Oxidative Stress and AKT-Associated Angiogenesis in a Zebrafish Model and Its Potential Application for Withanolides
Abstract
1. Introduction
2. Oxidative Stress Studies in the Zebrafish Model
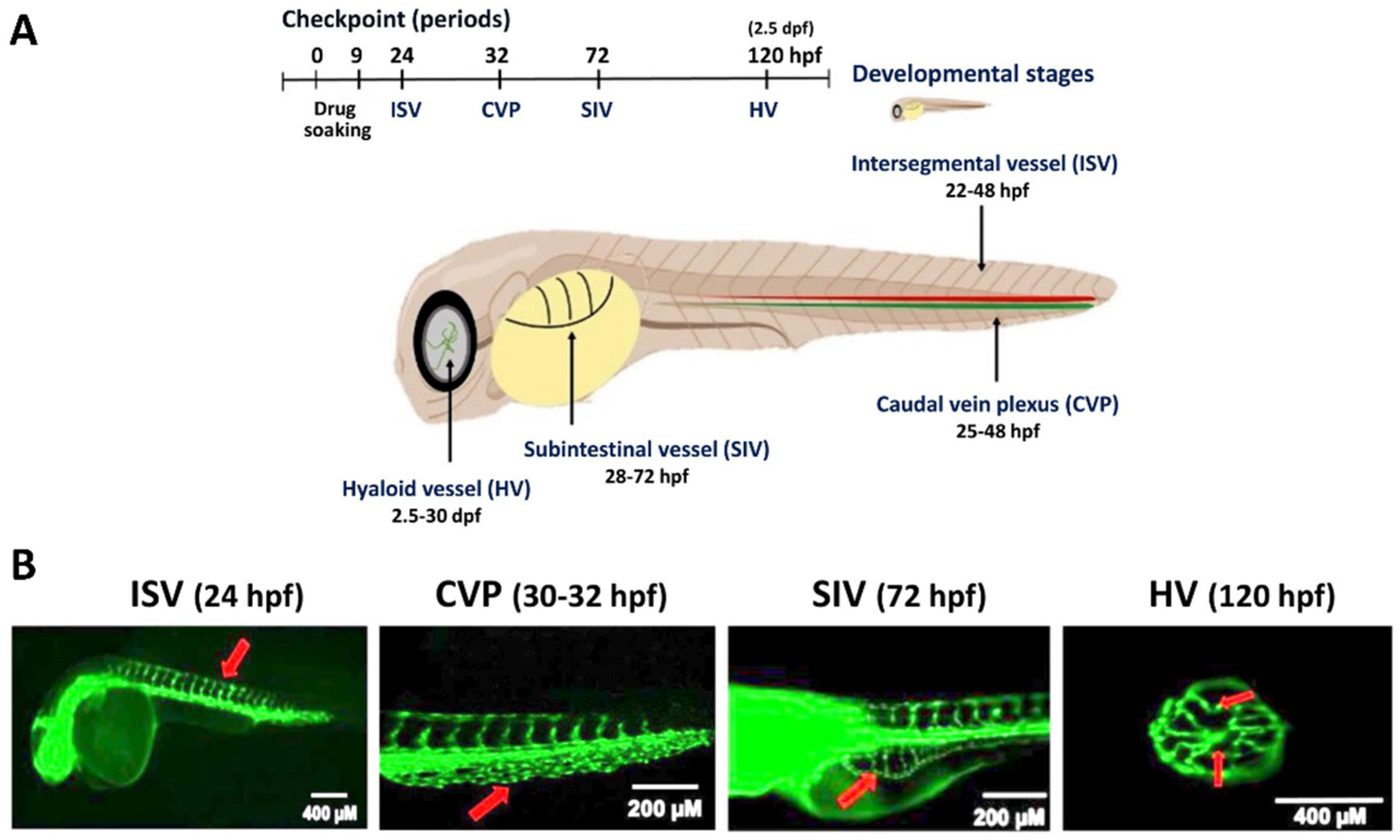
3. Angiogenesis Applications in the Zebrafish Model
4. Developmental and Xenograft Tumor Angiogenesis of Zebrafish
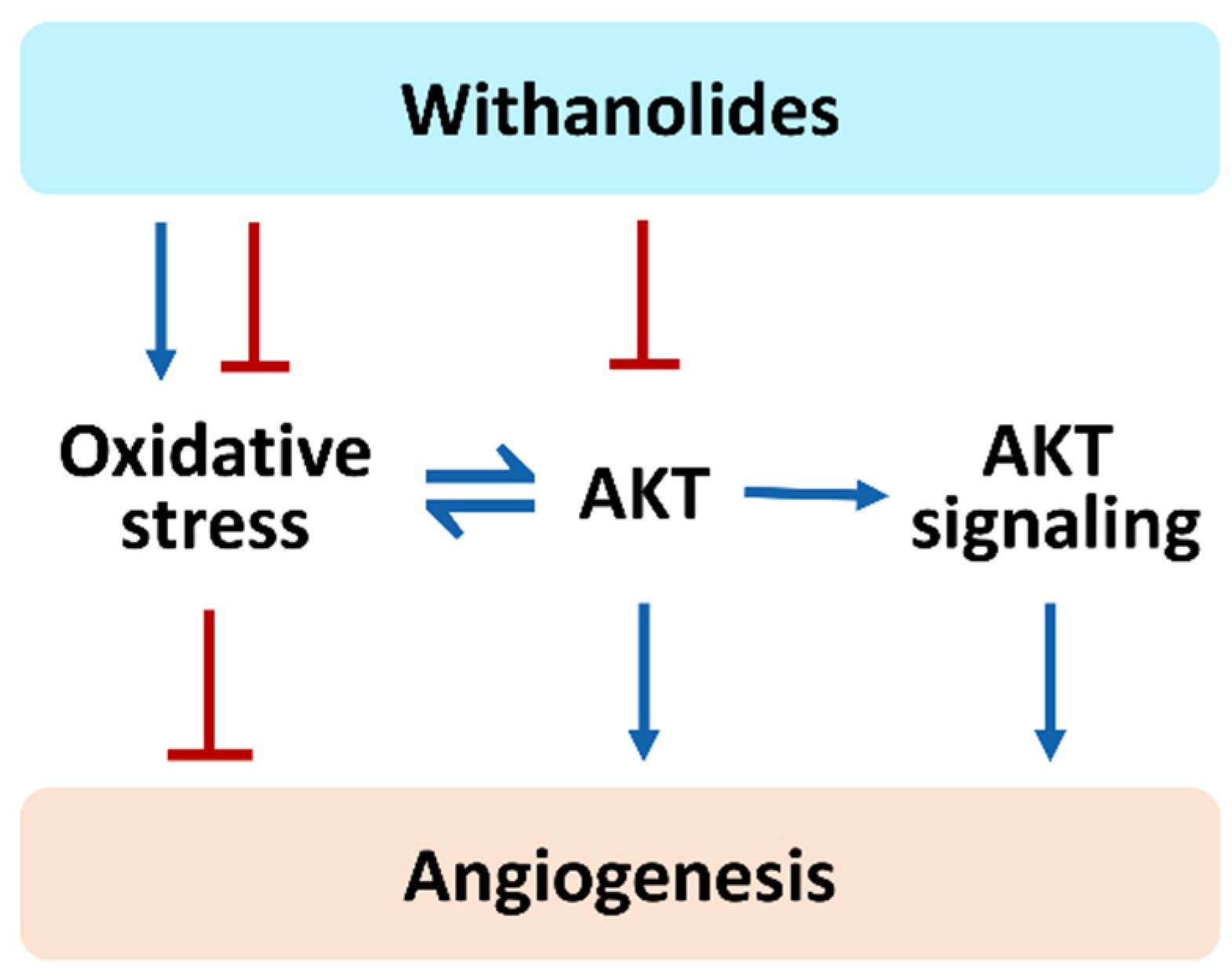
5. Role of Oxidative Stress in the Angiogenesis of the Zebrafish Model
6. AKT Plays a Vital Role in Regulating Migration and Angiogenesis
7. Role of AKT in Oxidative Stress Studies of the Zebrafish Model
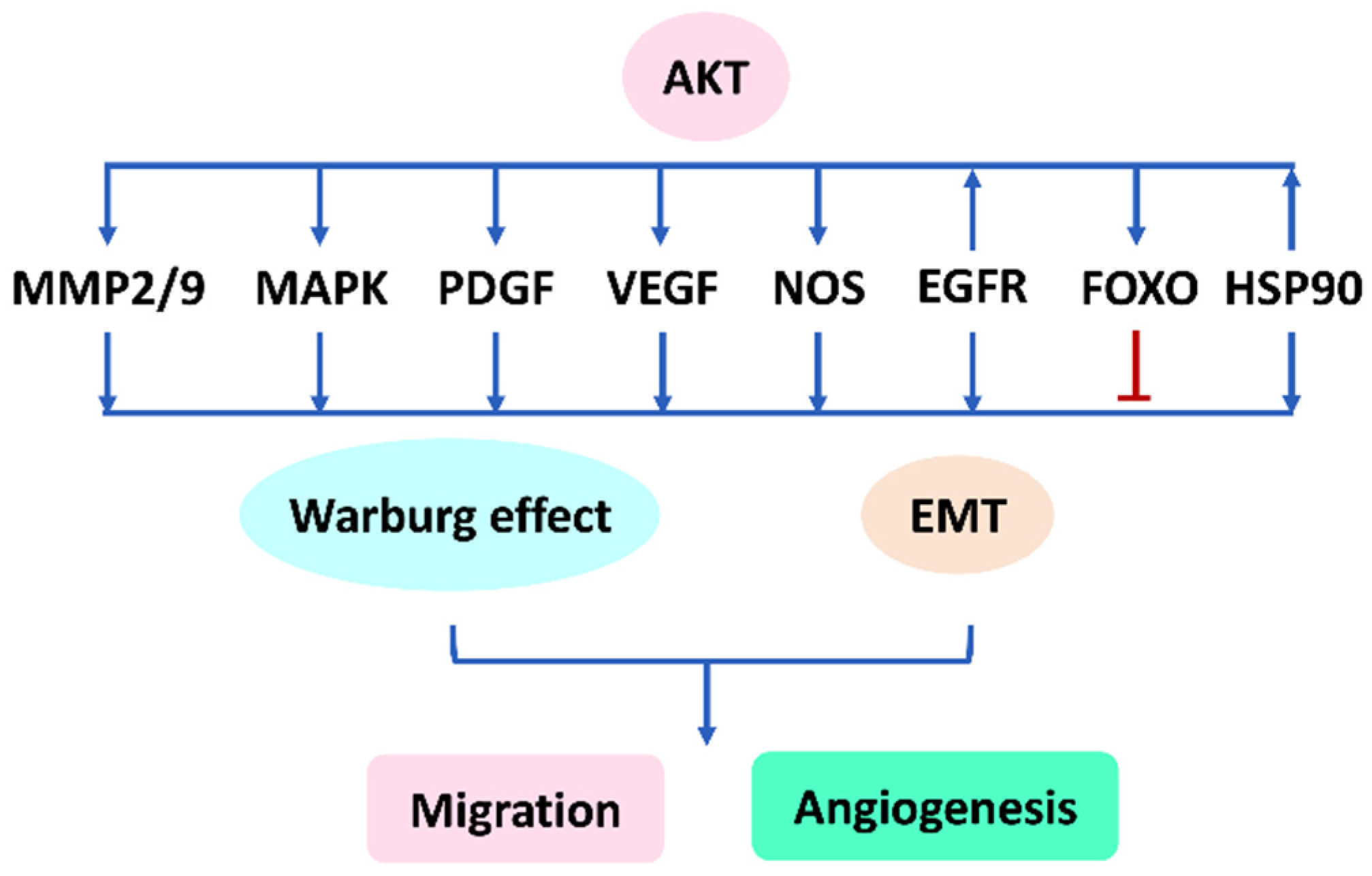
8. The AKT Network Plays a Vital Role in Regulating Angiogenesis
8.1. The Network between AKT, MMP-2/9, EMT, and Migration
8.2. AKT Regulates Several Migration- and Angiogenesis-Related Genes to Modulate EMT
8.3. AKT Network, Warburg Effect, Migration, and Angiogenesis
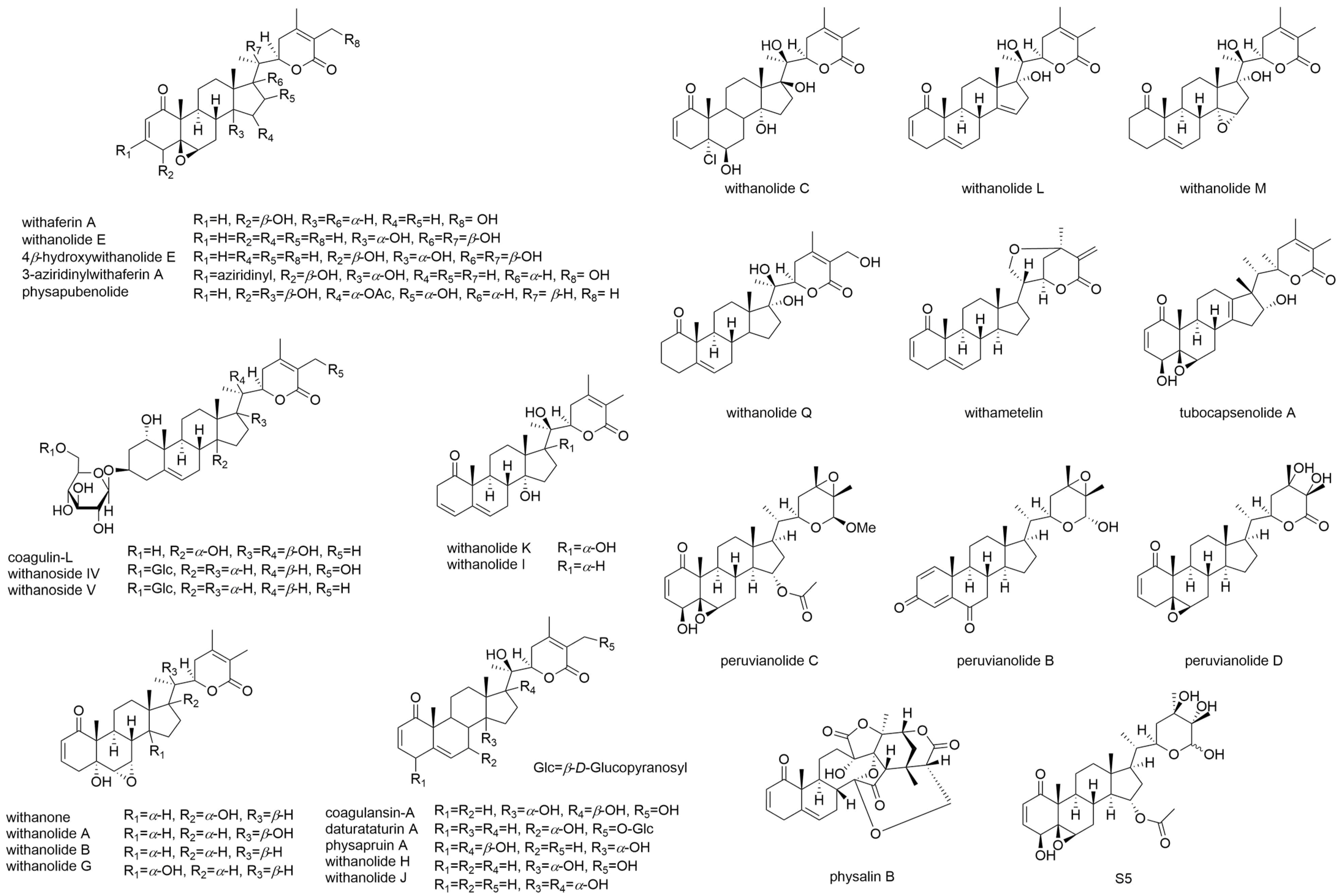
9. Overview of the Current Developments for Oxidative Stress, AKT, AKT Signaling, and Angiogenesis of Selected Withanolides
10. Withanolides May Have Dual Functions in Regulating Oxidative Stress
11. AKT-Modulating Effects of Withanolides
| Drugs | AKT | Cells | References |
|---|---|---|---|
| Withaferin A | inactivation | head and neck cancer | [114] |
| ovarian cancer | [115] | ||
| lung cancer | [116] | ||
| melanoma | [117] | ||
| inactivation/activation | breast cancer | [121] | |
| Tubocapsenolide A | inactivation | breast cancer | [103] |
| Withametelin | inactivation | myeloid leukemia | [118] |
| Coagulansin-A | inactivation | myeloid leukemia | [118] |
| Daturataturin A | inactivation | keratinocytes | [119] |
| Physapubenolide | inactivation | liver cancer | [120] |
12. The Role of Oxidative Stress and AKT in Regulating Angiogenesis by Withanolides Needs Further Investigation
13. AKT Inhibitors Have the Potential for Anti-Migration and Anti-Angiogenesis Effects, but These Effects Are Rarely Reported in Withanolides
14. AKT Signaling Inhibitors Have the Potential for Anti-Migration and Anti-Angiogenesis Effects, but These Effects Are Rarely Reported in Withanolides
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roussos, E.T.; Condeelis, J.S.; Patsialou, A. Chemotaxis in cancer. Nat. Rev. Cancer 2011, 11, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.K.; Tennant, A.H.; Conolly, R.B.; Prince, K.; Stevens, J.S.; DeMarini, D.M.; Martin, B.L.; Thompson, L.C.; Gilmour, M.I.; Cascio, W.E.; et al. High-throughput video processing of heart rate responses in multiple wild-type embryonic zebrafish per imaging field. Sci. Rep. 2019, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L.; Bradford, Y.M.; Frazer, K.; Howe, D.G.; Paddock, H.; Ramachandran, S.; Singer, A.; Toro, S.; Van Slyke, C.E.; Eagle, A.E.; et al. ZFIN, The zebrafish model organism database: Updates and new directions. Genesis 2015, 53, 498–509. [Google Scholar] [CrossRef]
- Sprague, J.; Bayraktaroglu, L.; Clements, D.; Conlin, T.; Fashena, D.; Frazer, K.; Haendel, M.; Howe, D.G.; Mani, P.; Ramachandran, S.; et al. The Zebrafish Information Network: The zebrafish model organism database. Nucleic Acids Res. 2006, 34, D581–D585. [Google Scholar] [CrossRef]
- Pritchett, D.; Brennan, C.H. Classical and operant conditioning in larval zebrafish. In Behavioral and Neural Genetics of Zebrafish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–122. [Google Scholar]
- Rubinstein, A.L. Zebrafish: From disease modeling to drug discovery. Curr. Opin. Drug Discov. Dev. 2003, 6, 218–223. [Google Scholar]
- Sassen, W.A.; Köster, R.W. A molecular toolbox for genetic manipulation of zebrafish. Adv. Genom. Genet. 2015, 5, 151. [Google Scholar]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Wang, F. Environmental relevant concentrations of triclosan affected developmental toxicity, oxidative stress, and apoptosis in zebrafish embryos. Environ. Toxicol. 2022; in press. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukherjee, S.; Chattopadhyay, A.; Bhattacharya, S. Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: Expression of antioxidant genes. Ecotoxicol. Environ. Saf. 2014, 107, 1–8. [Google Scholar] [CrossRef]
