Human Umbilical Cord Mesenchymal Stem Cells: Current Literature and Role in Periodontal Regeneration
Abstract
:1. Introduction
2. Etiopathophysiology of Periodontal Tissue and Its Degeneration
2.1. The Dental Plaque and Calculus
2.2. Immunological Perspectives
T Cells: Friend or Foe?
2.3. Susceptibility of PDs
2.4. Impact of Genetics and Epigenetics
3. Regenerative Mechanisms of Periodontal Tissues
3.1. Cementum Regeneration
3.2. Periodontal Ligament
3.3. Alveolar Bone
4. Dental and Non-Dental Stem Cells for Regenerating Periodontal Apparatus
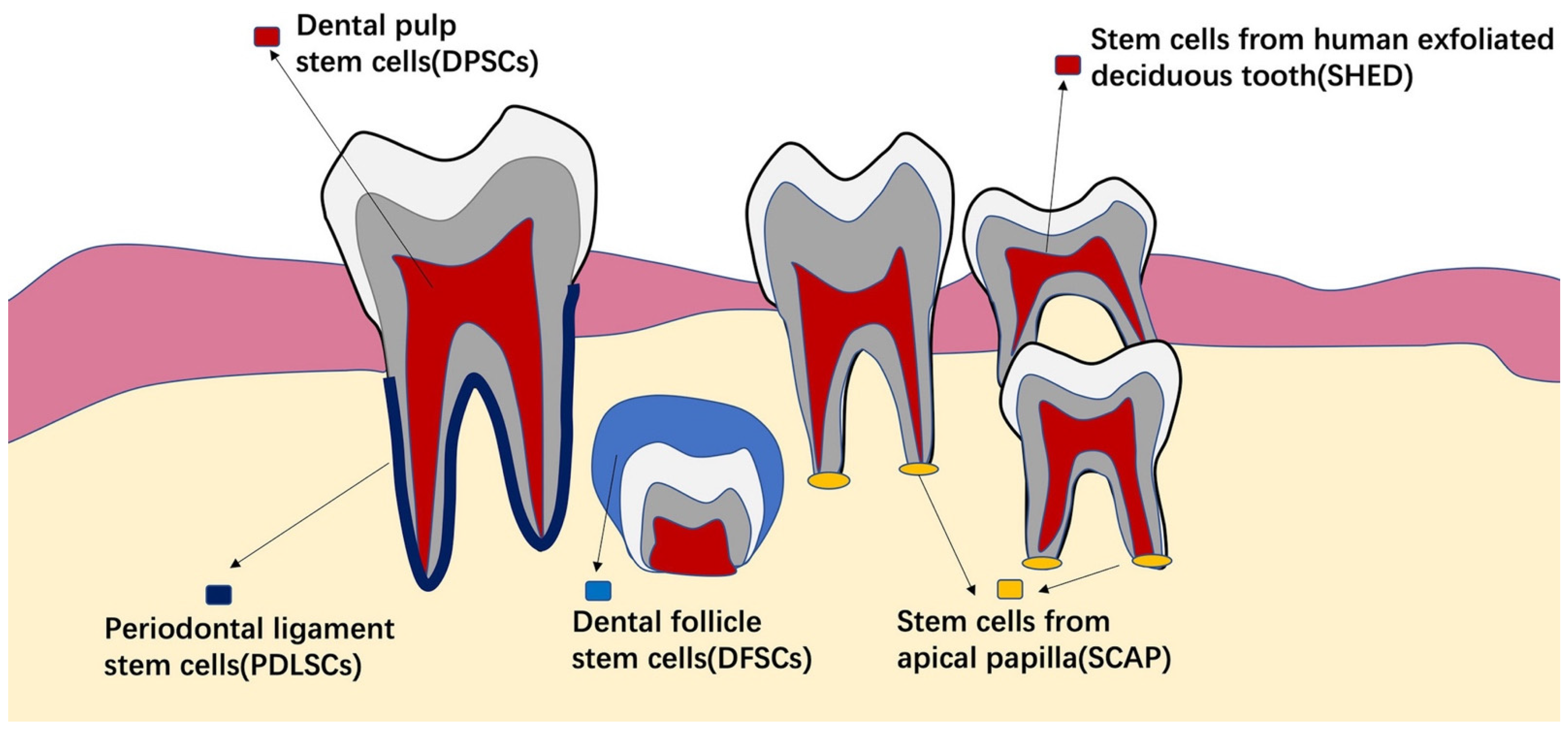
4.1. Stem Cells from Dental Tissues
4.2. Stem Cells from Non-Dental Tissues
5. Umbilical Cord Stem Cells
5.1. Differentiation, Proliferation, Pluripotency, and Senescence Characteristics of Human UC-MSCs Compared to Other Stem Cells
5.2. Umbilical Cord Stem Cell Applications
6. Role in Periodontal Regeneration and Potential Clinical Applications
7. Challenges and Issues Pertaining to Human UC-MSCs
8. Conclusion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet. 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Villar, C.C.; Cochran, D.L. Regeneration of periodontal tissues: Guided tissue regeneration. Dent. Clin. N. Am. 2010, 54, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Gottlow, J.; Karring, T.; Lindhe, J. The regenerative potential of the periodontal ligament: An experimental study in the monkey. J. Clin. Periodontol. 1982, 9, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Sun, H.-H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Husain, S.; Lone, M.A.; Lone, M.A.; Akhlaq, H.; Zafar, M.S. Clinical effectiveness of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide grafts for regeneration of periodontal defects: A systematic review and meta-analysis. Regen. Med. 2020, 15, 2379–2395. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A. Comparing Nanohydroxyapatite Graft and Other Bone Grafts in the Repair of Periodontal Infrabony Lesions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 12021. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; Gunsolley, J.C. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann. Periodontol. 2003, 8, 227–265. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A.; Javed, F. Nanocrystalline hydroxyapatite in regeneration of periodontal intrabony defects: A systematic review and meta-analysis. Ann. Anat. 2022, 240, 151877. [Google Scholar] [CrossRef]
- Takeda, K.; Shiba, H.; Mizuno, N.; Hasegawa, N.; Mouri, Y.; Hirachi, A.; Yoshino, H.; Kawaguchi, H.; Kurihara, H. Brain-derived neurotrophic factor enhances periodontal tissue regeneration. Tissue Eng. 2005, 11, 1618–1629. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, C.; Huang, S.; Wu, X.; Zhao, Y.; Pan, C.; Wang, H.; Liu, J.; Li, Q.; Kou, Y. Efficacy of adjunctive bioactive materials in the treatment of periodontal intrabony defects: A systematic review and meta-analysis. BioMed Res. Int. 2018, 2018, 8670832. [Google Scholar] [CrossRef]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: A systematic review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Piccirillo, A.; Perillo, F.; Cecoro, G.; Nastri, L.; Guida, L. Enamel matrix derivative and autogenous bone graft for periodontal regeneration of intrabony defects in humans: A systematic review and meta-analysis. Materials 2019, 12, 2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, M.S.; Pisani, F.; De Vito, D.; Lone, M.A.; Almasri, M. Long-term Clinical Performance of Regeneration versus Conservative Surgery in the Treatment of Infra-bony Defects: A Systematic Review. J. Int. Acad. Periodontol. 2021, 23, 31–56. [Google Scholar] [PubMed]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration–intrabony defects: A systematic review from the AAP regeneration workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; De Buitrago, J.G.; Reddy, M.S. Periodontal regeneration–furcation defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S108–S130. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.S.; Zafar, M.S.; Pisani, F.; Lone, M.A.; Malik, Y.R. Critical features of periodontal flaps with regard to blood clot stability: A review. J. Oral Biosci. 2021, 63, 111–119. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. 2000 2015, 68, 282–307. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: A novel approach to limit morbidity. J. Clin. Periodontol. 2007, 34, 87–93. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163. [Google Scholar] [CrossRef]
- Harrel, S.K. A minimally invasive surgical approach for periodontal regeneration: Surgical technique and observations. J. Periodontol. 1999, 70, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Franceschetti, G.; Calura, G. Single-flap approach with buccal access in periodontal reconstructive procedures. J. Periodontol. 2009, 80, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Buduneli, N.; Cortellini, P. Entire papilla preservation technique in the regenerative treatment of deep intrabony defects: 1-Year results. J. Clin. Periodontol. 2017, 44, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Buduneli, N.; Cortellini, P. Clinical outcomes of the entire papilla preservation technique with and without biomaterials in the treatment of isolated intrabony defects: A randomized controlled clinical trial. J. Clin. Periodontol. 2020, 47, 470–478. [Google Scholar] [CrossRef]
- Hammarström, L. The role of enamel matrix proteins in the development of cementum and periodontal tissues. Ciba Found. Symp. 1997, 205, 246–255, discussion 255–260. [Google Scholar]
- Trombelli, L.; Farina, R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J. Clin. Periodontol. 2008, 35, 117–135. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef]
- Yan, X.-Z.; Van Den Beucken, J.J.; Both, S.K.; Yang, P.-S.; Jansen, J.A.; Yang, F. Biomaterial strategies for stem cell maintenance during in vitro expansion. Tissue Eng. Part B Rev. 2014, 20, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef]
