Functional Role of microRNAs in Regulating Cardiomyocyte Death
Abstract
1. Introduction
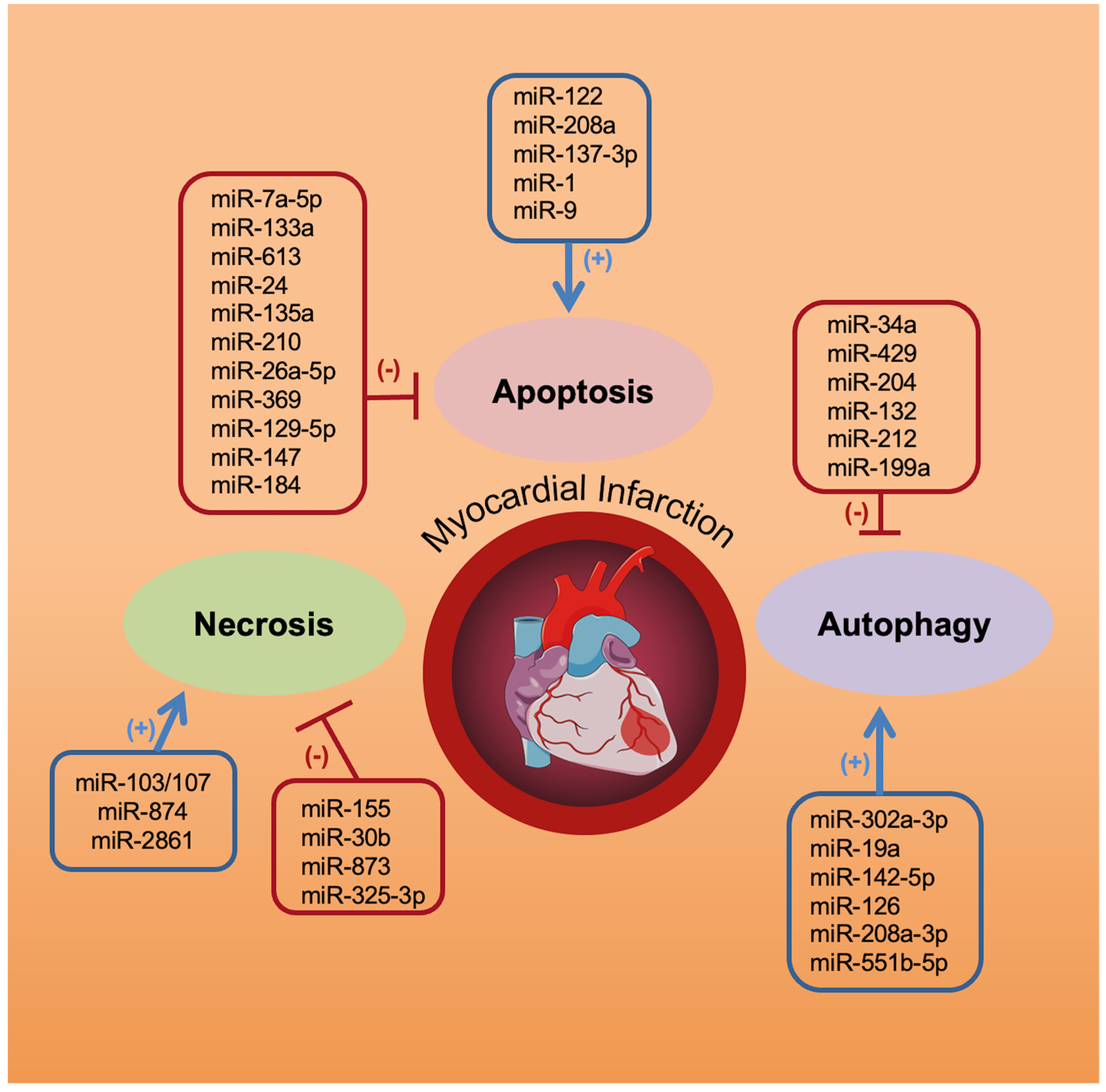
2. miRNAs and Cardiomyocyte Necrosis
3. miRNAs and Cardiomyocyte Apoptosis
4. miRNAs and Cardiomyocyte Autophagy
| Mechanisms | miRNA | Target Gene(s) | References |
|---|---|---|---|
| Apoptosis | miR-1 | Bcl2 | [57] |
| miR-7a-5p | VDAC1 | [61] | |
| miR-9 | CDK8 | [63] | |
| miR-24 | Mapk14 | [64] | |
| miR-26a-5p | PTEN/PI3K/AKT | [68] | |
| miR-29b-3p | SPARC/TGF-β1/SMAD | [76] | |
| miR-122 | GATA-4 | [58] | |
| miR-129-5p | HMGB1 | [70] | |
| miR-133a | TAGLN2, HSP60, HSP70, Apaf1, Caspase3,8,9 | [53,54,55,56] | |
| miR-135a | TLR4 | [65] | |
| miR-137-3p | KLF15 | [60] | |
| miR-142-3p | HMGB1, Rac1 | [75] | |
| miR-147 | HIPK2 | [71] | |
| miR-184 | FBXO28 | [72] | |
| miR-208a | APC | [59] | |
| miR-210 | E2F3 | [67] | |
| miR-223 | PARP-1 | [73] | |
| miR-369 | TRPV3 | [69] | |
| miR-613 | PDCD10 | [62] | |
| Necrosis | miR-30b | CypD | [18] |
| miR-103/107 | FADD | [15] | |
| miR-155 | RIP1 | [23] | |
| miR-325-3p | PIPK3 | [22] | |
| miR-874 | Caspase-8 | [20] | |
| miR-873 | P53 | [16] | |
| miR-2861 | ANT1 | [26] | |
| Autophagy | miR-17-5p | STAT3 | [106] |
| miR-19a | Bim | [103] | |
| miR-26a | Usp15 | [109] | |
| miR-30e-3p | Egr-1 | [104] | |
| miR-34a | Lc3-II, p62, TNF-α | [90] | |
| miR-126 | Beclin-1 | [96] | |
| miR-132 | FoxO3 | [101] | |
| miR-142-5p | Beclin-1 | [95] | |
| miR-145 | FRS2 | [110] | |
| miR-199a | HSPA5/MAPK-mTOR | [100] | |
| miR-204 | SIRT1 | [98,99] | |
| miR-208a-3p | PDCD4 | [94] | |
| miR-212 | FoxO3 | [101] | |
| miR-302a-3p | FoxO3 | [102] | |
| miR-429 | MO25/LKB1/AMPK | [91] | |
| miR-542-5p | ATG7 | [92] | |
| miR-551b-5p | PCDH17 | [107] |
5. Clinical Studies: miRNAs as Biomarkers and Potential Therapies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santulli, G. MicroRNA: From Molecular Biology to Clinical Practice; Santulli, G., Ed.; Springer Nature: New York, NY, USA, 2016. [Google Scholar]
- Yoshida, T.; Asano, Y.; Ui-Tei, K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Noncoding RNA 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Kansakar, U.; Donkor, K.; Wilson, S.; Jankauskas, S.; Mone, P.; Wang, X.; Lombardi, A.; Santulli, G. Cardiac remodeling after myocardial infarction: Functional contribution of microRNAs to inflammation and fibrosis. Front. Cardiovasc. Med. 2022; in press. [Google Scholar]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of cell death in heart disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Li, L.; Tong, A.; Zhang, Q.; Wei, Y.; Wei, X. The molecular mechanisms of MLKL-dependent and MLKL-independent necrosis. J. Mol. Cell Biol. 2021, 13, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kist, M.; Vucic, D. Cell death pathways: Intricate connections and disease implications. EMBO J. 2021, 40, e106700. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, A. Programmed necrosis in heart disease: Molecular mechanisms and clinical implications. J. Mol. Cell. Cardiol. 2018, 116, 125–134. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Zhang, M.; Zhang, Y. Programmed necrosis in cardiomyocytes: Mitochondria, death receptors and beyond. Br. J. Pharmacol. 2019, 176, 4319–4339. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, P.; Li, J. Necroptosis: An emerging form of programmed cell death. Crit. Rev. Oncol. Hematol. 