- Sun, M.; Ding, R.; Ma, Y.; Sun, Q.; Ren, X.; Sun, Z.; Duan, J. Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere 2021, 282, 131124. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Hu, D.; Wang, Z.; Wang, L.; Wu, T.; Wu, Z.; Mohan, C.; Peng, A. Identification of apoptosis and macrophage migration events in paraquat-induced oxidative stress using a zebrafish model. Life Sci. 2016, 157, 116–124. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, C.Y.; Hu, C.H.; Pai, T.W.; Chen, Y.R.; Wang, W.D. Integrated hypoxia signaling and oxidative stress in developmental neurotoxicity of benzo[a]pyrene in zebrafish embryos. Antioxidants 2020, 9, 731. [Google Scholar] [CrossRef]
- Garcia-Caballero, M.; Quesada, A.R.; Medina, M.A.; Mari-Beffa, M. Fishing anti(lymph)angiogenic drugs with zebrafish. Drug Discov. Today 2018, 23, 366–374. [Google Scholar] [CrossRef]
- Vogt, A.; Cholewinski, A.; Shen, X.; Nelson, S.G.; Lazo, J.S.; Tsang, M.; Hukriede, N.A. Automated image-based phenotypic analysis in zebrafish embryos. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 656–663. [Google Scholar] [CrossRef]
- Rezzola, S.; Paganini, G.; Semeraro, F.; Presta, M.; Tobia, C. Zebrafish (Danio rerio) embryo as a platform for the identification of novel angiogenesis inhibitors of retinal vascular diseases. Biochim. Biophys. Acta 2016, 1862, 1291–1296. [Google Scholar] [CrossRef]
- Chavez, M.N.; Aedo, G.; Fierro, F.A.; Allende, M.L.; Egana, J.T. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front. Physiol. 2016, 7, 56. [Google Scholar] [CrossRef]
- Moshal, K.S.; Ferri-Lagneau, K.F.; Leung, T. Zebrafish model: Worth considering in defining tumor angiogenesis. Trends Cardiovasc. Med. 2010, 20, 114–119. [Google Scholar] [CrossRef]
- Tobia, C.; De Sena, G.; Presta, M. Zebrafish embryo, a tool to study tumor angiogenesis. Int. J. Dev. Biol. 2011, 55, 505–509. [Google Scholar] [CrossRef]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef]
- Santoso, F.; Sampurna, B.P.; Lai, Y.H.; Liang, S.T.; Hao, E.; Chen, J.R.; Hsiao, C.D. Development of a simple ImageJ-based method for dynamic blood flow tracking in zebrafish embryos and its application in drug toxicity evaluation. Inventions 2019, 4, 65. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Zhang, W.; Qian, Z.; Xiang, Y. Monitoring antiangiogenesis of bevacizumab in zebrafish. Drug Des. Dev. Ther. 2018, 12, 2423–2430. [Google Scholar] [CrossRef]
- McKinney, M.C.; Weinstein, B.M. Using the zebrafish to study vessel formation. Methods Enzymol. 2008, 444, 65–97. [Google Scholar]
- Tshering, G.; Pimtong, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Anti-angiogenic effects of beta-eudesmol and atractylodin in developing zebrafish embryos. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 243, 108980. [Google Scholar] [CrossRef]
- Alvarez, Y.; Cederlund, M.L.; Cottell, D.C.; Bill, B.R.; Ekker, S.C.; Torres-Vazquez, J.; Weinstein, B.M.; Hyde, D.R.; Vihtelic, T.S.; Kennedy, B.N. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev. Biol. 2007, 7, 114. [Google Scholar] [CrossRef]
- Isogai, S.; Lawson, N.D.; Torrealday, S.; Horiguchi, M.; Weinstein, B.M. Angiogenic network formation in the developing vertebrate trunk. Development 2003, 130, 5281–5290. [Google Scholar] [CrossRef]
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef]
- Liu, F.Y.; Hsu, T.C.; Choong, P.; Lin, M.H.; Chuang, Y.J.; Chen, B.S.; Lin, C. Uncovering the regeneration strategies of zebrafish organs: A comprehensive systems biology study on heart, cerebellum, fin, and retina regeneration. BMC Syst. Biol. 2018, 12, 29. [Google Scholar] [CrossRef]
- Dewan, A.; Liu, M.; Hartman, S.; Zhang, S.S.; Liu, D.T.; Zhao, C.; Tam, P.O.; Chan, W.M.; Lam, D.S.; Snyder, M.; et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314, 989–992. [Google Scholar] [CrossRef]
- Nicoli, S.; Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007, 2, 2918–2923. [Google Scholar] [CrossRef]
- Chung, S.Y.; Chao, T.C.; Su, Y. The stemness-high human colorectal cancer cells promote angiogenesis by producing higher amounts of angiogenic cytokines via activation of the Egfr/Akt/Nf-kappaB pathway. Int. J. Mol. Sci. 2021, 22, 1355. [Google Scholar] [CrossRef]
- Chiavacci, E.; Rizzo, M.; Pitto, L.; Patella, F.; Evangelista, M.; Mariani, L.; Rainaldi, G. The zebrafish/tumor xenograft angiogenesis assay as a tool for screening anti-angiogenic miRNAs. Cytotechnology 2015, 67, 969–975. [Google Scholar] [CrossRef][Green Version]
- Ji, G.; Gu, J.; Guo, M.; Zhou, L.; Wang, Z.; Shi, L.; Gu, A. A systematic comparison of the developmental vascular toxicity of bisphenol A and its alternatives in vivo and in vitro. Chemosphere 2022, 291, 132936. [Google Scholar] [CrossRef]
