1. Introduction
In the natural environment, constant bacterial growth is seldom found, in contrast to laboratory conditions, when bacteria are cultured in rich media at an optimal temperature. In a nutrition-rich medium, bacteria can sustain a continuous, relatively fast, balanced growth for a given time [
1,
2]. According to the literature, the characteristic bacterial growth curve pattern comprises four phases: lag, exponential growth, stationary phase, and death phase. During the lag phase, bacteria in nutrient-rich medium need to adapt to the new environment in order to start cellular metabolism; during this, there is a little or no cell reproduction that takes place. The lag duration has often been considered erratic and unpredictable [
3]. At the end of the lag phase, the cell concentration starts increasing, and the population enters the exponential growth phase [
4]. Since bacterial cells divide by binary fission, the growth rate of cultures is logarithmic and characterized by the number of doublings, that is, the generation time [
5]. During the exponential (or logarithmic) phase, bacterial reproduction occurs at a maximal rate characteristic for the given growth condition. It is believed that the growth rate depends directly on the culture medium that is slower in nutrient-poor and faster in nutrient-rich conditions [
2]. If cells in the exponential growth phase are inoculated into fresh medium, the lag phase is bypassed, and the logarithmic phase continues. Unicellular populations grown in batch culture, after a while, reach a point when the growth rate decreases, and the increase in cell number ceases; this is defined as the stationary phase. The stationary phase is characterized, usually, by a dynamic equilibrium between the numbers of dividing and dying cells, resulting in a constant cell number represented by a plateau in the growth curve [
6]. The state-of-the-art is consistent, in that, when nutrients become limited or other environmental conditions restrict growth, the bacterial cultures enter the stationary phase [
1,
2]. However, this description does not distinguish between the metabolically active or inactive cells because it just simply refers to a culture that shows no increment in the cell number [
6]. When the environment cannot maintain the population, cultures in the stationary phase accumulate toxic products of catabolism that lead to a decrease in the number of viable cells defined as the death phase. Nowadays, a fifth phase is distinguished, termed as the long-term stationary phase, in which the minority of the population could hold their viability for several weeks and even months [
7]. For example,
Escherichia coli (
E. coli) could be maintained in batch culture for long periods of time (more than five years without the addition of nutrients) [
7].
According to the state-of-the-art, the transition from the logarithmic phase to the stationary phase is driven by the σ
38 (σ
S)-factor, encoded by the
rpoS gene in
E. coli cells [
8], whose transcription is repressed by the phosphorylated form of the ArcA regulator in the exponential phase [
9]. The transition to stationary phase is not limited only to the activation of σ
38, but other alterations of metabolism occur, e.g., hibernation of ribosomes regulated by RelA via the synthesis of the molecule ppGpp (alarmone) as a response to growing starvation and adaptation to long-term survival [
10]. The increasing nutrient deprivation in the stationary phase induces decrement or loss of the colony forming units (CFU) in standard plating assays that can be considered as a transition to the death phase [
7]. The loss of the proliferating capability raises the question of the bacterial life span and aging. Ref. [
11] reported that bacteria can fail to divide on laboratory media, but they still appear viable. This phenomenon is termed viable, but not culturable (VBNC) [
11,
12,
13,
14]. The non-proliferative cells seem to be in a growth-arrested, G
0-like state that is incompatible with colony formation on nutrient agar plates [
1].
The theory that cell growth can be controlled by direct cell–cell contact mediated interaction was raised in unicellular algae [
15], and it was described in
E. coli by the CdiA-CdiB system, defined as contact-dependent growth inhibition (CDI). The growth inhibition was dependent on the growth state of the inhibitory cells, occurring in the logarithmic phase, but not in the stationary phase. Therefore, this system cannot be implicated in the development of stationary cell concentration [
16,
17]. CDI systems are suggested mostly to play roles in bacterial competition to hinder the growth of neighboring microbes [
18].