- Yu, N.; Oortgiesen, D.A.; Bronckers, A.L.; Yang, F.; Walboomers, X.F.; Jansen, J.A. Enhanced periodontal tissue regeneration by periodontal cell implantation. J. Clin. Periodontol. 2013, 40, 698–706. [Google Scholar] [CrossRef]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Morsczeck, C.; Moehl, C.; Götz, W.; Heredia, A.; Schäffer, T.; Eckstein, N.; Sippel, C.; Hoffmann, K. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol. Int. 2005, 29, 567–575. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Burguera, E.F.; Xu, H.H.; Amin, N.; Ryou, H.; Arola, D.D. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate–chitosan–biodegradable fiber scaffolds. Biomaterials 2010, 31, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Can, A.; Karahuseyinoglu, S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.G.B.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P.V. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Chaparro, P.; Gonçalves, C.; Figueiredo, L.; Faveri, M.; Lobão, E.; Tamashiro, N.; Duarte, P.; Feres, M. Newly identified pathogens associated with periodontitis: A systematic review. J. Dent. Res. 2014, 93, 846–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Chaparro, P.J.; Duarte, P.M.; Shibli, J.A.; Montenegro, S.; Lacerda Heluy, S.; Figueiredo, L.C.; Faveri, M.; Feres, M. The current weight of evidence of the microbiologic profile associated with peri-implantitis: A systematic review. J. Periodontol. 2016, 87, 1295–1304. [Google Scholar] [CrossRef]
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontol. 2000 2016, 72, 30–53. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.-K.; Poulsen, K.; Væth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Amaliya, A.; Laine, M.L.; Delanghe, J.R.; Loos, B.G.; Van Wijk, A.J.; Van der Velden, U. Java project on periodontal diseases: Periodontal bone loss in relation to environmental and systemic conditions. J Clin. Periodontol. 2015, 42, 325–332. [Google Scholar] [CrossRef]
- Mombelli, A.; Casagni, F.; Madianos, P.N. Can presence or absence of periodontal pathogens distinguish between subjects with chronic and aggressive periodontitis? A systematic review. J. Clin. Periodontol. 2002, 29, 10–21. [Google Scholar] [CrossRef]
- Pillet, S.; Pozzetto, B.; Roblin, X. Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J. Gastroenterol. 2016, 22, 2030. [Google Scholar] [CrossRef]
- Slots, J. Periodontal herpesviruses: Prevalence, pathogenicity, systemic risk. Periodontol. 2000 2015, 69, 28–45. [Google Scholar] [CrossRef]
- Yong-wei, F.; Yong-qing, G.; Hong-zhi, X. Valacyclovir as an adjunct to full-mouth scaling and root planing of advanced chronic periodontitis: A randomized clinical trail. Shanghai J. Stomatol. 2014, 23, 103–106. [Google Scholar]
- Sunde, P.T.; Olsen, I.; Enersen, M.; Grinde, B. Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J. Clin. Virol. 2008, 42, 176–178. [Google Scholar] [CrossRef]
- Mäntylä, P.; Stenman, M.; Kinane, D.F.; Tikanoja, S.; Luoto, H.; Salo, T.; Sorsa, T. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J. Periodontal Res. 2003, 38, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Demuth, D.R.; Gorr, S.U.; Hajishengallis, G.N.; Martin, M.H. Human variability in innate immunity. Periodontol. 2000 2007, 45, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinane, D.F.; Hajishengallis, G. Polymicrobial infections, biofilms, and beyond. J. Clin. Periodontol. 2009, 36, 404–405. [Google Scholar] [CrossRef]
- Benakanakere, M.; Kinane, D.F. Innate cellular responses to the periodontal biofilm. Front Oral Biol. 2012, 15, 41–55. [Google Scholar]
- Graves, D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gemmell, E.; Marshall, R.I.; Seymour, G.J. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol. 2000 1997, 14, 112–143. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Gemmell, E.; Seymour, G.J. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol. 2000 2004, 35, 21–41. [Google Scholar] [CrossRef]
- Aranha, A.M.F.; Repeke, C.E.; Garlet, T.P.; Vieira, A.E.; Campanelli, A.P.; Trombone, A.P.F.; Letra, A.; Silva, R.M.; Garlet, G.P. Evidence supporting a protective role for th9 and th22 cytokines in human and experimental periapical lesions. J. Endod. 2013, 39, 83–87. [Google Scholar] [CrossRef]
- Eskan, M.A.; Jotwani, R.; Abe, T.; Chmelar, J.; Lim, J.-H.; Liang, S.; Ciero, P.A.; Krauss, J.L.; Li, F.; Rauner, M. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012, 13, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Figueredo, C.; Lira-Junior, R.; Love, R. T and B cells in periodontal disease: New functions in a complex scenario. Int. J. Mol. Sci. 2019, 20, 3949. [Google Scholar] [CrossRef] [Green Version]
- Abbas, F.; Van der Velden, U.; Hart, A.; Moorer, W.; Vroom, T.M.; Scholte, G. Bleeding/plaque ratio and the development of gingival inflammation. J. Clin. Periodontol. 1986, 13, 774–782. [Google Scholar] [CrossRef]
- Winkel, E.; Abbas, F.; Van der Velden, U.; Vroom, T.M.; Scholte, G.; Hart, A. Experimental gingivitis in relation to age in individuals not susceptible to periodontal destruction. J. Clin. Periodontol. 1987, 14, 499–507. [Google Scholar] [CrossRef]
- Kinane, D.; Attström, R. Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J. Clin. Periodontol. 2005, 32, 130–131. [Google Scholar] [CrossRef]
- Joss, A.; Adler, R.; Lang, N.P. Bleeding on probing. A parameter for monitoring periodontal conditions in clinical practice. J. Clin. Periodontol. 1994, 21, 402–408. [Google Scholar] [CrossRef]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing an indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef]