- Chen, G.; Jia, Z.; Wang, L.; Hu, T. Effect of acute exposure of saxitoxin on development of zebrafish embryos (Danio rerio). Environ. Res. 2020, 185, 109432. [Google Scholar] [CrossRef]
- Chen, G.; Wang, L.; Li, W.; Zhang, Q.; Hu, T. Nodularin induced oxidative stress contributes to developmental toxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 194, 110444. [Google Scholar] [CrossRef]
- Shao, X.; Hu, Z.; Hu, C.; Bu, Q.; Yan, G.; Deng, P.; Lv, L.; Wu, D.; Deng, Y.; Zhao, J.; et al. Taurine protects methamphetamine-induced developmental angiogenesis defect through antioxidant mechanism. Toxicol. Appl. Pharmacol. 2012, 260, 260–270. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; He, S.; Lou, Q.; Zhai, G.; Shi, C.; Yin, Z.; Zheng, F. Deletion of narfl leads to increased oxidative stress mediated abnormal angiogenesis and digestive organ defects in zebrafish. Redox Biol. 2020, 28, 101355. [Google Scholar] [CrossRef]
- Huang, C.L.; Jian, X.; Yuh, C.H. Wnk1-Osr1/Spak kinase cascade is important for angiogenesis. Trans. Am. Clin. Climatol. Assoc. 2020, 131, 140–146. [Google Scholar]
- Sie, Z.L.; Li, R.Y.; Sampurna, B.P.; Hsu, P.J.; Liu, S.C.; Wang, H.D.; Huang, C.L.; Yuh, C.H. WNK1 kinase stimulates angiogenesis to promote tumor growth and metastasis. Cancers 2020, 12, 575. [Google Scholar] [CrossRef]
- Kozlov, S.V.; Waardenberg, A.J.; Engholm-Keller, K.; Arthur, J.W.; Graham, M.E.; Lavin, M. Reactive oxygen species (ROS)-activated ATM-dependent phosphorylation of cytoplasmic substrates identified by large-scale phosphoproteomics screen. Mol. Cell. Proteom. MCP 2016, 15, 1032–1047. [Google Scholar] [CrossRef]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Lab. Investig. J. Tech. Methods Pathol. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/mTOR in oral cancer: Mechanisms and advances in clinical trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Xu, C.; Hu, D.M.; Zhu, Q. eEF1A2 promotes cell migration, invasion and metastasis in pancreatic cancer by upregulating MMP-9 expression through Akt activation. Clin. Exp. Metastasis 2013, 30, 933–944. [Google Scholar] [CrossRef]
- Liu, C.J.; Yang, J.H.; Huang, F.Z.; Nie, W.P.; Liu, C.P.; Mao, X.H.; Yin, X.M.; Shen, X.B.; Peng, C.; Chen, M.F.; et al. Glutathione-s-transferase A 4 (GSTA4) suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting AKT pathway. Am. J. Transl. Res. 2017, 9, 301–315. [Google Scholar]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Tseng, H.L.; Li, C.J.; Huang, L.H.; Chen, C.Y.; Tsai, C.H.; Lin, C.N.; Hsu, H.Y. Quercetin 3-O-methyl ether protects FL83B cells from copper induced oxidative stress through the PI3K/Akt and MAPK/Erk pathway. Toxicol. Appl. Pharmacol. 2012, 264, 104–113. [Google Scholar] [CrossRef]
- Hu, Y.R.; Ma, H.; Zou, Z.Y.; He, K.; Xiao, Y.B.; Wang, Y.; Feng, M.; Ye, X.L.; Li, X.G. Activation of Akt and JNK/Nrf2/NQO1 pathway contributes to the protective effect of coptisine against AAPH-induced oxidative stress. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 85, 313–322. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Sa, F.; Chong, C.M.; Wang, Y.; Zhou, Z.Y.; Chang, R.C.; Chan, S.W.; Hoi, P.M.; Yuen Lee, S.M. Schisantherin A protects against 6-OHDA-induced dopaminergic neuron damage in zebrafish and cytotoxicity in SH-SY5Y cells through the ROS/NO and AKT/GSK3beta pathways. J. Ethnopharmacol. 2015, 170, 8–15. [Google Scholar] [CrossRef]
- Zou, Z.C.; Fu, J.J.; Dang, Y.Y.; Zhang, Q.; Wang, X.F.; Chen, H.B.; Jia, X.J.; Lee, S.M.; Li, C.W. Pinocembrin-7-methylether protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity via modulating Nrf2 induction through AKT and ERK pathways. Neurotox. Res. 2021, 39, 1323–1337. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Jin, U.H.; Suh, S.J.; Chang, H.W.; Son, J.K.; Lee, S.H.; Son, K.H.; Chang, Y.C.; Kim, C.H. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J. Cell. Biochem. 2008, 104, 15–26. [Google Scholar] [CrossRef]
- Cheng, C.S.; Chen, J.X.; Tang, J.; Geng, Y.W.; Zheng, L.; Lv, L.L.; Chen, L.Y.; Chen, Z. Paeonol inhibits pancreatic cancer cell migration and invasion through the inhibition of TGF-beta1/Smad signaling and epithelial-mesenchymal-transition. Cancer Manag. Res. 2020, 12, 641–651. [Google Scholar] [CrossRef]
- Gu, J.; Cui, C.F.; Yang, L.; Wang, L.; Jiang, X.H. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial-mesenchymal transition via the Wnt/beta-catenin pathway. Oncol. Res. 2019, 27, 193–202. [Google Scholar] [CrossRef]