The experiments were carried out on cyanobacteria, a well characterized strain of
Synechococcus elongatus PCC7942 (
S. elongatus), which is an autotrophic, photosynthetic, Gram-negative prokaryotic microorganism carrying three to eight chromosomal copies per cell, in contrast to more traditionally studied bacteria, such as
E. coli or
Bacillus subtilis, which typically contain only one or two complete chromosomal copies [
19]. Cyanobacteria are oxygenic, photosynthetic Gram-negative bacteria, converting solar energy into chemical energy. They are the most abundant microorganisms in aquatic environments and play a key role in the global carbon cycle. It is estimated that these photosynthetic microbes are responsible for at least 50% of carbon fixation in the oceans [
20]. They are usually considered as ancestors of chloroplasts in higher plants.
We acknowledge that the stationary phase could be achieved by starvation, toxins, or abiotic stress. However, we investigated the development of so-called native stationary phases in optimal conditions, which are not due to the listed ones. In normal laboratorial conditions, the depletion of nutrients occurs rarely, and the spent medium could provide enough nutrients to enable the growth of cultures and reach the normal stationary plateau more times. Here, we challenge a generally accepted view of bacterial growth in batch culture in the case of S. elongatus. We examined the exponential and stationary growth phases in good conditions and concluded that the stationary phase could not be due to nutrient depletion, toxins, or contact inhibition. With serial dilutions of stationary populations used for inoculations, we could define a minimal stationary cell concentration (MSCC) point. Similar results were gained from studies of other unicellular organisms, as well. Proteomic analysis sheds light on the fact that the stationary-phase cells changed their phenotype. Overall, we should reconsider the old principles of bacterial growth.
2. Materials and Methods
2.1. Cell Culture and Growth Conditions
S. elongatus PCC7942 cells were grown photoautotrophically in BG-11 medium [
21], supplemented with 10 mM HEPES–NaOH (pH 8). The media used for the growth of
E. coli (
Escherichia coli K12, DH5alphaF’ (
F’/endA1 hsdR17 (rk−mk+) supE44 thi-1 recA1 gyrA (Nalr) relA1 D(laclZYA-argF)U169 deoR (ϕ
80dlacD(lacZ) M15)) were the following: LB (pepton 10 g L
−1, yeast extract 5 g L
−1, NaCl 10 g L
−1), 2YT (tryptone 16 g L
−1, yeast extract 10 g L
−1, NaCl 5 g L
−1), and SOC (tryptone 20 g L
−1, yeast extract 5 g L
−1, NaCl 0.5 g L
−1, glucose 20 mM). The
E. coli was cultivated in LB, unless otherwise stated. The bacteria were grown in Erlenmeyer flasks on a rotary shaker by shaking
E. coli at 200 rpm, at 37 °C. Additionally,
S. elongatus was rotated at 110 rpm at 29 °C under continuous illumination at 40 μmol photons m
−2 s
−1 white light intensity. The centrifugation of the cultures was carried out with 4000×
g for 10 min in sterile conditions using an Eppendorf 5910 R centrifuge (Eppendorf, Hamburg, Germany).