- Löe, H.; Theilade, E.; Jensen, S.B. Experimental gingivitis in man. J. Periodontol. 1965, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Theilade, E.; Wright, W.; Jensen, S.B.; Löe, H. Experimental gingivitis in man: II. A longitudinal clinical and bacteriological investigation. J. Periodontal Res. 1966, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Scapoli, C.; Tatakis, D.N.; Grassi, L. Modulation of clinical expression of plaque-induced gingivitis: Effects of personality traits, social support and stress. J. Clin. Periodontol. 2005, 32, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Tatakis, D.N.; Scapoli, C.; Bottega, S.; Orlandini, E.; Tosi, M. Modulation of clinical expression of plaque-induced gingivitis: II. Identification of “high-responder” and “low-responder” subjects. J. Clin. Periodontol. 2004, 31, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Tatakis, D.N.; Trombelli, L. Modulation of clinical expression of plaque-induced gingivitis: I. Background review and rationale. J. Clin. Periodontol. 2004, 31, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Scapoli, C.; Mamolini, E.; Trombelli, L. Role of IL-6, TNF-A and LT-A variants in the modulation of the clinical expression of plaque-induced gingivitis. J. Clin. Periodontol. 2007, 34, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Minenna, L.; Carrieri, A.; Scapoli, C.; Tatakis, D.N. Experimental gingivitis: Reproducibility of plaque accumulation and gingival inflammation parameters in selected populations during a repeat trial. J. Clin. Periodontol. 2008, 35, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Scapoli, C.; Carrieri, A.; Giovannini, G.; Calura, G.; Farina, R. Interleukin-1β levels in gingival crevicular fluid and serum under naturally occurring and experimentally induced gingivitis. J. Clin. Periodontol. 2010, 37, 697–704. [Google Scholar] [CrossRef]
- Offenbaceer, S.; Odle, B.; Van Dyke, T. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J. Periodontal Res. 1986, 21, 101–112. [Google Scholar] [CrossRef]
- Stashenko, P. The role of immune cytokines in the pathogenesis of periapical lesions. Dent. Traumatol. 1990, 6, 89–96. [Google Scholar] [CrossRef]
- Wilton, J.; Griffiths, G.; Curtis, M.; Maiden, M.; Gillett, I.; Wilson, D.; Sterne, J.; Johnson, N. Detection of high-risk groups and individuals for periodontal diseases: Systemic predisposition and markers of general health. J. Clin. Periodontol. 1988, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Seymour, R.A.; Steele, J.G.; Robertson, P.; Butler, T.J.; Thomason, J.M. Prevalence of gingival overgrowth induced by calcium channel blockers: A community-based study. J. Periodontol. 1999, 70, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, S.-W.; Jiang, S.-Y. Relationship between gingival inflammation and pregnancy. Mediators Inflamm. 2015, 2015, 623427. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.; Lopez, M.; Rua-Dobles, A. Periodontal changes by HIV serostatus in a cohort of homosexual and bisexual men. J. Clin. Periodontol. 1992, 19, 794–801. [Google Scholar] [CrossRef]
- Kinane, D.F.; Lappin, D.F. Immune processes in periodontal disease: A review. Ann. Periodontol. 2002, 7, 62–71. [Google Scholar] [CrossRef]
- Van der Velden, U.; Abbas, F.; Armand, S.; Loos, B.; Timmerman, M.; Van der Weijden, G.; Van Winkelhoff, A.; Winkel, E. Java project on periodontal diseases. The natural development of periodontitis: Risk factors, risk predictors and risk determinants. J. Clin. Periodontol. 2006, 33, 540–548. [Google Scholar] [CrossRef]
- Shapira, L.; Wilensky, A.; Kinane, D.F. Effect of genetic variability on the inflammatory response to periodontal infection. J. Clin. Periodontol. 2005, 32, 72–86. [Google Scholar] [CrossRef]
- Kinane, D.F.; Shiba, H.; Hart, T.C. The genetic basis of periodontitis. Periodontol. 2000 2005, 39, 91–117. [Google Scholar] [CrossRef]
- Baylin, S.B. DNA methylation and gene silencing in cancer. Nat. Clin. Pract. Oncol. 2005, 2, S4–S11. [Google Scholar] [CrossRef]
- Benakanakere, M.; Abdolhosseini, M.; Hosur, K.; Finoti, L.; Kinane, D. TLR2 promoter hypermethylation creates innate immune dysbiosis. J. Dent. Res. 2015, 94, 183–191. [Google Scholar] [CrossRef]
- De Jong, T.; Bakker, A.; Everts, V.; Smit, T. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; McCulloch, C.A. Information generation and processing systems that regulate periodontal structure and function. Periodontol. 2000 2013, 63, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, K.-H.; Lee, Y.-M.; Seol, Y.-J. Advanced engineering strategies for periodontal complex regeneration. Materials 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.D.; Jang, A.T.; Kurylo, M.P.; Hurng, J.; Yang, F.; Yang, L.; Pal, A.; Chen, L.; Ho, S.P. Periodontal ligament entheses and their adaptive role in the context of dentoalveolar joint function. Dent. Mater. 2017, 33, 650–666. [Google Scholar] [CrossRef]
- Lee, J.H.; Pryce, B.A.; Schweitzer, R.; Ryder, M.I.; Ho, S.P. Differentiating zones at periodontal ligament–bone and periodontal ligament–cementum entheses. J. Periodontal Res. 2015, 50, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Lin, J.D.; Fong, J.I.; Ryder, M.I.; Ho, S.P. The adaptive nature of the bone-periodontal ligament-cementum complex in a ligature-induced periodontitis rat model. BioMed Res. Int. 2013, 2013, 876316. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal wound healing and tissue regeneration: A narrative review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef]
- Foster, B.L.; Nagatomo, K.J.; Nociti Jr, F.H.; Fong, H.; Dunn, D.; Tran, A.B.; Wang, W.; Narisawa, S.; Millán, J.L.; Somerman, M.J. Central role of pyrophosphate in acellular cementum formation. PLoS ONE 2012, 7, e38393. [Google Scholar] [CrossRef] [Green Version]
- Matalová, E.; Lungová, V.; Sharpe, P. Development of tooth and associated structures. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier: Amsterdam, The Netherlands, 2015; pp. 335–346. [Google Scholar]
- Foster, B.L.; Popowics, T.E.; Fong, H.K.; Somerman, M.J. Advances in defining regulators of cementum development and periodontal regeneration. Curr. Top. Dev. Biol. 2007, 78, 47–126. [Google Scholar] [PubMed]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol. 2000 2015, 67, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Hoz, L.; Romo, E.; Zeichner-David, M.; Sanz, M.; Nuñez, J.; Gaitán, L.; Mercado, G.; Arzate, H. Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol. Int. 2012, 36, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Schroeder, H.E. Cementogenesis reviewed: A comparison between human premolars and rodent molars. Anat. Rec. 1996, 245, 267–292. [Google Scholar] [CrossRef]