2012, 82, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, C.; Zhao, Y.; Ponnusamy, M.; Li, P.; Wang, K. Role of noncoding RNAs in regulation of cardiac cell death and cardiovascular diseases. Cell. Mol. Life Sci. 2018, 75, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhang, X.J.; Li, Q.; Wang, K.; Wang, Y.; Jiao, J.Q.; Feng, C.; Teng, S.; Zhou, L.Y.; Gong, Y.; et al. MicroRNA-103/107 Regulate Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury Through Targeting FADD. Circ. Res. 2015, 117, 352–363. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Liu, C.Y.; An, T.; Zhang, J.; Zhou, L.Y.; Wang, M.; Dong, Y.H.; Li, N.; Gao, J.N.; et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016, 23, 1394–1405. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshida, K. Mechanical insights into the regulation of programmed cell death by p53 via mitochondria. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; An, T.; Zhou, L.Y.; Liu, C.Y.; Zhang, X.J.; Feng, C.; Li, P.F. E2F1-regulated miR-30b suppresses Cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ. 2015, 22, 743–754. [Google Scholar] [CrossRef]
- Feng, S.; Yang, Y.; Mei, Y.; Ma, L.; Zhu, D.E.; Hoti, N.; Castanares, M.; Wu, M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007, 19, 2056–2067. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Zhou, L.Y.; Ding, S.L.; Long, B.; Liu, C.Y.; Sun, T.; Fan, Y.Y.; Sun, L.; Li, P.F. miR-874 regulates myocardial necrosis by targeting caspase-8. Cell Death Dis. 2013, 4, e709. [Google Scholar] [CrossRef]
- Xin, Z.; Ma, Z.; Jiang, S.; Wang, D.; Fan, C.; Di, S.; Hu, W.; Li, T.; She, J.; Yang, Y. FOXOs in the impaired heart: New therapeutic targets for cardiac diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 486–498. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, B.J.; Ma, M.; Yu, K.; Zhang, Q.; Zhang, X.W. MicroRNA-325-3p protects the heart after myocardial infarction by inhibiting RIPK3 and programmed necrosis in mice. BMC Mol. Biol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Liu, J.; van Mil, A.; Vrijsen, K.; Zhao, J.; Gao, L.; Metz, C.H.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J. Cell. Mol. Med. 2011, 15, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Gambardella, J.; Sardu, C.; Lombardi, A.; Santulli, G. Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. Noncoding RNA 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wang, N.; Bisetto, S.; Yi, B.; Sheu, S.S. Downregulation of adenine nucleotide translocator 1 exacerbates tumor necrosis factor-alpha-mediated cardiac inflammatory responses. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H39–H48. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Li, N.; Li, L.; Liu, C.Y.; Dong, Y.H.; Gao, J.N.; Zhou, L.Y.; Wang, C.Q.; Li, P.F. MicroRNA-2861 regulates programmed necrosis in cardiomyocyte by impairing adenine nucleotide translocase 1 expression. Free Radic. Biol. Med. 2016, 91, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gerschenson, L.E.; Geske, F.J. Virchow and apoptosis. Am. J. Pathol. 2001, 158, 1543. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Kim, N.H.; Kang, P.M. Apoptosis in cardiovascular diseases: Mechanism and clinical implications. Korean Circ. J. 2010, 40, 299–305. [Google Scholar] [CrossRef]
- Lee, Y.; Gustafsson, A.B. Role of apoptosis in cardiovascular disease. Apoptosis 2009, 14, 536–548. [Google Scholar] [CrossRef]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef]