2.2. Measurement of Optical Density
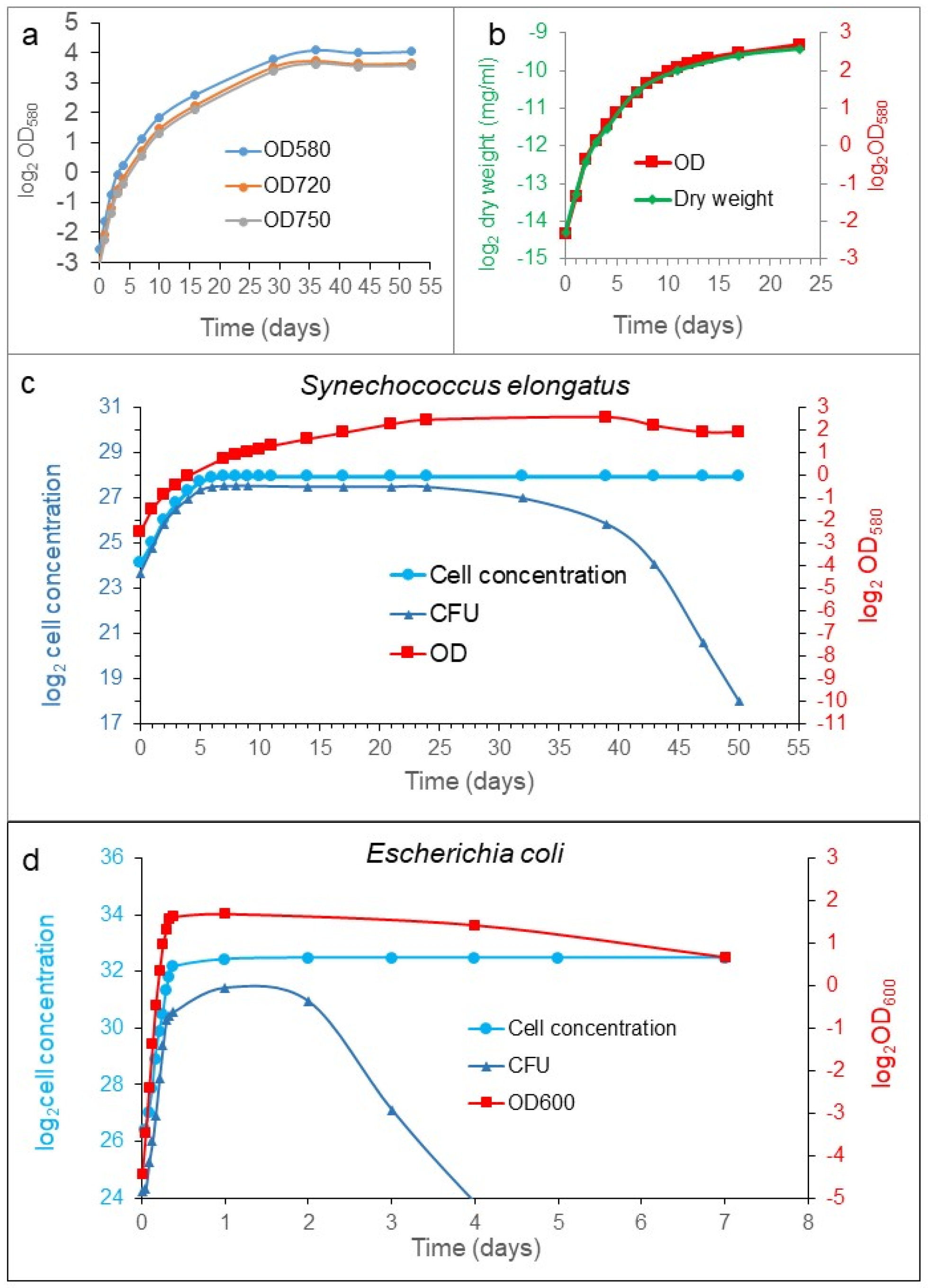
The optical density of the cyanobacterial cultures was determined daily by measuring OD at 580 nm in the case of S. elongatus and at 600 nm in the case of E. coli using a Nicolet Evolution 500 spectrophotometer (Thermo Electron, Cambridge, UK). In the case of S. elongatus, all of the OD measurements were executed at 730 and 750 nm, as well, but the curves were very similar to each other. Because the values were bigger at 580 nm, we represented these values.
2.3. Cell Enumeration
For the assessment of cell concentration, a Burker counting chamber was used. The depth of the counting chamber was 0.1 mm, and the counting area was 0.04 mm2. Cells were counted daily under the microscope (Nikon Eclipse Ni-E, Nikon Corporation, Tokyo, Japan). The cell concentration was calculated at a 250 × 1000 × dilution rate (cell number/mL).