- Bar-Kana, I.; Savion, N.; Narayanan, A.; Pitaru, S. Cementum attachment protein manifestation is restricted to the mineralized tissue forming cells of the periodontium. Eur. J. Oral Sci. 1998, 106, 357–364. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, M.; Monsarrat, P.; Blasco-Baque, V.; Loubières, P.; Burcelin, R.; Casteilla, L.; Planat-Bénard, V.; Kémoun, P. Periodontal tissue regeneration using syngeneic adipose-derived stromal cells in a mouse model. Stem Cells Transl. Med. 2017, 6, 656–665. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Crossman, J.; Elyasi, M.; El-Bialy, T.; Mir, C.F. Cementum regeneration using stem cells in the dog model: A systematic review. Arch. Oral Biol. 2018, 91, 78–90. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhang, X.J.; Dang, N.N.; Ma, Z.F.; Xu, L.; Wu, J.J.; Sun, Y.J.; Duan, Y.Z.; Lin, Z.; Jin, Y. Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J. Periodontal Res. 2009, 44, 199–210. [Google Scholar] [CrossRef]
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, A.C.F.; Sarra, G.; de Oliveira, N.K.; Moreira, M.S.; Deboni, M.C.Z.; Marques, M.M. Cell sheets of human dental pulp stem cells for future application in bone replacement. Clin. Oral Investig. 2019, 23, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Yorukoglu, A.C.; Kiter, A.; Akkaya, S.; Satiroglu-Tufan, N.L.; Tufan, A.C. A concise review on the use of mesenchymal stem cells in cell sheet-based tissue engineering with special emphasis on bone tissue regeneration. Stem Cells Int. 2017, 2017, 2374161. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Washio, K.; Yoshida, T.; Ishikawa, I.; Ando, T.; Yamato, M.; Okano, T. Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Reg. Med. 2015, 9, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomedicine. 2015, 10, 7031. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhang, Q.; Zhang, Y.; Cen, L.; Wang, J. PDL regeneration via cell homing in delayed replantation of avulsed teeth. J. Transl. Med. 2015, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bartold, P.M.; Shi, S.; Gronthos, S. Stem cells and periodontal regeneration. Periodontol. 2000 2006, 40, 164–172. [Google Scholar] [CrossRef]
- Catón, J.; Bostanci, N.; Remboutsika, E.; De Bari, C.; Mitsiadis, T.A. Future dentistry: Cell therapy meets tooth and periodontal repair and regeneration. J. Cell. Mol. Med. 2011, 15, 1054–1065. [Google Scholar] [CrossRef] [Green Version]
- Mitsiadis, T.A.; Woloszyk, A.; Jiménez-Rojo, L. Nanodentistry: Combining nanostructured materials and stem cells for dental tissue regeneration. Nanomedicine 2012, 7, 1743–1753. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zong, W.; Xu, X.; Chen, J. Improved biphasic calcium phosphate combined with periodontal ligament stem cells may serve as a promising method for periodontal regeneration. Am. J. Transl. Res. 2018, 10, 4030. [Google Scholar] [PubMed]
- Xu, X.Y.; Li, X.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Concise review: Periodontal tissue regeneration using stem cells: Strategies and translational considerations. Stem Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of adipose-derived stem cells and their exo as adjunctive therapy to nonsurgical periodontal treatment: A histologic and histomorphometric study in rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J. Cell. Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontol. 2000 2009, 51, 208–219. [Google Scholar] [CrossRef]
- Siaili, M.; Chatzopoulou, D.; Gillam, D. An overview of periodontal regenerative procedures for the general dental practitioner. Saudi Dent. J. 2018, 30, 26–37. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Almutairi, A.S. Case Report: Managing the postoperative exposure of a non-resorbable membrane surgically. F1000Res 2018, 7, 685. [Google Scholar] [CrossRef] [Green Version]
- Pretzl, B.; Kim, T.S.; Holle, R.; Eickholz, P. Long-term results of guided tissue regeneration therapy with non-resorbable and bioabsorbable barriers. IV. A case series of infrabony defects after 10 years. J. Periodontol. 2008, 79, 1491–1499. [Google Scholar] [CrossRef]
- Corinaldesi, G.; Lizio, G.; Badiali, G.; Morselli-Labate, A.M.; Marchetti, C. Treatment of intrabony defects after impacted mandibular third molar removal with bioabsorbable and non-resorbable membranes. J. Periodontol. 2011, 82, 1404–1413. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng. Part A 2014, 20, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollati, D.; Morra, M.; Cassinelli, C.; Cascardo, G. Implantable Devices Having Antibacterial Properties and Multifunctional Surfaces. U.S. Patent 20130197660A1, 19 June 2018. [Google Scholar]
- Nyamsuren, E.; Bayarchimeg, B.; Urjinlkham, J.; Oyun-enkh, P.; Kh, O.; Batsuuri, M.; Lu, S.-l. Efficacy of natural biopolymer chitosan membrane for guided tissue regeneration. Innovation 2018, 12, 16–20. [Google Scholar]
- Cui, J.; Jiang, B.; Liang, J.; Sun, C.; Lan, J.; Sun, X.; Huang, H.; Sun, K.; Xu, X. Preparation and characterization of chitosan/β-GP membranes for guided bone regeneration. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 241–245. [Google Scholar] [CrossRef]
- Xue, Y.; Hong, X.; Gao, J.; Shen, R.; Ye, Z. Preparation and biological characterization of the mixture of poly (lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomedicine 2019, 14, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Lausch, A.J.; Chong, L.C.; Uludag, H.; Sone, E.D. Multiphasic collagen scaffolds for engineered tissue interfaces. Adv. Funct. Mater. 2018, 28, 1804730. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 055004. [Google Scholar] [CrossRef]
