRastoldo, G., Marouane, E., El-Mahmoudi, N., Péricat, D., Watabe, I., Lapotre, A., Tonetto, A., López-Juárez, A., El-Ahmadi, A., Caron, P., Fraysse, M.-J. E., Chabbert, C., Zwergal, A., & Tighilet, B. (2022). L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells, 11(4), 684. https://doi.org/10.3390/cells11040684