2.4. Dry Mass Measurement
The dry weight (g/L) was monitored daily by filtering an aliquot of the culture (5–10 mL depending on the density) with pre-weighted glass microfiber filters (Whatman, Darmstadt, Germany, GF/C, 24 mm, pore size 1.2 μm). The filter was then dried at 80 °C for 5 h or to constant weight, prior to gross weight determination.
2.5. Determination of Colony Forming Units (CFU)
The cell suspensions of the bacterial cultures were serially diluted with sterile growth medium. Aliquots (0.1 mL) of the adequate dilutions were mixed with the melted (35 °C) soft agarose (2 mL) and poured onto the pre-warmed solid bottom agar in Petri dishes. The plates were incubated at 29 °C under continuous illumination at 30 μmol photons m−2 s−1 light intensity in the case of S. elongatus and in dark at 37 °C in the case of E. coli. The E. coli plates were evaluated the next day. After 3 weeks of incubation of S. elongatus plates, the number of colonies was counted. All the experiments were performed in triplicate, and results are expressed as an average of the triplicate samples with standard deviation.
2.6. Dye Exclusion Methods
If cells taking up trypan blue or propidium iodide (Merck/SigmaAldrich, Budapest, Hungary) are considered non-viable. We followed the manufacturer’s suggestions, with minor modifications. Shortly, 0.4% solution of trypan blue was prepared in BG11 medium buffered isotonic salt solution at pH 7.5. An amount of 0.1 mL of trypan blue stock solution was administered to 0.1 mL of cells, and it was examined immediately under a microscope at low magnification. An amount of 10 mg/mL propidium iodide was administered to E. coli cells. The detection happened at 540/490 nm excitation. The number of the total and that of the blue-stained or red fluorescent cells were characterized.
2.7. Fluorescent Microscopy
A Nikon Eclipse Ni-E (Nikon Corporation, Tokyo, Japan) was complemented with a CoolLED PE-4000 light source. Micrographs were taken by a Nikon D750 camera (Nikon Corporation, Japan).
2.8. Proteomics Analysis
2.8.1. Sample Preparation for Affinity-Based Proteomics
Total proteins from
S. elongatus were extracted, as described [
22]. Total protein extracts (4 mg/IP) were immunopurified using anti-GFP antibody-coupled magnetic beads of 50 nm (MACS
® Technology, Miltenyi, Bergisch Gladbach, Germany), digested in column with trypsin and analyzed in a single run on the mass spectrometer [
23]. The resulting tryptic peptide mixture was desalted prior to LC-MS/MS analysis on a C18 ZipTip (Omix C18 100 μL tips, Varian, Santa Clara, CA, USA), and the purified peptide mixture was analyzed by LC-MS/MS using a nanoflow RP-HPLC (LC program: linear gradient of 3–40% B in 100 min, solvent A: 0.1% formic acid in water, solvent B: 0.1% formic acid in acetonitrile), which was on-line coupled to a linear ion trap Orbitrap (Orbitrap-Fusion Lumos, Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer operating in positive ion mode. Data acquisition was carried out in a data-dependent fashion, and the 20 most abundant, multiply charged ions were selected from each MS survey for MS/MS analysis (MS spectra were acquired in the Orbitrap, and CID spectra were acquired in the linear ion trap).
2.8.2. Data Interpretation
Raw data were converted into peak lists using the in-house Proteome Discoverer (v1.4) and searched against the Uniprot Synechococcus elongatus PCC7942 (Syne7) database (downloaded 12 June 2019, 2653 proteins) using our in-cloud Protein Prospector search engine (v5.15.1) with the following parameters: enzyme: trypsin, with a maximum of 2 missed cleavages; mass accuracies: 5 ppm for precursor ions and 0.6 Da for fragment ions (both monoisotopic); fixed modification: carbamidomethylation of Cys residues; variable modifications: acetylation of protein N-termini; Met oxidation; cyclization of N-terminal Gln residues, allowing a maximum of 2 variable modifications per peptide. Acceptance criteria: minimum scores: 22 and 15; maximum E values: 0.01 and 0.05 for protein and peptide identifications, respectively. Spectral counting was used to estimate relative abundance of individual proteins in the non-antibody and GFP negative controls and in the anti-GFP immuno-purified samples.