- Mahajan, A.; Kedige, S. Periodontal bone regeneration in intrabony defects using osteoconductive bone graft versus combination of osteoconductive and osteostimulative bone graft: A comparative study. Dent. Res. J. 2015, 12, 25. [Google Scholar] [CrossRef]
- Zhou, M.; Geng, Y.-m.; Li, S.-y.; Yang, X.-b.; Che, Y.-j.; Pathak, J.L.; Wu, G. Nanocrystalline hydroxyapatite-based scaffold adsorbs and gives sustained release of osteoinductive growth factor and facilitates bone regeneration in mice ectopic model. J. Nanomater. 2019, 2019, 1202159. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Xu, Y.; Zhang, T.; Ma, Y.; Liu, J.; Yuan, B.; Chen, X.; Zhou, P.; Zhao, X.; Pang, F. Mesenchymal stem cell sheets: A new cell-based strategy for bone repair and regeneration. Biotechnol. Lett. 2019, 41, 305–318. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.; Bartold, P. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L. Regeneration of periodontal tissue: Bone replacement grafts. Dent. Clin. N. Am. 2010, 54, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Keith Jr, J.D.; Petrungaro, P.; Leonetti, J.A.; Elwell Jr, C.W.; Zeren, K.J.; Caputo, C.; Nikitakis, N.G.; Schöpf, C.; Warner, M.M. Clinical and histologic evaluation of a mineralized block allograft: Results from the developmental period (2001-2004). Int. J. Periodontics Restorative Dent. 2006, 26, 321–327. [Google Scholar]
- Piattelli, A.; Scarano, A.; Corigliano, M.; Piattelli, M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: A histological and histochemical study in man. Biomaterials 1996, 17, 1127–1131. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Wang, F.; Wang, Z. Maxillary sinus floor augmentation and dental implant placement using dentin matrix protein-1 gene-modified bone marrow stromal cells mixed with deproteinized boving bone: A comparative study in beagles. Arch. Oral Biol. 2016, 64, 102–108. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.H.; Takagi, S.; Chow, L.C. In-situ hardening hydroxyapatite-based scaffold for bone repair. J. Mater. Sci. Mater. Med. 2006, 17, 437–445. [Google Scholar] [CrossRef]
- Belal, M.H.; Al-Noamany, F.A.; El-Tonsy, M.M.; El-Guindy, H.M.; Ishikawa, I. Treatment of human class II furcation defects using connective tissue grafts, bioabsorbable membrane, and resorbable hydroxylapatite: A comparative study. J. Int. Acad. Periodontol. 2005, 7, 114–128. [Google Scholar]
- Zhang, Y.; Xu, H.H. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J. Biomed. Mater. Res. A 2005, 75, 832–840. [Google Scholar] [CrossRef]
- Hayashi, C.; Kinoshita, A.; Oda, S.; Mizutani, K.; Shirakata, Y.; Ishikawa, I. Injectable calcium phosphate bone cement provides favorable space and a scaffold for periodontal regeneration in dogs. J. Periodontol. 2006, 77, 940–946. [Google Scholar] [CrossRef]
- Chu, K.; Oshida, Y.; Hancock, E.; Kowolik, M.J.; Barco, T.; Zunt, S. Hydroxyapatite/PMMA composites as bone cements. Biomed. Mater. Eng. 2004, 14, 87–105. [Google Scholar] [PubMed]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Tissue Engineering: Toward Strong and Tough Glass and Ceramic Scaffolds for Bone Repair (Adv. Funct. Mater. 44/2013). Adv. Funct. Mater. 2013, 23, 5460. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Li, Y.-C.; Young, T.-H.; Chen, M.-H. Novel microinjector for carrying bone substitutes for bone regeneration in periodontal diseases. J. Formos. Med. Assoc. 2016, 115, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon Jr, C.G.; Khatri, C.A.; Wight, S.A.; Wang, F.W. Preliminary report on the biocompatibility of a moldable, resorbable, composite bone graft consisting of calcium phosphate cement and poly (lactide-co-glycolide) microspheres. J. Orthop. Res. 2002, 20, 473–482. [Google Scholar] [CrossRef]
- Brown, W.E.; Chow, L.C. Combinations of Sparingly Soluble Calcium Phosphates in Slurries and Pastes as Mineralizers and Cements. U.S. Patent 4612053A, 16 September 1986. [Google Scholar]
- Takagi, S.; Chow, L.C.; Hirayama, S.; Eichmiller, F.C. Properties of elastomeric calcium phosphate cement–chitosan composites. Dent. Mater. 2003, 19, 797–804. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Villalona, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef]
- Wang, P.; Song, Y.; Weir, M.D.; Sun, J.; Zhao, L.; Simon, C.G.; Xu, H.H. A self-setting iPSMSC-alginate-calcium phosphate paste for bone tissue engineering. Dent. Mater. 2016, 32, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Grosfeld, E.-C.; Hoekstra, J.W.M.; Herber, R.-P.; Ulrich, D.J.; Jansen, J.A.; van den Beucken, J.J. Long-term biological performance of injectable and degradable calcium phosphate cement. Biomed. Mater. 2016, 12, 015009. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Wang, P.; Wang, L.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ren, K.; Zhao, L.; Xu, H.H. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiadis, T.; Feki, A.; Papaccio, G.; Catón, J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 2011, 23, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W.; Man, Y.; Xu, H.H. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015, 18, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, C.; Li, C.; Weir, M.D.; Wang, P.; Reynolds, M.A.; Zhao, L.; Xu, H.H. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweikle, M.; Zinn, T.; Lund, R.; Tiainen, H. Injectable synthetic hydrogel for bone regeneration: Physicochemical characterisation of a high and a low pH gelling system. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 67–76. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, K.; Liu, X.; Reynolds, M.A.; Bao, C.; Wang, P.; Zhao, L.; Xu, H.H. Novel hiPSC-based tri-culture for pre-vascularization of calcium phosphate scaffold to enhance bone and vessel formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 296–304. [Google Scholar] [CrossRef]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal tissues, maxillary jaw bone, and tooth regeneration approaches: From animal models analyses to clinical applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of nanomaterials in dentistry: Interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano 2015, 9, 2255–2289. [Google Scholar] [CrossRef] [Green Version]