- Lee, E.R.; Kim, J.Y.; Kang, Y.J.; Ahn, J.Y.; Kim, J.H.; Kim, B.W.; Choi, H.Y.; Jeong, M.Y.; Cho, S.G. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim. Biophys. Acta 2006, 1763, 958–968. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Peng, S.Y.; Hsiao, C.C.; Lan, T.H.; Yen, C.Y.; Farooqi, A.A.; Cheng, C.M.; Tang, J.Y.; Yu, T.J.; Yeh, Y.C.; Chuang, Y.T.; et al. Pomegranate extract inhibits migration and invasion of oral cancer cells by downregulating matrix metalloproteinase-2/9 and epithelial-mesenchymal transition. Environ. Toxicol. 2020, 35, 673–682. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, H.; Hong, C.; Chen, Z.; Deng, X.; Wang, A.; Yang, F.; Yang, L.; Chen, C.; Qin, X. PDGF promotes the Warburg effect in pulmonary arterial smooth muscle cells via activation of the PI3K/AKT/mTOR/HIF-1alpha signaling pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 1603–1613. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lin, Y.C.; Lin, C.C.; Chen, W.M.; Chen, B.P.C.; Lee, H. High glucose induces VEGF-C expression via the LPA1/3-Akt-ROS-LEDGF signaling axis in human prostate cancer PC-3 cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 597–611. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Yasui, H.; Kakudo, K.; Nozaki, M. Regulation of cell migration via the EGFR signaling pathway in oral squamous cell carcinoma cells. Oncol. Lett. 2017, 13, 930–936. [Google Scholar] [CrossRef]
- Lo, H.W.; Hsu, S.C.; Xia, W.; Cao, X.; Shih, J.Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67, 9066–9076. [Google Scholar] [CrossRef]
- Pan, C.W.; Jin, X.; Zhao, Y.; Pan, Y.; Yang, J.; Karnes, R.J.; Zhang, J.; Wang, L.; Huang, H. AKT-phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1-MAPK interaction. EMBO J. 2017, 36, 995–1010. [Google Scholar] [CrossRef]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Long, T.E.; Park, W.; Landry, J.C.; Taliaferro-Smith, L.; Farris, A.B.; Diaz, R.; El-Rayes, B.F. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol. Carcinog. 2015, 54, 1147–1158. [Google Scholar] [CrossRef]
- Liu, T.; Yin, H. PDK1 promotes tumor cell proliferation and migration by enhancing the Warburg effect in non-small cell lung cancer. Oncol. Rep. 2017, 37, 193–200. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Soro-Arnaiz, I.; De Bock, K. The Warburg effect in endothelial cells and its potential as an anti-angiogenic target in cancer. Front. Cell Dev. Biol. 2018, 6, 100. [Google Scholar] [CrossRef]
- Sonveaux, P.; Copetti, T.; De Saedeleer, C.J.; Vegran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frerart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Wang, F.; Qi, X.M.; Wertz, R.; Mortensen, M.; Hagen, C.; Evans, J.; Sheinin, Y.; James, M.; Liu, P.; Tsai, S.; et al. p38gamma MAPK is essential for aerobic glycolysis and pancreatic tumorigenesis. Cancer Res. 2020, 80, 3251–3264. [Google Scholar] [CrossRef]
- Lim, S.O.; Li, C.W.; Xia, W.; Lee, H.H.; Chang, S.S.; Shen, J.; Hsu, J.L.; Raftery, D.; Djukovic, D.; Gu, H.; et al. EGFR signaling enhances aerobic glycolysis in triple-negative breast cancer cells to promote tumor growth and immune escape. Cancer Res. 2016, 76, 1284–1296. [Google Scholar] [CrossRef]
- Caneba, C.A.; Yang, L.; Baddour, J.; Curtis, R.; Win, J.; Hartig, S.; Marini, J.; Nagrath, D. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014, 5, e1302. [Google Scholar] [CrossRef]
- Dong, Z.; Zhong, X.; Lei, Q.; Chen, F.; Cui, H. Transcriptional activation of SIRT6 via FKHRL1/FOXO3a inhibits the Warburg effect in glioblastoma cells. Cell. Signal. 2019, 60, 100–113. [Google Scholar] [CrossRef]
- Deacon, K.; Onion, D.; Kumari, R.; Watson, S.A.; Knox, A.J. Elevated SP-1 transcription factor expression and activity drives basal and hypoxia-induced vascular endothelial growth factor (VEGF) expression in non-small cell lung cancer. J. Biol. Chem. 2012, 287, 39967–39981. [Google Scholar] [CrossRef]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.; Liu, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef]
- Yang, J.S.; Lin, C.W.; Su, S.C.; Yang, S.F. Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 191–200. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef]
- Ha, G.H.; Park, J.S.; Breuer, E.K. TACC3 promotes epithelial-mesenchymal transition (EMT) through the activation of PI3K/Akt and ERK signaling pathways. Cancer Lett. 2013, 332, 63–73. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Condelli, V.; Crispo, F.; Pietrafesa, M.; Lettini, G.; Matassa, D.S.; Esposito, F.; Landriscina, M.; Maddalena, F. HSP90 molecular chaperones, metabolic rewiring, and epigenetics: Impact on tumor progression and perspective for anticancer therapy. Cells 2019, 8, 532. [Google Scholar] [CrossRef]