3. Results
3.1. Characterization of the Growth of the Bacterial Population
To monitor bacterial cell growth, different measurements can be used, such as optical density (OD), dry weight, bacterial enumeration (cell counting), and colony-forming ability (CFU). We analyzed the growth of
S. elongatus and
Escherichia coli populations in batch culture. We compared the growth curves determined with different methods. All the processes characterized the exponential phase similarly (
Figure 1). However, they diverged from each other significantly from the transition between the exponential and stationary phases (
Figure 1c). The stationary phase is defined as constant cell concentration, so the cell concentration of the population was halted upon reaching the plateau, as it was expected. Meanwhile, the OD and the dry weight kept on increasing in rich medium, which means that the cells were elongating and not dividing (see later). However, the CFU showed a robust difference from the previous ones during the examined period. In the early exponential phase, the CFU curve moved similarly as the cell concentration. However, in the transition phase between the exponential and stationary phases, the increment was decelerated, later stopped, showing a plateau, and then it was dropping down steeply to reach a very low value characteristic for the organism and the medium (
Figure 1c); sometimes, it reached several ppm (parts pro mill) of the stationary cell concentration. The exhaustion of the nutrient must influence the CFU value in time, obviously. However, the decrease in CFU we observed occurred with a supplemented fresh stocks of nutrients.
Taken together, four ways of characterizations gave similar results for the exponential phase, but they became different from each other, starting from the transition phase. The cell concentration was more or less constant, OD values and dry weight increased parallelly, and the CFU had a maximum plateau at the transition phase. Then, it dropped down suddenly in the stationary phase, achieving a low, but constant, value for a while. The so-called death phase could not be detected with dye exclusion experiments during the investigation (three months) in well-fed cultures. The reduction in the colony-forming cells do not mean cell death, necessarily.
3.2. The Stationary Phase Is Neither Due to Nutrient Deprivation Nor Toxins
According to long standing theories, the stationary phase is due to nutrient depletion and/or some toxin accumulation. We do not argue that it can happen, however, in lab conditions. Usually, nutrition deprivation rarely occurs. To prove this,
S. elongatus cells were inoculated into 1/2×, 1×, or 2× BG11 media, setting the starting cell density to 0.2 OD
580 (10 × 10
6 cells/mL), and
E. coli cells were inoculated into Luria-Bertani (LB) broth, 2YT, and SOC media. The extinction of the cultures was monitored daily at 580 nm and hourly at 600 nm for
E. coli. In all media, there were no significant differences among the growth curves (
Figure 2a,b). Furthermore, we tested whether toxic metabolites could accumulate during the growth phase. To achieve this,
S. elongatus populations were grown in regular batch culture and in a special batch culture, wherein the medium was changed daily. The cell concentration of the cultures was monitored daily in a Burker chamber. No differences were detected in the growth rate and in the stationary plateau, either (
Supplementary Figure S1).
We were interested in how the population would grow in spent medium. Therefore, the population was grown in a batch culture after reaching the stationary phase at ~4 OD
580 for
S. elongatus and ~3 OD for
E. coli. The cells were removed by centrifugation, and the cell free, spent media were re-inoculated, adjusted with the stationary phase culture to ~0.5 OD
580 in the case of
S. elongatus, and, with regard to the original stationary phase culture related to 0.04 OD
600 in the case of
E. coli (again), the bacterial growth was followed. The population also reached ~4 OD
580/3 OD
600 in both cases after the second inoculation in the spent medium, too (
Figure 2c,d). We repeated these experiments at least three times in both cases.