- Polini, A.; Bai, H.; Tomsia, A.P. Dental applications of nanostructured bioactive glass and its composites. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhou, T.; Lin, S.; Shi, S.; Lin, Y. Nanomaterials for craniofacial and dental tissue engineering. J. Dent. Res. 2017, 96, 725–732. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ma, S.; Gao, Y.; Zuo, Y.; Hu, J. Enhancement of bone formation by BMP-7 transduced MSCs on biomimetic nano-hydroxyapatite/polyamide composite scaffolds in repair of mandibular defects. J. Biomed. Mater. Res. Part A 2010, 95, 973–981. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhang, F.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ma, J.; Gu, N.; Xu, H.H. Gold nanoparticles in injectable calcium phosphate cement enhance osteogenic differentiation of human dental pulp stem cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 35–45. [Google Scholar] [CrossRef]
- Yang, J.W.; Shin, Y.Y.; Seo, Y.; Kim, H.-S. Therapeutic functions of stem cells from oral cavity: An update. Int. J. Mol. Sci. 2020, 21, 4389. [Google Scholar] [CrossRef]
- Sybil, D.; Jain, V.; Mohanty, S.; Husain, S.A. Oral stem cells in intraoral bone formation. J. Oral Biosci. 2020, 62, 36–43. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of oral-derived stem cells in dental and maxillofacial applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The emerging role of stem cells in regenerative dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Tian, W.; Pan, J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019, 52, e12572. [Google Scholar] [CrossRef] [Green Version]
- Tziafas, D.; Kodonas, K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J. Endod. 2010, 36, 781–789. [Google Scholar] [CrossRef]
- Graziano, A.; d’Aquino, R.; Angelis, M.G.C.D.; De Francesco, F.; Giordano, A.; Laino, G.; Piattelli, A.; Traini, T.; De Rosa, A.; Papaccio, G. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J. Cell. Physiol. 2008, 214, 166–172. [Google Scholar] [CrossRef]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Papaccio, G.; Graziano, A.; d’Aquino, R.; Graziano, M.F.; Pirozzi, G.; Menditti, D.; De Rosa, A.; Carinci, F.; Laino, G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: A cell source for tissue repair. J. Cell. Physiol. 2006, 208, 319–325. [Google Scholar] [CrossRef]
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal ligament stem cells: Current knowledge and future perspectives. Stem Cells Dev. 2019, 28, 995–1003. [Google Scholar] [CrossRef]
- Liu, L.; Michowski, W.; Kolodziejczyk, A.; Sicinski, P. The cell cycle in stem cell proliferation, pluripotency and differentiation. Nat. Cell Biol. 2019, 21, 1060–1067. [Google Scholar] [CrossRef]
- Su, W.-T.; Chiou, W.-L.; Yu, H.-H.; Huang, T.-Y. Differentiation potential of SHEDs using biomimetic periosteum containing dexamethasone. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1036–1045. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory role of stem cells from human exfoliated deciduous teeth on periodontal regeneration. Tissue Eng. Part A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Chrepa, V.; Pitcher, B.; Henry, M.A.; Diogenes, A. Survival of the apical papilla and its resident stem cells in a case of advanced pulpal necrosis and apical periodontitis. J. Endod. 2017, 43, 561–567. [Google Scholar] [CrossRef]
- Nada, O.A.; El Backly, R.M. Stem cells from the apical papilla (SCAP) as a tool for endogenous tissue regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Nadiri, A.; Kuchler-Bopp, S.; Perrin-Schmitt, F.; Peters, H.; Lesot, H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006, 12, 2069–2075. [Google Scholar] [CrossRef]
- Hu, B.; Unda, F.; Bopp-Kuchler, S.; Jimenez, L.; Wang, X.; Haikel, Y.; Wang, S.; Lesot, H. Bone marrow cells can give rise to ameloblast-like cells. J. Dent. Res. 2006, 85, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Kawaguchi, H.; Hirachi, A.; Takeda, K.; Mizuno, N.; Nishimura, M.; Koike, C.; Tsuji, K.; Iba, H.; Kato, Y. Behavior of transplanted bone marrow–derived mesenchymal stem cells in periodontal defects. J. Periodontol. 2006, 77, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rossi, F.M.; Putnins, E.E. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials 2010, 31, 8574–8582. [Google Scholar] [CrossRef]
- Ohazama, A.; Modino, S.; Miletich, I.; Sharpe, P. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Inanç, B.; Elçin, A.E.; Elçin, Y.M. In vitro differentiation and attachment of human embryonic stem cells on periodontal tooth root surfaces. Tissue Eng. Part A 2009, 15, 3427–3435. [Google Scholar] [CrossRef]
- Ning, F.; Guo, Y.; Tang, J.; Zhou, J.; Zhang, H.; Lu, W.; Gao, Y.; Wang, L.; Pei, D.; Duan, Y. Differentiation of mouse embryonic stem cells into dental epithelial-like cells induced by ameloblasts serum-free conditioned medium. Biochem. Biophys. Res. Commun. 2010, 394, 342–347. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Wang, B.; Lin, N.H.; Laslett, A.L.; Gronthos, S.; Bartold, P.M. Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J. Periodontal Res. 2011, 46, 438–447. [Google Scholar] [CrossRef]
- Yan, X.; Qin, H.; Qu, C.; Tuan, R.S.; Shi, S.; Huang, G.T.-J. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010, 19, 469–480. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Alatyyat, S.M.; Alasmari, H.M.; Aleid, O.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Umbilical cord stem cells: Background, processing and applications. Tissue Cell 2020, 65, 101351. [Google Scholar] [CrossRef]