- Haunstetter, A.; Izumo, S. Apoptosis: Basic mechanisms and implications for cardiovascular disease. Circ. Res. 1998, 82, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Orogo, A.M.; Gustafsson, A.B. Cell death in the myocardium: My heart won’t go on. IUBMB Life 2013, 65, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Leibowitz, B.; Yu, J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol. Ther. 2010, 9, 417–422. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Amgalan, D.; Garner, T.P.; Pekson, R.; Jia, X.F.; Yanamandala, M.; Paulino, V.; Liang, F.G.; Corbalan, J.J.; Lee, J.; Chen, Y.; et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat. Cancer 2020, 1, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Kwong, J.Q.; Burr, A.R.; Sargent, M.A.; Elrod, J.W.; Peixoto, P.M.; Martinez-Caballero, S.; Osinska, H.; Cheng, E.H.; Robbins, J.; et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife 2013, 2, e00772. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Kilbride, S.M.; Prehn, J.H. Central roles of apoptotic proteins in mitochondrial function. Oncogene 2013, 32, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Qu, Y.; Chen, H.; Qian, J. Insight into long noncoding RNA-miRNA-mRNA axes in myocardial ischemia-reperfusion injury: The implications for mechanism and therapy. Epigenomics 2019, 11, 1733–1748. [Google Scholar] [CrossRef]
- Santulli, G. microRNAs Distinctively Regulate Vascular Smooth Muscle and Endothelial Cells: Functional Implications in Angiogenesis, Atherosclerosis, and In-Stent Restenosis. Adv. Exp. Med. Biol. 2015, 887, 53–77. [Google Scholar] [CrossRef]
- Wronska, A.; Kurkowska-Jastrzebska, I.; Santulli, G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. 2015, 213, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Balamurali, D.; Stoll, M. Non-Coding RNA Databases in Cardiovascular Research. Noncoding RNA 2020, 6, 35. [Google Scholar] [CrossRef]
- Gandhi, S.; Ruehle, F.; Stoll, M. Evolutionary Patterns of Non-Coding RNA in Cardiovascular Biology. Noncoding RNA 2019, 5, 15. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Li, A.Y.; Yang, Q.; Yang, K. miR-133a mediates the hypoxia-induced apoptosis by inhibiting TAGLN2 expression in cardiac myocytes. Mol. Cell. Biochem. 2015, 400, 173–181. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xiao, J.; Ren, A.J.; Zhang, Y.F.; Zhang, H.; Chen, M.; Xie, B.; Gao, X.G.; Wang, Y.W. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J. Biomed. Sci. 2011, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lu, Y.; Pan, Z.; Chu, W.; Luo, X.; Lin, H.; Xiao, J.; Shan, H.; Wang, Z.; Yang, B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J. Cell Sci. 2007, 120, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Dakhlallah, D.; Zhang, J.; Yu, L.; Marsh, C.B.; Angelos, M.G.; Khan, M. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J. Cardiovasc. Pharmacol. 2015, 65, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, J.; Sun, Y.; Wu, Z.; Liu, Z.; Huang, G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int. Heart J. 2009, 50, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guo, J.; Li, J.; Bai, C.; Dong, Y. Downregulation of miR-122 attenuates hypoxia/reoxygenation (H/R)-induced myocardial cell apoptosis by upregulating GATA-4. Biochem. Biophys. Res. Commun. 2016, 478, 1416–1422. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Zhang, Z.; Lu, Y.; Lu, X. MiR-208a aggravates H2O2-induced cardiomyocyte injury by targeting APC. Eur. J. Pharmacol. 2019, 864, 172668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Qiu, Z.; Gao, Y. MiR-137-3p exacerbates the ischemia-reperfusion injured cardiomyocyte apoptosis by targeting KLF15. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1013–1024. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Xuan, F. MiR-7a-5p Attenuates Hypoxia/Reoxygenation-Induced Cardiomyocyte Apoptosis by Targeting VDAC1. Cardiovasc. Toxicol. 2021, 22, 108–117. [Google Scholar] [CrossRef]