Importantly, the cultures reached the same plateau in spent medium, and in enriched medium, as well. It means that there was no toxin, and no deprivation of nutrient that could affect the growth rate during the exponential phase or the cell concentration of the stationary plateau. Further, the richness of media did not affect the stationary cell concentrations. Therefore, we concluded that the regulation of the transition from the exponential to the stationary phase was due to something other than exhaustion of media or toxins.
3.3. Characterization of the Stationary Phase; the Minimal Stationary Cell Concentration
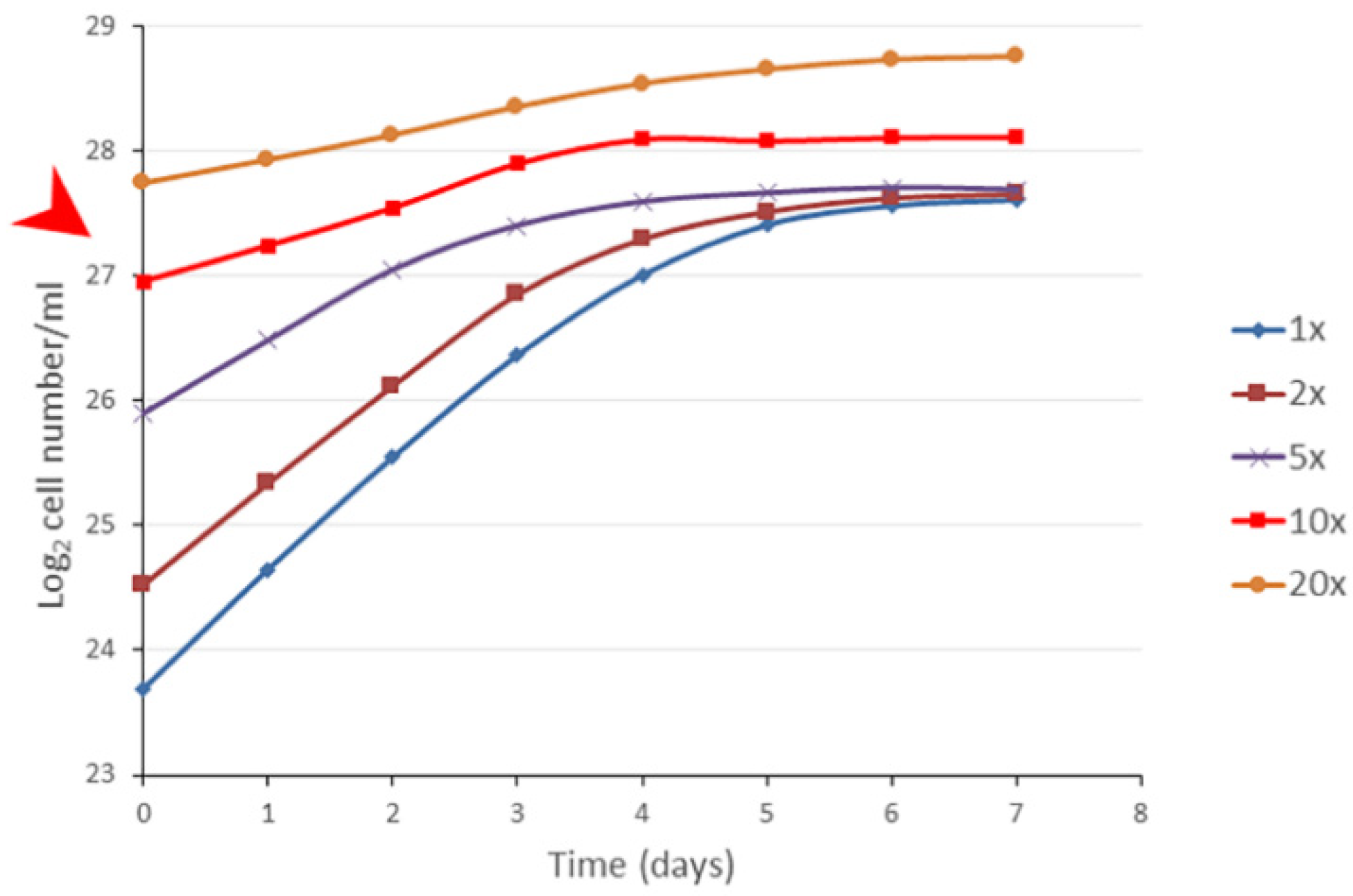
We wanted to see how the growth of a population was influenced by the different starting cell concentrations used for inoculation. Therefore, different dilutions of stationary populations of
S. elongatus were administered into fresh media, and the population growth was monitored (
Figure 3). Interestingly we found that, when the inoculation happened with 90%, 80%, and 70% of the original stationary cell concentration, the cell concentrations remained almost still. At dilution of the stationary phase cells at 60% or below, all populations grew, reaching normal stationary cell concentrations (~220 × 10
6/mL). This means that there was a limit to the dilutions of the non-growing populations that was considered a minimal stationary cell concentration (MSCC). This experiment could unravel the nature of the transition from the exponential to the stationary phase (
Figure 3).
There was an additional result, namely, the dilutions closer to the MSCC originated flatter exponential curves, which means that the generation times are longer and longer. Importantly, the generation time should not be considered as a constant value. In the case of higher dilutions (e.g., 10×, 20×), the differences were not so significant.
According to the state-of-the-art, there are two possible models to explain the nature of the stationary phase: (1) the stationary phase would be due to a dynamic steady state of the continuous division and cells death or (2) contact inhibition among cells.