- Misra, L.; Lal, P.; Sangwan, R.S.; Sangwan, N.S.; Uniyal, G.C.; Tuli, R. Unusually sulfated and oxygenated steroids from Withania somnifera. Phytochemistry 2005, 66, 2702–2707. [Google Scholar] [CrossRef]
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Wang, K.W. Natural bioactive new withanolides. Mini Rev. Med. Chem. 2020, 20, 1101–1117. [Google Scholar] [CrossRef]
- Huang, M.; He, J.X.; Hu, H.X.; Zhang, K.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; Ren, D.M.; Shen, T. Withanolides from the genus Physalis: A review on their phytochemical and pharmacological aspects. J. Pharm. Pharmacol. 2020, 72, 649–669. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Rao, A.S.; Nandal, A.; Kumar, S.; Ganaie, S.A.; Narasihman, B. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J. Ethnopharmacol. 2021, 270, 113704. [Google Scholar] [CrossRef]
- Yu, T.J.; Tang, J.Y.; Lin, L.C.; Lien, W.J.; Cheng, Y.B.; Chang, F.R.; Ou-Yang, F.; Chang, H.W. Withanolide C inhibits proliferation of breast cancer cells via oxidative stress-mediated apoptosis and DNA damage. Antioxidants 2020, 9, 873. [Google Scholar] [CrossRef]
- Bessalle, R.; Lavie, D. Withanolide C, a chlorinated withanolide from Withania somnifera. Phytochemistry 1992, 31, 3648–3651. [Google Scholar] [CrossRef]
- Sakurai, K.; Ishii, H.; Kobayashi, S.; Iwao, T. Isolation of 4 beta-hydroxywithanolide E, a new withanolide from Physalis peruviana L. Chem. Pharm. Bull. 1976, 24, 1403–1405. [Google Scholar] [CrossRef]
- Fauré, M.; Lissi, E.; Torres, R.; Videla, L.A. Antioxidant activities of lignans and flavonoids. Phytochemistry 1990, 29, 3773–3775. [Google Scholar] [CrossRef]
- Senthil, K.; Thirugnanasambantham, P.; Oh, T.J.; Kim, S.H.; Choi, H.K. Free radical scavenging activity and comparative metabolic profiling of in vitro cultured and field grown Withania somnifera roots. PLoS ONE 2015, 10, e0123360. [Google Scholar] [CrossRef]
- Kumar, S.; Seal, C.J.; Howes, M.J.; Kite, G.C.; Okello, E.J. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and beta-amyloid(1-42)-induced cytotoxicity in differentiated PC12 cells. Phytother. Res. PTR 2010, 24, 1567–1574. [Google Scholar] [CrossRef]
- Devkar, S.T.; Jagtap, S.D.; Katyare, S.S.; Hegde, M.V. Estimation of antioxidant potential of individual components present in complex mixture of Withania somnifera (Ashwagandha) root fraction by thin-layer chromatography-2,2-diphenyl-1-picrylhdrazyl method. Jpc-J. Planar. Chromat. 2014, 27, 157–161. [Google Scholar] [CrossRef]
- Dar, N.J.; Bhat, J.A.; Satti, N.K.; Sharma, P.R.; Hamid, A.; Ahmad, M. Withanone, an active constituent from Withania somnifera, affords protection against NMDA-induced excitotoxicity in neuron-like cells. Mol. Neurobiol. 2017, 54, 5061–5073. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Reddy, S.S.; Chauhan, P.; Maurya, P.; Saini, D.; Yadav, P.P.; Barthwal, M.K. Coagulin-L ameliorates TLR4 induced oxidative damage and immune response by regulating mitochondria and NOX-derived ROS. Toxicol. Appl. Pharmacol. 2016, 309, 87–100. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, J.; Zhu, X.Y.; Zhang, Y.Q.; Ye, S.T.; Leng, Y.R.; Yang, T.; Zhang, H.; Kong, L.Y. Physalin B ameliorates nonalcoholic steatohepatitis by stimulating autophagy and NRF2 activation mediated improvement in oxidative stress. Free. Radic. Biol. Med. 2021, 164, 1–12. [Google Scholar] [CrossRef]
- Khan, I.; Lee, K.L.; Fakruzzaman, M.; Song, S.H.; Ihsan ul, H.; Mirza, B.; Yan, C.G.; Kong, I.K. Coagulansin-A has beneficial effects on the development of bovine embryos in vitro via HSP70 induction. Biosci. Rep. 2016, 36, e00310. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chang, F.R.; Huang, Z.Y.; Chen, J.H.; Wu, Y.C.; Wu, C.C. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J. Biol. Chem. 2008, 283, 17184–17193. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Zhang, C.; Luo, J.G.; Kong, L.Y. Downregulation of TIGAR sensitizes the antitumor effect of physapubenolide through increasing intracellular ROS levels to trigger apoptosis and autophagosome formation in human breast carcinoma cells. Biochem. Pharmacol. 2017, 143, 90–106. [Google Scholar] [CrossRef]