- Szepesi, Á.; Matula, Z.; Szigeti, A.; Várady, G.; Szalma, J.; Szabó, G.; Uher, F.; Sarkadi, B.; Német, K. In vitro characterization of human mesenchymal stem cells isolated from different tissues with a potential to promote complex bone regeneration. Stem Cells Int. 2016, 2016, 3595941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliane, G.; Agnes, D.; Helene, E.; Dominique, T.; Gerard, S.; Pierre, L.; Scott, C.; Denis, E.; Joanne, K.; Judith, B. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling (1989). Cell. Ther. Transplant. 2011, 2, 1–6. [Google Scholar]
- Gluckman, E. Hematopoietic stem-cell transplants using umbilical-cord blood. Mass Med. Soc. 2001, 344, 1860–1861. [Google Scholar] [CrossRef]
- Revencu, T.; Trifan, V.; Nacu, L.; Gutium, T.; Globa, L.; Motoc, A.; Nacu, V. Collection, isolation and characterization of the stem cells of umbilical cord blood. Rom. J. Morphol. Embryol. 2013, 54, 291–297. [Google Scholar]
- Matsumoto, T.; Mugishima, H. Non-hematopoietic stem cells in umbilical cord blood. Int. J Stem Cells 2009, 2, 83. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, T.C.; Ferrari, H.F.; Garcia, A.F.; Novais, J.B.; Silva-Frade, C.; Ferrarezi, M.C.; Andrade, A.L.; Gameiro, R. Isolation and characterization of Wharton’s jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Reza, H.M. Short review on human umbilical cord lining epithelial cells and their potential clinical applications. Stem Cell Res. Ther. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Jo, C.H.; Kim, H.-R.; Hwang, Y.-i. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef]
- Subbarayan, R.; Murugan Girija, D.; Mukherjee, J.; Mamidanna, S.R.R.; Ranga Rao, S. Comparision of gingival and umbilical cord stem cells based on its modulus and neuronal differentiation. J. Cell. Biochem. 2017, 118, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, G.; Xing, Z.; Zhan, J.; Ma, J. Comparative evaluation of the osteogenic capacity of human mesenchymal stem cells from bone marrow and umbilical cord tissue. Exp. Ther. Med. 2019, 17, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Perez, R.; Monguio-Tortajada, M.; Gamez-Valero, A.; Rojas-Marquez, R.; Borras, F.E.; Roura, S.; Vives, J. Osteogenic commitment of Wharton’s jelly mesenchymal stromal cells: Mechanisms and implications for bioprocess development and clinical application. Stem Cell Res. Ther. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Das, A.; Barui, A.; Paul, R.R. Comparative evaluation of proliferative potential and replicative senescence associated changes in mesenchymal stem cells derived from dental pulp and umbilical cord. Cell Tissue Bank. 2021, 23, 157–170. [Google Scholar] [CrossRef]
- Tanhehco, Y.C.; Bhatia, M. Hematopoietic stem cell transplantation and cellular therapy in sickle cell disease: Where are we now? Curr. Opin. Hematol. 2019, 26, 448–452. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Z.; Wu, Y.; Yang, X.; Cao, Y.; Li, X.; Yan, B.; Li, S.; Da, W.; Wu, X. Clinical evaluation of haploidentical hematopoietic combined with human umbilical cord-derived mesenchymal stem cells in severe aplastic anemia. Eur. J. Med. Res. 2018, 23, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Y.; Sun, X.; Chen, J.; Qin, M.-Q.; Luan, Z.; Zhu, Y.-P.; Fang, J.-P. Hematopoietic stem cell transplantation for children with β-thalassemia major: Multicenter experience in China. World J. Pediatr. 2018, 14, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Dolstra, H.; Roeven, M.W.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Han, H.; Chae, G.T.; Lee, S.H.; Bo, S.; Yoon, J.H.; Lee, Y.S.; Lee, K.S.; Park, H.K.; Kang, K.S. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells 2006, 24, 1620–1626. [Google Scholar] [CrossRef]
- Ichim, T.E.; Solano, F.; Brenes, R.; Glenn, E.; Chang, J.; Chan, K.; Riordan, N.H. Placental mesenchymal and cord blood stem cell therapy for dilated cardiomyopathy. Reprod. Biomed. Online 2008, 16, 898–905. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Bennett, E.R.; Durham, R.J.; Volpi, J.J.; Wiese, J.R.; Frankel, M.; Shpall, E.; Wilson, J.M.; Troy, J.; Kurtzberg, J. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: Clinical outcomes from a phase I safety study. Stem Cells Transl. Med. 2018, 7, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Lee, S.; Shin, N.; Ko, Y.; Kim, D.; Lee, J.; Lee, W. Bone regeneration with umbilical cord blood mesenchymal stem cells in femoral defects of ovariectomized rats. Osteoporos. Sarcopenia 2018, 4, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Kong, J.; Dong, M.; Duan, H.; Chen, S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.M.; Abdelrahman, S.A.; Hussein, S.; Shalaby, S.M.; Mosaad, H.; Awad, A.M. Effect of human umbilical cord blood mesenchymal stem cells administered by intravenous or intravitreal routes on cryo-induced retinal injury. IUBMB life 2017, 69, 188–201. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Li, X.; Yu, H.; Zhou, Z. Therapeutic potential of umbilical cord blood cells for type 1 diabetes mellitus. J. Diabetes 2015, 7, 762–773. [Google Scholar] [CrossRef]
- Boroujeni, Z.N.; Aleyasin, A. Human umbilical cord-derived mesenchymal stem cells can secrete insulin in vitro and in vivo. Biotechnol. Appl. Biochem. 2014, 61, 82–92. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Han, Q.; Song, X.; Liu, J.; Dong, L.; Han, W.; Mu, Y. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res. Ther. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Boroujeni, M.E.; Gardaneh, M. Umbilical cord: An unlimited source of cells differentiable towards dopaminergic neurons. Neural Regen. Res. 2017, 12, 1186–1192. [Google Scholar] [CrossRef]
- Ebrahimi, M.J.; Aliaghaei, A.; Boroujeni, M.E.; Khodagholi, F.; Meftahi, G.; Abdollahifar, M.A.; Ahmadi, H.; Danyali, S.; Daftari, M.; Sadeghi, Y. Human Umbilical Cord Matrix Stem Cells Reverse Oxidative Stress-Induced Cell Death and Ameliorate Motor Function and Striatal Atrophy in Rat Model of Huntington Disease. Neurotox. Res. 2018, 34, 273–284. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, S.; Zhang, C.; Cao, W.; Liu, M.; Li, D.; Lv, P.; Xing, Q.; Qu, R.; Yao, N.; et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav. Brain Res. 2017, 320, 291–301. [Google Scholar] [CrossRef]