Dye exclusion experiments were performed to monitor whether continuous cell proliferation and death dynamically maintain a constant cell concentration of the stationary phase in E. coli. Trypan blue and propidium iodide were administered to the cultures. Theoretically, the living, intact cells exclude the dyes, and only dead cells absorb dyes. Neither significant cell proliferation, nor cell death, were detected during the period of the investigation corroborated by dye exclusion experiment. There was hardly any sign of cell destruction in the stationary population. Approximately 2 × 105 cells were monitored, and ~5 ppm and ~27 ppm dead E. coli cells were detected with trypan blue and propidium iodide, respectively.
Contact inhibition could also be excluded because dilutions did not induce cell proliferation until the MSCC. This dilution experiment was executed with other prokaryotic and eukaryotic unicellular organisms, too, and the MSCC could be identified in the examined cases. For ease of comparison between different microorganisms, the ratio of MSCC to stationary cell concentration was investigated. This value was characteristic for the different organisms, including eukaryotic unicellular ones, as well (
Table 1).
Taken together, in the case of the S. elongatus, we could find a minimal stationary cell concentration (MSCC), defined as a limit value of concentration as far as the diluted populations were still. However, below it, the population grew up. This value was ~70% of the native stationary cell concentration in the applied medium in the case of S. elongatus. Further, the stationary phase is not due to a dynamic equilibrium of a continuous cell division and death. MSCC could only be determined by serial dilutions of stationary-phase cells used as inoculates.
3.4. The Bacterial Cell Concentration Alone Is Not Enough to Set the Stationary Phase
Exponential phase cells (~25 × 10
6/mL) were concentrated by centrifugation and adjusted to the concentration of 25, 50, 75, 250, and 450 × 10
6/mL, and they were suspended in fresh BG11 to check the population growth. If the inoculates were below the MSCC value, the population reached the original (native) plateau of stationary phase; where the cell concentration reached or exceeded the MSCC value, then the cell concentration approximately doubled, and proliferation stopped (
Figure 4). The doubling mechanism was independent from the age of the exponential populations applied for the concentration. Interestingly, the growth rate of the concentrated populations was altered, decreasing with higher concentrations, meaning that the so-called generation time (doubling time of the given population) cannot be a constant value. It depended on the inoculating concentrations. Importantly, our statement is for populations, not for individual cell proliferation.