- Yu, T.J.; Cheng, Y.B.; Lin, L.C.; Tsai, Y.H.; Yao, B.Y.; Tang, J.Y.; Chang, F.R.; Yen, C.H.; Ou-Yang, F.; Chang, H.W. Physalis peruviana-derived physapruin A (PHA) inhibits breast cancer cell proliferation and induces oxidative-stress-mediated apoptosis and DNA damage. Antioxidants 2021, 10, 393. [Google Scholar] [CrossRef]
- Tang, J.Y.; Huang, H.W.; Wang, H.R.; Chan, Y.C.; Haung, J.W.; Shu, C.W.; Wu, Y.C.; Chang, H.W. 4beta-Hydroxywithanolide E selectively induces oxidative DNA damage for selective killing of oral cancer cells. Environ. Toxicol. 2018, 33, 295–304. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsiao, Y.H.; Ng, K.L.; Kuo, Y.H.; Lim, Y.P.; Hsieh, W.T. Physalin A attenuates inflammation through down-regulating c-Jun NH2 kinase phosphorylation/Activator Protein 1 activation and up-regulating the antioxidant activity. Toxicol. Appl. Pharmacol. 2020, 402, 115115. [Google Scholar] [CrossRef]
- He, H.; Zang, L.H.; Feng, Y.S.; Chen, L.X.; Kang, N.; Tashiro, S.; Onodera, S.; Qiu, F.; Ikejima, T. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-kappaB survival pathway in A375-S2 cells. J. Ethnopharmacol. 2013, 148, 544–555. [Google Scholar] [CrossRef]
- Kang, N.; Jian, J.F.; Cao, S.J.; Zhang, Q.; Mao, Y.W.; Huang, Y.Y.; Peng, Y.F.; Qiu, F.; Gao, X.M. Physalin A induces G2/M phase cell cycle arrest in human non-small cell lung cancer cells: Involvement of the p38 MAPK/ROS pathway. Mol. Cell. Biochem. 2016, 415, 145–155. [Google Scholar] [CrossRef]
- Baitharu, I.; Jain, V.; Deep, S.N.; Shroff, S.; Sahu, J.K.; Naik, P.K.; Ilavazhagan, G. Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia. PLoS ONE 2014, 9, e105311. [Google Scholar] [CrossRef]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis Int. J. Program. Cell Death 2007, 12, 2115–2133. [Google Scholar] [CrossRef]
- Chang, H.W.; Li, R.N.; Wang, H.R.; Liu, J.R.; Tang, J.Y.; Huang, H.W.; Chan, Y.H.; Yen, C.Y. Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front. Physiol. 2017, 8, 634. [Google Scholar] [CrossRef]
- Chien, T.M.; Wu, K.H.; Chuang, Y.T.; Yeh, Y.C.; Wang, H.R.; Yeh, B.W.; Yen, C.H.; Yu, T.J.; Wu, W.J.; Chang, H.W. Withaferin A triggers apoptosis and DNA damage in bladder cancer J82 cells through oxidative stress. Antioxidants 2021, 10, 1063. [Google Scholar] [CrossRef]
- Samadi, A.K.; Tong, X.; Mukerji, R.; Zhang, H.; Timmermann, B.N.; Cohen, M.S. Withaferin A, a cytotoxic steroid from Vassobia breviflora, induces apoptosis in human head and neck squamous cell carcinoma. J. Nat. Prod. 2010, 73, 1476–1481. [Google Scholar] [CrossRef]
- Zhang, X.; Samadi, A.K.; Roby, K.F.; Timmermann, B.; Cohen, M.S. Inhibition of cell growth and induction of apoptosis in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural withanolide Withaferin A. Gynecol. Oncol. 2012, 124, 606–612. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; Jiang, J.; Sun, C.; Wang, X.; Shen, M.; Tian, R.; Shi, C.; Xu, M.; Peng, F.; et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 2015, 357, 219–230. [Google Scholar] [CrossRef]
- Samadi, A.K.; Cohen, S.M.; Mukerji, R.; Chaguturu, V.; Zhang, X.; Timmermann, B.N.; Cohen, M.S.; Person, E.A. Natural withanolide withaferin A induces apoptosis in uveal melanoma cells by suppression of Akt and c-MET activation. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2012, 33, 1179–1189. [Google Scholar] [CrossRef]
- Akhtar, N.; Baig, M.W.; Haq, I.U.; Rajeeve, V.; Cutillas, P.R. Withanolide metabolites inhibit PI3K/AKT and MAPK pro-survival pathways and induce apoptosis in acute myeloid leukemia cells. Biomedicines 2020, 8, 333. [Google Scholar] [CrossRef]
- Wei, Z.; Li, T.; Sun, Y.; Su, H.; Zeng, Y.; Wang, Q.; Kuang, H. Daturataturin A, a withanolide in Datura metel L., induces HaCaT autophagy through the PI3K-Akt-mTOR signaling pathway. Phytother. Res. PTR 2021, 35, 1546–1558. [Google Scholar] [CrossRef]
- Ma, T.; Fan, B.Y.; Zhang, C.; Zhao, H.J.; Han, C.; Gao, C.Y.; Luo, J.G.; Kong, L.Y. Metabonomics applied in exploring the antitumour mechanism of physapubenolide on hepatocellular carcinoma cells by targeting glycolysis through the Akt-p53 pathway. Sci. Rep. 2016, 6, 29926. [Google Scholar] [CrossRef]
- Wang, H.C.; Hu, H.H.; Chang, F.R.; Tsai, J.Y.; Kuo, C.Y.; Wu, Y.C.; Wu, C.C. Different effects of 4beta-hydroxywithanolide E and withaferin A, two withanolides from Solanaceae plants, on the Akt signaling pathway in human breast cancer cells. Phytomed. Int. J. Phytother. Phytopharm. 2019, 53, 213–222. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, R.; Gan, L.; Lau, W.B.; Cao, X.; Zhao, J.; Ma, X.; Christopher, T.A.; Lopez, B.L.; Wang, Y. Withaferin A inhibits apoptosis via activated Akt-mediated inhibition of oxidative stress. Life Sci. 2018, 211, 91–101. [Google Scholar] [CrossRef]