- Wu, Z.; Qi, Y.; Guo, Z.; Li, P.; Zhou, D. miR-613 suppresses ischemia-reperfusion-induced cardiomyocyte apoptosis by targeting the programmed cell death 10 gene. BioSci. Trends 2016, 10, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.; Tan, G.; Fan, Z.; Xiao, J.; Shi, C.; Lin, Z. MicroRNA-9 facilitates hypoxia-induced injury and apoptosis in H9c2 cells via targeting CDK8. J. BioSci. 2021, 46, 16. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Q.; Zhang, Z.S.; Wang, X.Y.; Shi, J.Z.; Yang, X.B. MiR-24 Protects Cardiomyocytes Against Hypoxia/Reoxygenation-Induced Injury Through Regulating Mitogen-Activated Protein Kinase 14. Int. Heart J. 2020, 61, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xie, B.; Zhang, Z.; Yan, J.; Cheng, M.; Zhou, Y. MiR-135a Protects against Myocardial Injury by Targeting TLR4. Chem. Pharm. Bull. 2021, 69, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.A.; Goluszko, E.; Chen, H.Z.; Leone, G.; Post, S.; Lozano, G.; Chen, Z.; Chauchereau, A. E2F3 is a mediator of DNA damage-induced apoptosis. Mol. Cell Biol. 2010, 30, 524–536. [Google Scholar] [CrossRef][Green Version]
- Bian, W.S.; Shi, P.X.; Mi, X.F.; Sun, Y.Y.; Yang, D.D.; Gao, B.F.; Wu, S.X.; Fan, G.C. MiR-210 protects cardiomyocytes from OGD/R injury by inhibiting E2F3. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 743–749. [Google Scholar] [CrossRef]
- Xing, X.; Guo, S.; Zhang, G.; Liu, Y.; Bi, S.; Wang, X.; Lu, Q. miR-26a-5p protects against myocardial ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e9106. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Huang, W. MicroRNA-369 attenuates hypoxia-induced cardiomyocyte apoptosis and inflammation via targeting TRPV3. Braz. J. Med. Biol. Res. 2021, 54, e10550. [Google Scholar] [CrossRef]
- Chen, Z.X.; He, D.; Mo, Q.W.; Xie, L.P.; Liang, J.R.; Liu, L.; Fu, W.J. MiR-129-5p protects against myocardial ischemia-reperfusion injury via targeting HMGB1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4440–4450. [Google Scholar] [CrossRef]
- Wu, C.G.; Huang, C. MicroRNA-147 inhibits myocardial inflammation and apoptosis following myocardial infarction via targeting HIPK2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6279–6287. [Google Scholar] [CrossRef]
- Zou, J.F.; Wu, X.N.; Shi, R.H.; Sun, Y.Q.; Qin, F.J.; Yang, Y.M. Inhibition of microRNA-184 reduces H2O2-mediated cardiomyocyte injury via targeting FBXO28. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11251–11258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, Y.; Xu, Y.; Jin, W.; Li, H. MicroRNA-223 protects neonatal rat cardiomyocytes and H9c2 cells from hypoxia-induced apoptosis and excessive autophagy via the Akt/mTOR pathway by targeting PARP-1. J. Mol. Cell. Cardiol. 2018, 118, 133–146. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, Z.; Jin, L.; Zhang, F.; Peng, X.; Xiao, Y.; Wang, X.; Lyu, Q.; Cai, X. lncRNA TUG1-Mediated Mir-142-3p Downregulation Contributes to Metastasis and the Epithelial-to-Mesenchymal Transition of Hepatocellular Carcinoma by Targeting ZEB1. Cell. Physiol. Biochem. 