Taken together, if exponential phase cells were concentrated, they began proliferating no matter the concentration. However, the MSCC seemed a special point. Importantly, the cell divisions were not stopped by the cell concentration alone, meaning that the cell concentration by oneself is not enough to set the stationary phase.
3.5. The Stationary Phase Cells Are Different from the Dividing Cells
It was believed for a long time that, upon starvation, the eubacteria can enter a developmental program that results in metabolically different, more resistant cells [
6]. Proteomic analyses were carried out on the
S. elongatus cells of exponential, stationary, and late stationary phases to unravel the difference. The total protein content of the exponential-phase cells was five-fold higher than that of stationary-phase cells. Therefore, prior the analysis, it was normalized. We performed a FtsZ-GFP-based affinity proteomic analysis to monitor the alterations between the exponential and stationary phases. FtsZ interacted with approximately ~1100 proteins of a total (S1) of ~2660 proteins of
S. elongatus, and, thus, we could exclude the very abundant photosynthetic proteins, which would overload the mass spectrometric detection. We could classify the detected proteins into four groups: (I) proteins whose expression did not change during the phases, considered constitutive proteins (
Table S2 of Supplementary), (II) a group containing those proteins that were characteristic for the exponential phase, (III) a group comprised of the proteins typical for the stationary phase, and (IV) is characteristic by the late stationary phase (
Figure 5).
Table 2 shows the most important differences in the proteomics among the exponential and stationary and late stationary phases that we detected.
It has long been known that the protein composition of ribosomes is a sensitive sensor for the bacterial phases [
24]. Well marked and characterized changes in the alternative use of some ribosome proteins are known, e.g., S6, S21, L12, L31, and L36 in
E. coli [
25,
26,
27,
28]. In our experiments, all of the ribosomal proteins could be pulled down from the exponential lysate, so the ribosomal heterogeneity was able to be checked. S6, L7/12, L18, L20, and L29 proteins were missing or significantly under-represented in the stationary phase in
S. elongatus (
Table 2).
Without the claim of completeness, the proteomic data indicated that proteins involved in DNA replication and repair (e.g., helicases, gyrases, topoisomerases) were missing or underrepresented in the stationary phase (
Table 2;
Table S2 of Supplementary). Interestingly, well known cell division proteins, or proteins involved in cytokinesis, could be found with similar intensity in all phases, e.g., CDV3, MinD, Ftn2, etc. (
Table 2.)
According to the data, we strongly believe that the stationary population consisted of differentiated cells, and a part of the population lost the capability of CFU. The proliferating populations could be distinguished from the stationary phase cells by phenotypic characters (
Figure 6) and by proteomics. Proteomics of the late stationary phase cells (three months old) were altered very much from the exponential, and they were less altered than the early stationary phase cells (
Table 2).
Taken together, there were characteristic proteomic differences among the studied groups that corroborated that genetic regulation controls the change in the phenotype to stationary state. According to our new model, the exponential phase cells [Exp] differentiate to stationary [Stat], then to late stationary, and those are called VBNC cells (cells that do not have CFU); this is the bacterial G0 in our nomenclature: [Exp]→[Stat]→G0.
3.6. Altered Phenotypes of Exponential Phase and Stationary-Phase Cells
Using fluorescence microscopy, the native, exponentially dividing
S. elongatus cells could be distinguished from the differentiated non-proliferative cells by their size and auto-fluorescence at 525 nm excitation (
Figure 6). Since the inoculating culture necessarily contained stationary G0 cells, the presence of stationary phase cells in the early population were not surprising; with the development of the exponential population, the concentration of the stationary phase cells decreased, and it was hardly detectable for a while.