Exploring the Process of Neutrophil Transendothelial Migration Using Scanning Ion-Conductance Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of EA.hy926 Cells
2.2. Isolation of Neutrophil Granulocytes
2.3. S. aureus Culturing
2.4. Cultivation of a Monolayer of Endothelial Cells on the Cell Culture Inserts
2.5. Study of Transendothelial Migration of Neutrophil Granulocytes in the Model of Experimental Septicopyemia
2.6. Scanning and Measuring of Cell Rigidity by the Method of Scanning Ion-Conductance Microscopy
2.7. Statistical Analysis
3. Results
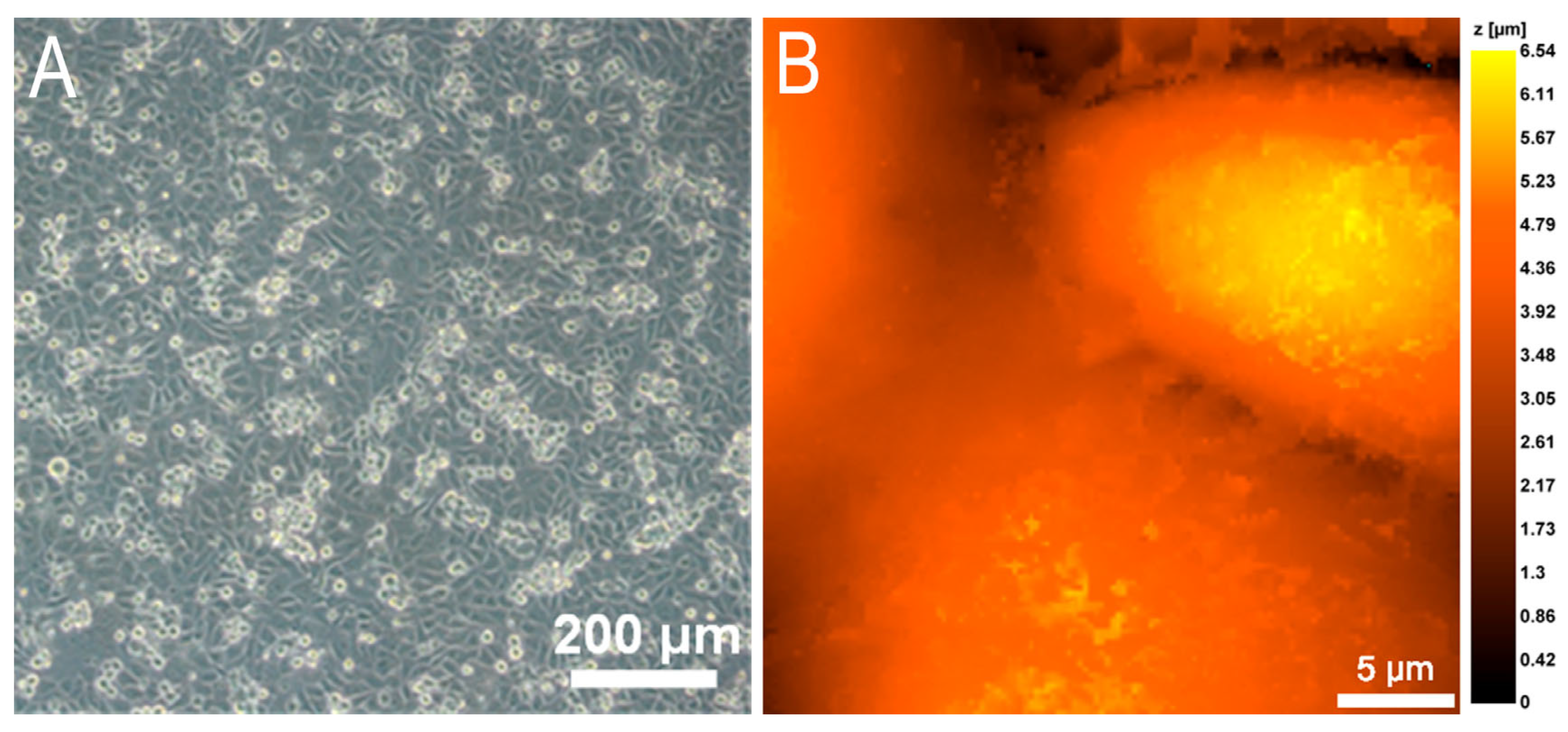
3.1. The Endothelial Cell Monolayer for Investigation of Neutrophil Transendothelial Migration
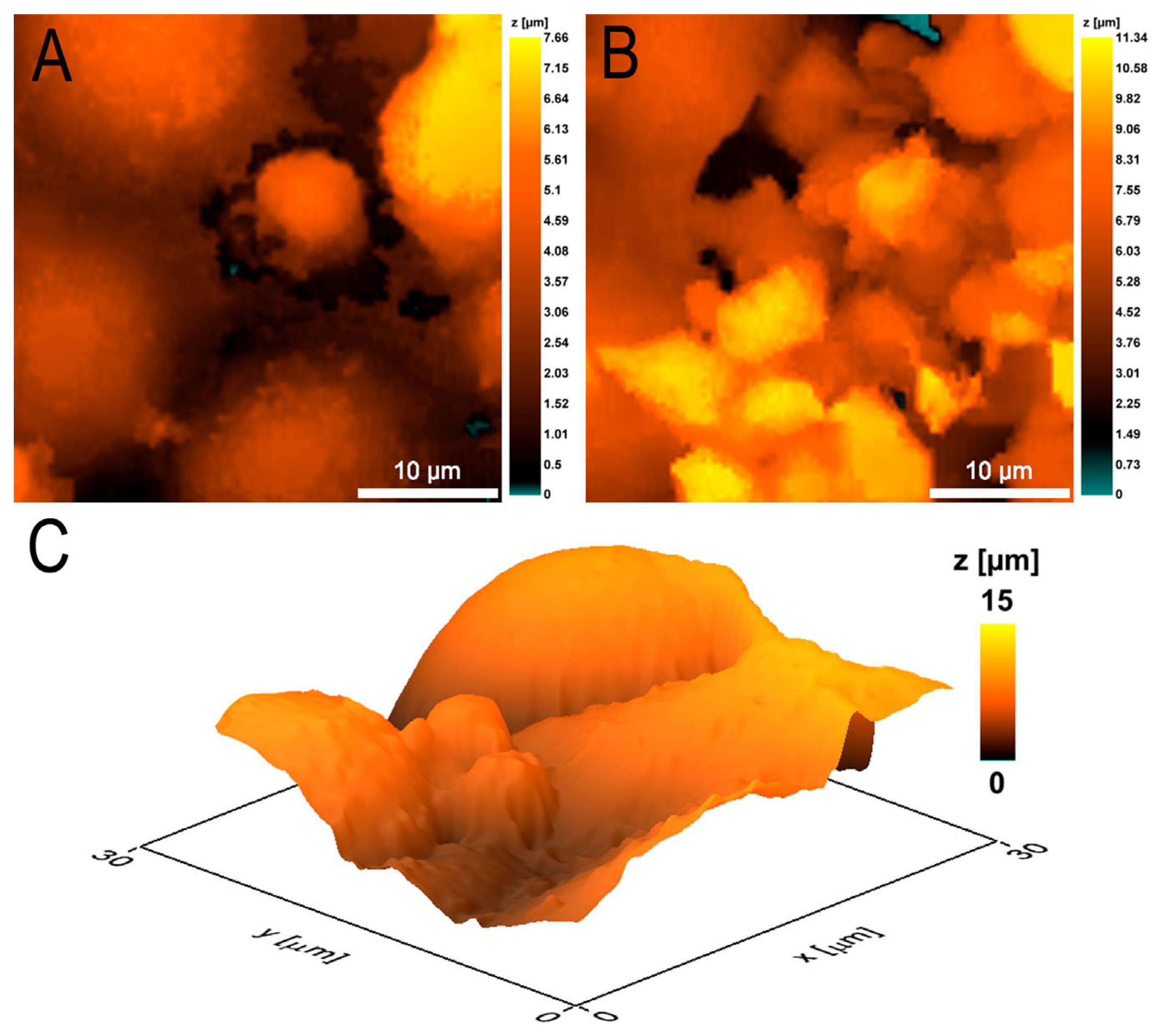
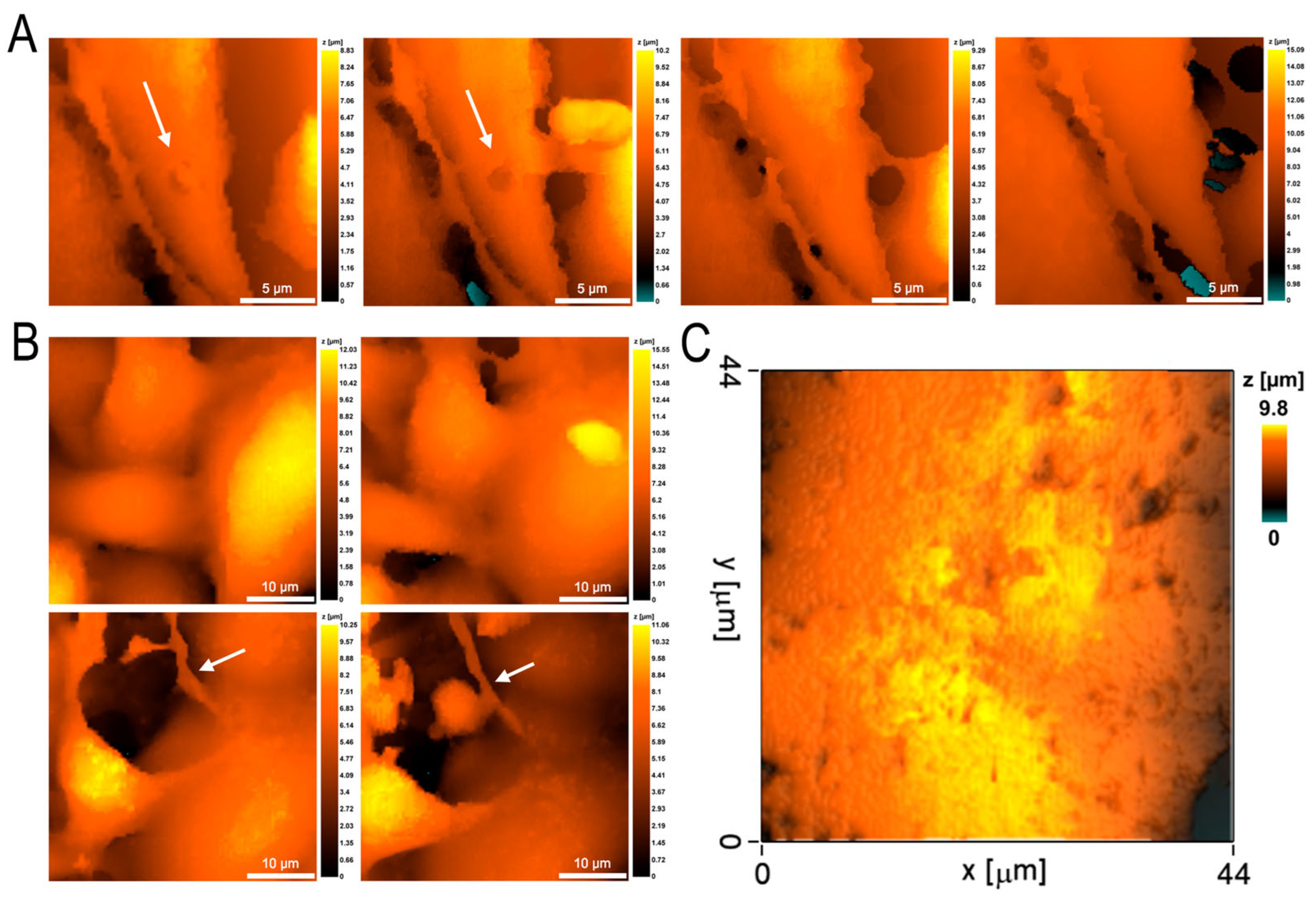
3.2. Morphometric Studies of EA.hy926 Culture Monolayer by Scanning Ion-Conductance Microscopy
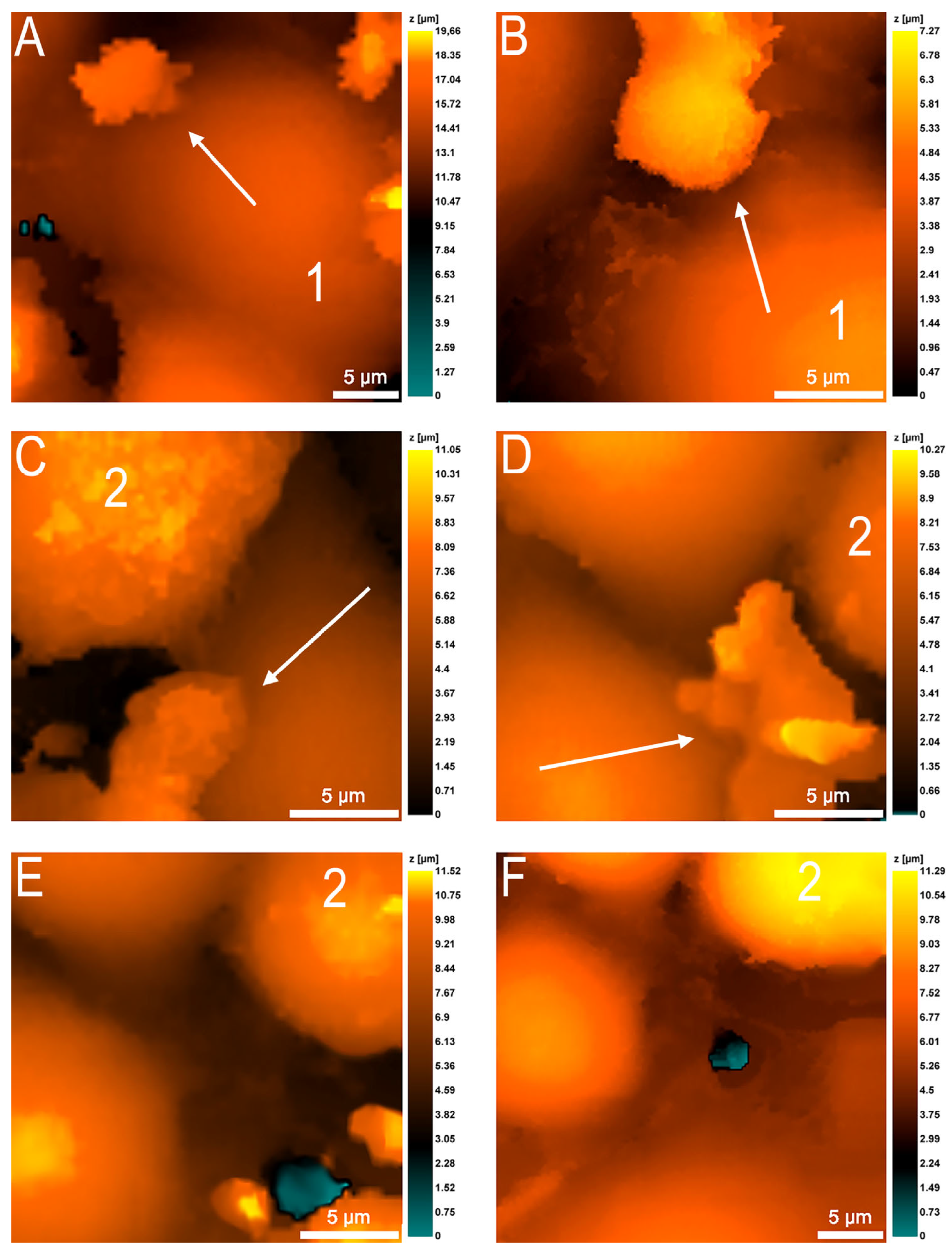
3.3. Changes in Neutrophil Granulocytes during Transendothelial Migration
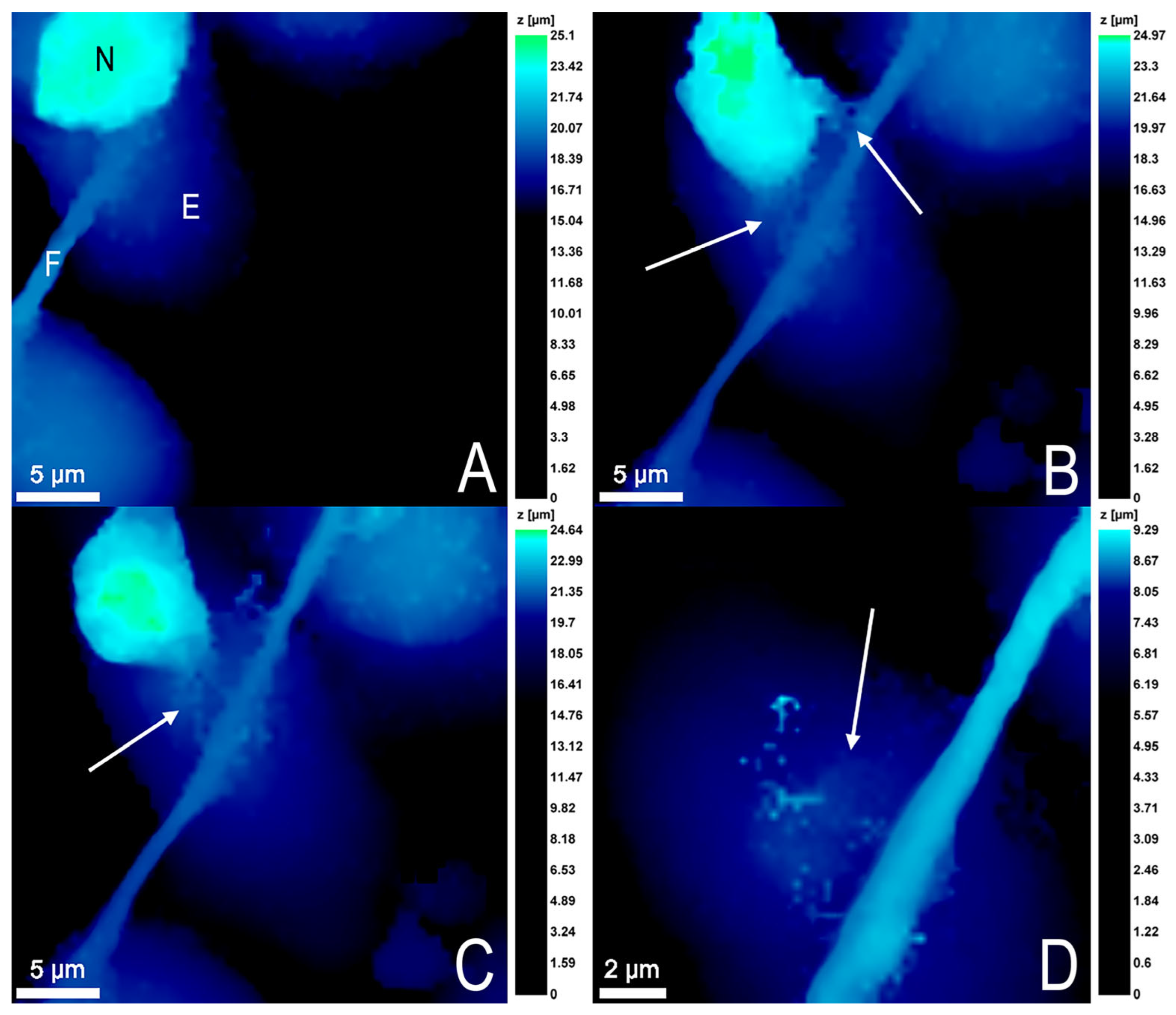