2018, 48, 1928–1941. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Y.; Lv, X.W.; Ye, Z.L.; Sun, Y.H.; Kong, B.H.; Qin, Z.B. Inhibition of lncRNA TUG1 upregulates miR-142-3p to ameliorate myocardial injury during ischemia and reperfusion via targeting HMGB1- and Rac1-induced autophagy. J. Mol. Cell. Cardiol. 2019, 133, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lei, D.; Bu, F.; Han, H.; Zhao, S.; Wang, Y. MicroRNA-29b-3p Targets SPARC Gene to Protect Cardiocytes against Autophagy and Apoptosis in Hypoxic-Induced H9c2 Cells. J. Cardiovasc. Transl. Res. 2019, 12, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Santulli, G.; Totary-Jain, H. Tailoring mTOR-based therapy: Molecular evidence and clinical challenges. Pharmacogenomics 2013, 14, 1517–1526. [Google Scholar] [CrossRef]
- Tungsukruthai, S.; Reamtong, O.; Roytrakul, S.; Sukrong, S.; Vinayanwattikun, C.; Chanvorachote, P. Targeting AKT/mTOR and Bcl-2 for Autophagic and Apoptosis Cell Death in Lung Cancer: Novel Activity of a Polyphenol Compound. Antioxidants 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, J.; Zhao, J.; Ma, N.; Kim, S.W.; Qiao, S.; Ma, X. Autophagy: The Last Defense against Cellular Nutritional Stress. Adv. Nutr. 2018, 9, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, M.Y.; Li, P.F.; Cao, J.M. MicroRNAs in Cardiac Autophagy: Small Molecules and Big Role. Cells 2018, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Hariharan, N.; Monden, Y.; Zablocki, D.; Sadoshima, J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr. Cardiol. 2011, 32, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, K.; Hu, P. The Role of Autophagy in Acute Myocardial Infarction. Front. Pharmacol. 2019, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Cardioprotective effects of autophagy: Eat your heart out, heart failure! Sci. Transl. Med. 2018, 10, aau0462. [Google Scholar] [CrossRef]
- Shao, H.; Yang, L.; Wang, L.; Tang, B.; Wang, J.; Li, Q. MicroRNA-34a protects myocardial cells against ischemia-reperfusion injury through inhibiting autophagy via regulating TNFalpha expression. Biochem. Cell Biol. 2018, 96, 349–354. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, F. Antagonism of miR-429 ameliorates anoxia/reoxygenation injury in cardiomyocytes by enhancing MO25/LKB1/AMPK mediated autophagy. Life Sci. 2019, 235, 116842. [Google Scholar] [CrossRef]
- Wang, F.; Min, X.; Hu, S.Y.; You, D.L.; Jiang, T.T.; Wang, L.; Wu, X. Hypoxia/reoxygenation-induced upregulation of miRNA-542-5p aggravated cardiomyocyte injury by repressing autophagy. Hum. Cell 2021, 34, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, X.; Wang, X.; Zhu, F.; Guo, C.; Wang, Q.; Shi, Y.; Wang, J.; Chen, Y.; Zhang, L. Tumor suppressor gene PDCD4 negatively regulates autophagy by inhibiting the expression of autophagy-related gene ATG5. Autophagy 2013, 9, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, N.; Lian, X.; Peng, F.; Zhang, H.; Gong, H. MiR-208a-3p aggravates autophagy through the PDCD4-ATG5 pathway in Ang II-induced H9c2 cardiomyoblasts. Biomed. Pharmacother 2018, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, Q.; Liu, X. MicroRNA-142-5p inhibits autophagy in cardiomyocytes in a mouse model of hypoxia/reoxygenation. FEBS Open Bio 2020, 12, 333. [Google Scholar] [CrossRef]
- Shi, C.C.; Pan, L.Y.; Peng, Z.Y.; Li, J.G. MiR-126 regulated myocardial autophagy on myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6971–6979. [Google Scholar] [CrossRef]