- Mohan, R.; Hammers, H.J.; Bargagna-Mohan, P.; Zhan, X.H.; Herbstritt, C.J.; Ruiz, A.; Zhang, L.; Hanson, A.D.; Conner, B.P.; Rougas, J.; et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Khuan Abby Ho, Y.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Ravindranath, P.P.; Mohan, R. Small molecule anti-angiogenic probes of the ubiquitin proteasome pathway: Potential application to choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4138–4145. [Google Scholar] [CrossRef]
- Yue, G.G.; Lee, J.K.; Kwok, H.F.; Cheng, L.; Wong, E.C.; Jiang, L.; Yu, H.; Leung, H.W.; Wong, Y.L.; Leung, P.C.; et al. Novel PI3K/AKT targeting anti-angiogenic activities of 4-vinylphenol, a new therapeutic potential of a well-known styrene metabolite. Sci. Rep. 2015, 5, 11149. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, C.; Lin, C.Y.; Chen, Y.H.; Lin, C.Y.; Chi, C.W.; Chen, Y.J.; Liu, S.C.; Chang, T.K.; Tang, C.H.; et al. Garcimultiflorone K inhibits angiogenesis through Akt/eNOS- and mTOR-dependent pathways in human endothelial progenitor cells. Phytomed. Int. J. Phytother. Phytopharm. 2019, 64, 152911. [Google Scholar] [CrossRef]
- Hung, T.W.; Chen, P.N.; Wu, H.C.; Wu, S.W.; Tsai, P.Y.; Hsieh, Y.S.; Chang, H.R. Kaempferol inhibits the invasion and migration of renal cancer cells through the downregulation of AKT and FAK pathways. Int. J. Med. Sci. 2017, 14, 984–993. [Google Scholar] [CrossRef]
- Song, M.; Wang, X.; Luo, Y.; Liu, Z.; Tan, W.; Ye, P.; Fu, Z.; Lu, F.; Xiang, W.; Tang, L.; et al. Cantharidin suppresses gastric cancer cell migration/invasion by inhibiting the PI3K/Akt signaling pathway via CCAT1. Chem.-Biol. Interact. 2020, 317, 108939. [Google Scholar] [CrossRef]
- Ahmed, H.; Ghoshal, A.; Jones, S.; Ellis, I.; Islam, M. Head and neck cancer metastasis and the effect of the local soluble factors, from the microenvironment, on signalling pathways: Is it all about the Akt? Cancers 2020, 12, 2093. [Google Scholar] [CrossRef]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion? J. Oral Pathol. Med. 2020, 49, 227–234. [Google Scholar] [CrossRef]
- Lai, H.; Fu, X.; Sang, C.; Hou, L.; Feng, P.; Li, X.; Chen, T. Selenadiazole derivatives inhibit angiogenesis-mediated human breast tumor growth by suppressing the VEGFR2-mediated ERK and AKT signaling pathways. Chem. Asian J. 2018, 13, 1447–1457. [Google Scholar] [CrossRef]
- Wang, P.; Tian, X.F.; Rong, J.B.; Liu, D.; Yi, G.G.; Tan, Q. Protein kinase B (akt) promotes pathological angiogenesis in murine model of oxygen-induced retinopathy. Acta Histochem. Cytochem. 2011, 44, 103–111. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the multifaceted therapeutic potential of withaferin A and its derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Gu, J.; Chen, C.; Wang, J.; Chen, T.; Yao, W.; Yan, T.; Liu, Z. Withaferin A exerts preventive effect on liver fibrosis through oxidative stress inhibition in a sirtuin 3-dependent manner. Oxidative Med. Cell. Longev. 2020, 2020, 2452848. [Google Scholar] [CrossRef]
- Prasanna Kumar, S.; Shilpa, P.; Salimath, B.P. Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor. Curr. Trends Biotechnol. Pharm. 2009, 3, 138–148. [Google Scholar]
- Dong, B.; An, L.; Yang, X.; Zhang, X.; Zhang, J.; Tuerhong, M.; Jin, D.Q.; Ohizumi, Y.; Lee, D.; Xu, J.; et al. Withanolides from Physalis peruviana showing nitric oxide inhibitory effects and affinities with iNOS. Bioorg. Chem. 2019, 87, 585–593. [Google Scholar] [CrossRef]
- Fan, Y.; Mao, Y.; Cao, S.; Xia, G.; Zhang, Q.; Zhang, H.; Qiu, F.; Kang, N. S5, a withanolide isolated from Physalis pubescens l., induces G2/M cell cycle arrest via the EGFR/P38 pathway in human melanoma A375 cells. Molecules 2018, 23, 3175. [Google Scholar] [CrossRef]
- Gu, M.; Yu, Y.; Gunaherath, G.M.; Gunatilaka, A.A.; Li, D.; Sun, D. Structure-activity relationship (SAR) of withanolides to inhibit Hsp90 for its activity in pancreatic cancer cells. Investig. New Drugs 2014, 32, 68–74. [Google Scholar] [CrossRef]
- Wang, H.C.; Tsai, Y.L.; Wu, Y.C.; Chang, F.R.; Liu, M.H.; Chen, W.Y.; Wu, C.C. Withanolides-induced breast cancer cell death is correlated with their ability to inhibit heat protein 90. PLoS ONE 2012, 7, e37764. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Saloni; Singh, H.; Kim, M.H.; Sharma, P.; Misra, S.; Khan, F. Molecular docking, QSAR and ADMET studies of withanolide analogs against breast cancer. Drug Des. Dev. Ther. 2017, 11, 1859–1870. [Google Scholar] [CrossRef]