- Galieva, L.R.; Mukhamedshina, Y.O.; Arkhipova, S.S.; Rizvanov, A.A. Human Umbilical Cord Blood Cell Transplantation in Neuroregenerative Strategies. Front. Pharmacol. 2017, 8, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chez, M.; Lepage, C.; Parise, C.; Dang-Chu, A.; Hankins, A.; Carroll, M. Safety and Observations from a Placebo-Controlled, Crossover Study to Assess Use of Autologous Umbilical Cord Blood Stem Cells to Improve Symptoms in Children with Autism. Stem Cells Transl. Med. 2018, 7, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar] [PubMed]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef]
- Yu, S.; Long, J.; Yu, J.; Du, J.; Ma, P.; Ma, Y.; Yang, D.; Fan, Z. Analysis of differentiation potentials and gene expression profiles of mesenchymal stem cells derived from periodontal ligament and Wharton’s jelly of the umbilical cord. Cells Tissues Organs 2013, 197, 209–223. [Google Scholar] [CrossRef] [PubMed]
- George, J.P.; Chakravarty, P.; Chowdhary, K.Y.; Purushothama, H.; Rao, J.A. Attachment and differentiation of human umbilical cord stem cells on to the tooth root surface with and without the use of fibroblast growth factor-an in vitro study. Int. J. Stem Cells 2015, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Hou, R.; Wang, Y.; Lu, B.; Zhang, J.; Feng, X.; Liu, Y.; Cao, Q. Fundamental study of application of umbilical cord mesenchymal stem cells to the periodontium to aid healing after autotransplantation of teeth. Br. J. Oral Maxillofac. Surg. 2014, 52, 501–506. [Google Scholar] [CrossRef]
- Kadam, S.; Gautam, S.; Dwivedi, A.; Jain, V. Treatment of gingival recession defect using human umbilical cord mesenchymal stem cells cultured on PCL based bone regenerating scaffold: A randomized controlled clinical study. Cytotherapy 2019, 21, S51. [Google Scholar] [CrossRef]
- Shang, F.; Liu, S.; Ming, L.; Tian, R.; Jin, F.; Ding, Y.; Zhang, Y.; Zhang, H.; Deng, Z.; Jin, Y. Human umbilical cord MSCs as new cell sources for promoting periodontal regeneration in inflammatory periodontal defect. Theranostics 2017, 7, 4370. [Google Scholar] [CrossRef]
- Sun, X.-C.; Wang, H.; Li, J.-h.; Zhang, D.; Yin, L.-Q.; Yan, Y.-F.; Ma, X.; Xia, H.-F. Repair of alveolar cleft bone defects by bone collagen particles combined with human umbilical cord mesenchymal stem cells in rabbit. Biomed. Eng. Online 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Chen, L.; Ye, L.; Cui, J.; Sun, Q.; Li, K.; Li, Z.; Liu, L. Human umbilical cord mesenchymal stem cells: A new therapeutic option for tooth regeneration. Stem Cells Int. 2015, 2015, 549432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacMillan, M.L.; Blazar, B.R.; DeFor, T.E.; Wagner, J.E. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: Results of a phase I–II clinical trial. Bone Marrow Transplant. 2009, 43, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, A.L.; Miller, D.; Berglund, A.; Schnabel, L.V.; Levine, G.J.; Antczak, D.F.; Watts, A.E. Cross-matching of allogeneic mesenchymal stromal cells eliminates recipient immune targeting. Stem Cells Transl. Med. 2020, 10, 694–710. [Google Scholar] [CrossRef]
- Yang, S.; Huang, S.; Feng, C.; Fu, X. Umbilical cord-derived mesenchymal stem cells: Strategies, challenges, and potential for cutaneous regeneration. Front. Med. 2012, 6, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.-Y. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. Rep. 2013, 9, 226–240. [Google Scholar] [CrossRef]



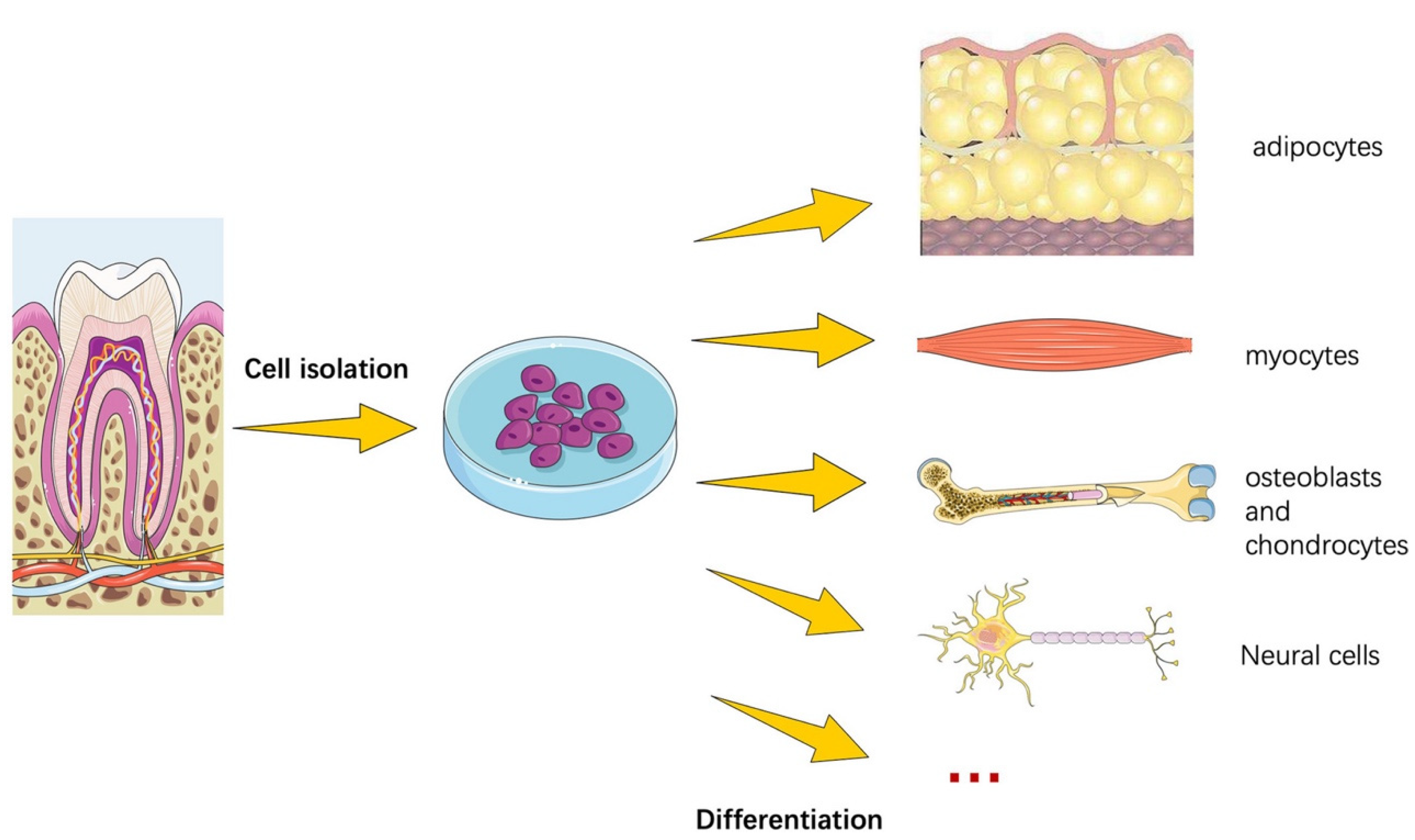

| Hematopoietic stem cells |
| Epithelial stem cells |
| Mesenchymal stem cells |
| Endothelial progenitors |
| Induced pluripotent stem cells |
| Clinical Application | Main Diseases | Type of hUCSCs Applied |
|---|---|---|
| Treatment of hematological diseases | Sickle cell anemia [218] | HSCs |
| Aplastic anemia [219] | HSCs | |
| Thalassemia [220] | HSCs | |
| Leukemia [221] | HSCs | |
| Treatment of cardiovascular diseases | Buerger’s disease [222] | MSCs |
| Dilated cardiomyopathy [223] | MSCs | |
| Stroke [224] | MSCs | |
| Bone regeneration | Osteoporosis [225] | MSCs |
| Congenital abnormalities, trauma, tumor resections, fractures as well as disorders such as arthritis | MSCs | |
| Treatment of eyesight diseases | Diabetic retinopathy-associated neurodegeneration [226] | MSCs |
| Traumatic optic neuropathy [227] | MSCs | |
| Treatment of metabolic disorders | Type 1 diabetes [228,229] | MSCs |
| Type 2 diabetes [230] | MSCs | |
| Treatment of neurodegenerative and neurodevelopmental disorders | Parkinson’s disease [231] | MSCs |
| Huntington’s disease [232] | MSCs | |
| Alzheimer’s disease [233] | MSCs | |
| Amyotrophic lateral sclerosis [234] | MSCs | |
| Autism [235] | MSCs | |
| Wound healing | Burn injuries [236] | MSCs |
| Chronic ulcers in diabetes [237] | MSCs | |
| HSCs (hematopoietic stem cells); MSCs (mesenchymal stem cells) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, M.S.; Shahzad, Z.; Tash, E.A.; Janjua, O.S.; Khan, M.I.; Zafar, M.S. Human Umbilical Cord Mesenchymal Stem Cells: Current Literature and Role in Periodontal Regeneration. Cells 2022, 11, 1168. https://doi.org/10.3390/cells11071168
Shaikh MS, Shahzad Z, Tash EA, Janjua OS, Khan MI, Zafar MS. Human Umbilical Cord Mesenchymal Stem Cells: Current Literature and Role in Periodontal Regeneration. Cells. 2022; 11(7):1168. https://doi.org/10.3390/cells11071168
Chicago/Turabian StyleShaikh, Muhammad Saad, Zara Shahzad, Esraa Abdulgader Tash, Omer Sefvan Janjua, Muhammad Ikram Khan, and Muhammad Sohail Zafar. 2022. "Human Umbilical Cord Mesenchymal Stem Cells: Current Literature and Role in Periodontal Regeneration" Cells 11, no. 7: 1168. https://doi.org/10.3390/cells11071168
APA StyleShaikh, M. S., Shahzad, Z., Tash, E. A., Janjua, O. S., Khan, M. I., & Zafar, M. S. (2022). Human Umbilical Cord Mesenchymal Stem Cells: Current Literature and Role in Periodontal Regeneration. Cells, 11(7), 1168. https://doi.org/10.3390/cells11071168