- Maejima, Y.; Isobe, M.; Sadoshima, J. Regulation of autophagy by Beclin 1 in the heart. J. Mol. Cell. Cardiol. 2016, 95, 19–25. [Google Scholar] [CrossRef]
- Qiu, R.; Li, W.; Liu, Y. MicroRNA-204 protects H9C2 cells against hypoxia/reoxygenation-induced injury through regulating SIRT1-mediated autophagy. Biomed. Pharmacother. 2018, 100, 15–19. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, X.; He, B.; Zhang, Y.; Kang, B.; Wang, Z.; Ni, X. MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J. Biomed. Sci. 2011, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, F.Y.; Zeng, Z.Y.; Cui, L.; Shen, J.; Song, X.W.; Li, P.; Zhao, X.X.; Qin, Y.W. MicroRNA-199a acts as a potential suppressor of cardiomyocyte autophagy through targeting Hspa5. Oncotarget 2017, 8, 63825–63834. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Lv, W.; Jiang, J.; Li, Y.; Fu, L.; Meng, F.; Li, J. MiR-302a-3p aggravates myocardial ischemia-reperfusion injury by suppressing mitophagy via targeting FOXO3. Exp. Mol. Pathol. 2020, 117, 104522. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Qian, J.Y.; Chen, Z.W.; Fu, M.Q.; Xu, J.F.; Xia, Y.; Ding, X.F.; Yang, X.D.; Cao, Y.Y.; Zou, Y.Z.; et al. Suppression of Bim by microRNA-19a may protect cardiomyocytes against hypoxia-induced cell death via autophagy activation. Toxicol. Lett. 2016, 257, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Khachigian, L.M. Early growth response-1 in the pathogenesis of cardiovascular disease. J. Mol. Med. 2016, 94, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, X.; Sun, Y.; Long, M.; Zheng, J.; Wu, W.; Li, L. miR-30e-3p Promotes Cardiomyocyte Autophagy and Inhibits Apoptosis via Regulating Egr-1 during Ischemia/Hypoxia. BioMed Res. Int. 2020, 2020, 7231243. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, Y.; Wu, J.; Song, J.; Lu, J. microRNA-17-5p downregulation inhibits autophagy and myocardial remodelling after myocardial infarction by targeting STAT3. Autoimmunity 2021, 55, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, W.; Zhang, W.; Wang, W.; Liu, T.; Zhou, X. LncRNA DCRF regulates cardiomyocyte autophagy by targeting miR-551b-5p in diabetic cardiomyopathy. Theranostics 2019, 9, 4558–4566. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.Y.; Zhou, L.Y.; Wang, J.X.; Wang, M.; Zhao, B.; Zhao, W.K.; Xu, S.J.; Fan, L.H.; Zhang, X.J.; et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat. Commun. 2015, 6, 6779. [Google Scholar] [CrossRef]
- Liang, H.; Su, X.; Wu, Q.; Shan, H.; Lv, L.; Yu, T.; Zhao, X.; Sun, J.; Yang, R.; Zhang, L.; et al. LncRNA 2810403D21Rik/Mirf promotes ischemic myocardial injury by regulating autophagy through targeting Mir26a. Autophagy 2020, 16, 1077–1091. [Google Scholar] [CrossRef]
- Higashi, K.; Yamada, Y.; Minatoguchi, S.; Baba, S.; Iwasa, M.; Kanamori, H.; Kawasaki, M.; Nishigaki, K.; Takemura, G.; Kumazaki, M.; et al. MicroRNA-145 repairs infarcted myocardium by accelerating cardiomyocyte autophagy. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1813–H1826. [Google Scholar] [CrossRef]
- Endo, K.; Naito, Y.; Ji, X.; Nakanishi, M.; Noguchi, T.; Goto, Y.; Nonogi, H.; Ma, X.; Weng, H.; Hirokawa, G.; et al. MicroRNA 210 as a biomarker for congestive heart failure. Biol. Pharm. Bull. 2013, 36, 48–54. [Google Scholar] [CrossRef]