- Azoitei, N.; Diepold, K.; Brunner, C.; Rouhi, A.; Genze, F.; Becher, A.; Kestler, H.; van Lint, J.; Chiosis, G.; Koren, J., 3rd; et al. HSP90 supports tumor growth and angiogenesis through PRKD2 protein stabilization. Cancer Res. 2014, 74, 7125–7136. [Google Scholar] [CrossRef]
- Bohonowych, J.E.; Gopal, U.; Isaacs, J.S. Hsp90 as a gatekeeper of tumor angiogenesis: Clinical promise and potential pitfalls. J. Oncol. 2010, 2010, 412985. [Google Scholar] [CrossRef]
| Drugs/Proteins | Activation | Inactivation | Migration Effects | Cells | References |
|---|---|---|---|---|---|
| Section 8.1. The Network between AKT, MMP-2/9, EMT, and Migration | |||||
| ARP100, AG-L-66085 | MMP-2/9 | inhibit | retinoblastoma | [56] | |
| Tanshinone IIA | AKT, MMP-9 | inhibit | aortic smooth muscle | [57] | |
| siRNA eEF1A2 | AKT, MMP-9 | inhibit | pancreatic cancer | [47] | |
| Paeonol | EMT | inhibit | pancreas cancer | [58] | |
| Emodin | EMT | inhibit | colon cancer | [59] | |
| Section 8.2. AKT Regulates Several Migration- and Angiogenesis-Related Genes to Modulate EMT | |||||
| MAPK | AKT | fibroblast, cancer | [60,61] | ||
| POMx | MMP-2/9, EMT | inhibit | oral cancer | [62] | |
| PDGF | AKT | promote | aortic smooth muscle | [63] | |
| High glucose | AKT, VEGF-C | prostate cancer | [64] | ||
| AKT | eNOS | endothelial cells | [65] | ||
| EGF | AKT | promote | oral cancer | [66] | |
| EGFR | EMT | breast cancer | [67] | ||
| AKT | FOXO1 | prostate cancer | [68] | ||
| circSTK40 | HSP90, AKT | breast cancer | [69] | ||
| HSP90 | EMT | promote | colorectal cancer | [70] | |
| Section 8.3. AKT Network, Warburg Effect, Migration, and Angiogenesis | |||||
| PDK1 | Warburg effect | promote | lung cancer | [71] | |
| High glycolysis | Warburg effect | angiogenesis | endothelial | [72] | |
| MCT1 | Warburg effect | angiogenesis | endothelial | [73] | |
| AKT | Warburg effect | cancer | [74] | ||
| p38γ MAPK | Warburg effect | pancreatic cancer | [75] | ||
| PDGF, AKT | Warburg effect | aortic smooth muscle | [63] | ||
| F1,6BP, EGFR | Warburg effect | breast cancer | [76] | ||
| NOS, HK2 | Warburg effect | ovarian cancer | [77] | ||
| FOXO3a | Warburg effect | glioblastoma | [78] | ||
| HIF-1α, VEGF | Warburg effect | lung cancer | [79] | ||
| HSP90, PKM2 | Warburg effect | liver cancer | [80] | ||
| Drugs | ROS | References |
|---|---|---|
| Coagulin-L | decrease | [100] |
| Physalin B | decrease | [101] |
| Coagulansin-A | decrease | [102] |
| Withanone | decrease | [98] |
| Tubocapsenolide A | increase | [103] |
| Physapubenolide | increase | [104] |
| Withanolide C | increase | [91] |
| Physapruin A | increase | [105] |
| 4β-Hydroxywithanolide E | increase | [106] |
| Physalin A | decrease/increase | [107,108,109] |
| Withaferin A | decrease/increase | [110,111,112,113] |
| Drugs | AKT Signaling | References |
|---|---|---|
| Withametelin | MAPK (ERK1/2) | [118] |
| Coagulansin-A | ||
| Withaferin A | PDGF | [135] |
| VEGF | [136] | |
| FOXO3a | [79] | |
| HSP90 | [139] | |
| Peruvianolide B | NOS | [137] |
| Peruvianolide C | ||
| Peruvianolide D | ||
| S5 | EGFR | [138] |
| 3-Aziridinylwithaferin A | HSP90 | [139] |
| Withanolide E | ||
| 4-Hydroxywithanolide E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.-Y.; Cheng, Y.-B.; Chuang, Y.-T.; Yang, K.-H.; Chang, F.-R.; Liu, W.; Chang, H.-W. Oxidative Stress and AKT-Associated Angiogenesis in a Zebrafish Model and Its Potential Application for Withanolides. Cells 2022, 11, 961. https://doi.org/10.3390/cells11060961
Tang J-Y, Cheng Y-B, Chuang Y-T, Yang K-H, Chang F-R, Liu W, Chang H-W. Oxidative Stress and AKT-Associated Angiogenesis in a Zebrafish Model and Its Potential Application for Withanolides. Cells. 2022; 11(6):961. https://doi.org/10.3390/cells11060961
Chicago/Turabian StyleTang, Jen-Yang, Yuan-Bin Cheng, Ya-Ting Chuang, Kun-Han Yang, Fang-Rong Chang, Wangta Liu, and Hsueh-Wei Chang. 2022. "Oxidative Stress and AKT-Associated Angiogenesis in a Zebrafish Model and Its Potential Application for Withanolides" Cells 11, no. 6: 961. https://doi.org/10.3390/cells11060961
APA StyleTang, J.-Y., Cheng, Y.-B., Chuang, Y.-T., Yang, K.-H., Chang, F.-R., Liu, W., & Chang, H.-W. (2022). Oxidative Stress and AKT-Associated Angiogenesis in a Zebrafish Model and Its Potential Application for Withanolides. Cells, 11(6), 961. https://doi.org/10.3390/cells11060961