- Watson, C.J.; Gupta, S.K.; O’Connell, E.; Thum, S.; Glezeva, N.; Fendrich, J.; Gallagher, J.; Ledwidge, M.; Grote-Levi, L.; McDonald, K.; et al. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur. J. Heart Fail. 2015, 17, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non-responders. Eur. J. Heart Fail. 2013, 15, 1277–1288. [Google Scholar] [CrossRef]
- Ovchinnikova, E.S.; Schmitter, D.; Vegter, E.L.; Ter Maaten, J.M.; Valente, M.A.; Liu, L.C.; van der Harst, P.; Pinto, Y.M.; de Boer, R.A.; Meyer, S.; et al. Signature of circulating microRNAs in patients with acute heart failure. Eur. J. Heart Fail. 2016, 18, 414–423. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Tomaniak, M.; Blaszczyk, O.; Koltowski, L.; Filipiak, K.J.; Sitkiewicz, D. Circulating microribonucleic acids miR-1, miR-21 and miR-208a in patients with symptomatic heart failure: Preliminary results. Arch. Cardiovasc. Dis. 2015, 108, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Gidlof, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Seronde, M.F.; Vausort, M.; Gayat, E.; Goretti, E.; Ng, L.L.; Squire, I.B.; Vodovar, N.; Sadoune, M.; Samuel, J.L.; Thum, T.; et al. Circulating microRNAs and Outcome in Patients with Acute Heart Failure. PLoS ONE 2015, 10, e0142237. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Lanfear, D.E.; de Ronde, M.W.J.; Lupon, J.; Leenders, J.J.; Liu, Z.; Zuithoff, N.P.A.; Eijkemans, M.J.C.; Zamora, E.; De Antonio, M.; et al. Prognostic value of circulating microRNAs on heart failure-related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur. J. Heart Fail. 2018, 20, 67–75. [Google Scholar] [CrossRef]
- Cakmak, H.A.; Coskunpinar, E.; Ikitimur, B.; Barman, H.A.; Karadag, B.; Tiryakioglu, N.O.; Kahraman, K.; Vural, V.A. The prognostic value of circulating microRNAs in heart failure: Preliminary results from a genome-wide expression study. J. Cardiovasc. Med. 2015, 16, 431–437. [Google Scholar] [CrossRef]
- Goren, Y.; Kushnir, M.; Zafrir, B.; Tabak, S.; Lewis, B.S.; Amir, O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 147–154. [Google Scholar] [CrossRef]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, I.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Du, J.; Cao, X.; Wang, Y.; Huang, Y.; Hu, S.; Zheng, Z. Plasma levels of microRNA-499 provide an early indication of perioperative myocardial infarction in coronary artery bypass graft patients. PLoS ONE 2014, 9, e104618. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Widera, C.; Gupta, S.K.; Lorenzen, J.M.; Bang, C.; Bauersachs, J.; Bethmann, K.; Kempf, T.; Wollert, K.C.; Thum, T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell. Cardiol. 2011, 51, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Grabmaier, U.; Clauss, S.; Gross, L.; Klier, I.; Franz, W.M.; Steinbeck, G.; Wakili, R.; Theiss, H.D.; Brenner, C. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction—The SITAGRAMI-miR analysis. Int. J. Cardiol. 2017, 244, 30–36. [Google Scholar] [CrossRef]
- Bauters, C.; Kumarswamy, R.; Holzmann, A.; Bretthauer, J.; Anker, S.D.; Pinet, F.; Thum, T. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int. J. Cardiol. 2013, 168, 1837–1840. [Google Scholar] [CrossRef]
- Wang, R.; Li, N.; Zhang, Y.; Ran, Y.; Pu, J. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern. Med. 2011, 50, 1789–1795. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, N.; Yang, J.; Liu, X.; Cao, X.; He, P.; Dong, X.; Qin, S.; Zhang, C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. 2010, 119, 87–95. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, M.; He, J.; Meng, W.; Ma, X.; Dong, S.; Meng, X.; Zhao, X.; Wang, X.; He, F. Circulating miR-208b and miR-34a are associated with left ventricular remodeling after acute myocardial infarction. Int. J. Mol. Sci. 2014, 15, 5774–5788. [Google Scholar] [CrossRef]
- Coskunpinar, E.; Cakmak, H.A.; Kalkan, A.K.; Tiryakioglu, N.O.; Erturk, M.; Ongen, Z. Circulating miR-221-3p as a novel marker for early prediction of acute myocardial infarction. Gene 2016, 591, 90–96. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gao, R.; Bei, Y.; Zhou, Q.; Zhou, Y.; Zhang, H.; Jin, M.; Wei, S.; Wang, K.; Xu, X.; et al. Circulating miR-30d Predicts Survival in Patients with Acute Heart Failure. Cell. Physiol. Biochem. 2017, 41, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Kafert-Kasting, S.; Thum, T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Mannhardt, I.; Eschenhagen, T. Engineering Cardiac Muscle Tissue: A Maturating Field of Research. Circ. Res. 2017, 120, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Breniere, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Prondzynski, M.; Lemoine, M.D.; Zech, A.T.; Horvath, A.; Di Mauro, V.; Koivumaki, J.T.; Kresin, N.; Busch, J.; Krause, T.; Kramer, E.; et al. Disease modeling of a mutation in alpha-actinin 2 guides clinical therapy in hypertrophic cardiomyopathy. EMBO Mol. Med. 2019, 11, e11115. [Google Scholar] [CrossRef]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asin, M.A.; Gonzalez-Alujas, M.T.; Perez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyongyosi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bar, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur. Heart J. 2021, 42, 192–201. [Google Scholar] [CrossRef]
| Disease | miRNA | Expression | References |
|---|---|---|---|
| Myocardial Infarction | miR-1 | Upregulation | [115,118] |
| miR-21 | Upregulation | [121] | |
| miR-29b | Upregulation | [115] | |
| miR-133 | Upregulation | [113,114] | |
| miR-208b | Upregulation | [119] | |
| miR-221-3p | Upregulation | [120] | |
| miR-328 | Upregulation | [117] | |
| miR-423 | Upregulation | [116] | |
| miR-499 | Upregulation | [112,113] | |
| Heart Failure | miR-18a | Upregulation | [122] |
| miR-21 | Upregulation | [123] | |
| miR-26b | Upregulation | [124] | |
| miR-30c | Upregulation | [125] | |
| miR-30d | Downregulation | [126] | |
| miR-126 | Downregulation | [127] | |
| miR-182 | Upregulation | [128] | |
| miR-210 | Upregulation | [129] | |
| miR-223 | Downregulation | [122] | |
| miR-423 | Downregulation | [130] | |
| miR-499 | Upregulation | [131] | |
| miR-652 | Downregulation | [122] | |
| miR-1254 | Upregulation | [132] | |
| miR-1306 | Downregulation | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kansakar, U.; Varzideh, F.; Mone, P.; Jankauskas, S.S.; Santulli, G. Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells 2022, 11, 983. https://doi.org/10.3390/cells11060983
Kansakar U, Varzideh F, Mone P, Jankauskas SS, Santulli G. Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells. 2022; 11(6):983. https://doi.org/10.3390/cells11060983
Chicago/Turabian StyleKansakar, Urna, Fahimeh Varzideh, Pasquale Mone, Stanislovas S. Jankauskas, and Gaetano Santulli. 2022. "Functional Role of microRNAs in Regulating Cardiomyocyte Death" Cells 11, no. 6: 983. https://doi.org/10.3390/cells11060983
APA StyleKansakar, U., Varzideh, F., Mone, P., Jankauskas, S. S., & Santulli, G. (2022). Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells, 11(6), 983. https://doi.org/10.3390/cells11060983