3.4. Endothelial Cell Changes during Transendothelial Migration of Neutrophils
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filippi, M.D. Neutrophil transendothelial migration: Updates and new perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Santoso, S.; Chavakis, T. Mechanisms of neutrophil transendothelial migration. Front. Biosci. 2009, 14, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.A.; Vlasova, K.Y.; Garanina, A.S.; Melnikov, P.A.; Potashnikova, D.M.; Vishnevskiy, D.A.; Vodopyanov, S.S.; Chekhonin, V.P.; Abakumov, M.A.; Majouga, A.G. Extravasating neutrophils open vascular barrier and improve liposomes delivery to tumors. ACS Nano 2019, 13, 12599–12612. [Google Scholar] [CrossRef] [PubMed]
- Pleskova, S.N.; Kriukov, R.N.; Bobyk, S.Z.; Boryakov, A.V.; Gorelkin, P.V.; Erofeev, A.S. Conditioning adhesive contacts between the neutrophils and the endotheliocytes by Staphylococcus aureus. J. Mol. Recognit. 2020, 30, e2846. [Google Scholar]
- Miron, A.; Spinozzi, D.; NíDhubhghaill, S.; Lie, J.T.; Oellerich, S.; Melles, G.R.J. In vitro endothelial cell migration from limbal edge-modified Quarter-DMEK grafts. PLoS ONE 2019, 14, e0225462. [Google Scholar] [CrossRef]
- Rabodzey, A.; Alcaide, P.; Luscinskas, F.W.; Ladoux, B. Mechanical forces induced by the transendothelial migration of human neutrophils. Biophys. J. 2008, 95, 1428–1438. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Bobyk, S.Z.; Kriukov, R.N.; Gorshkova, E.N.; Novikov, D.V.; Vasilchikov, P.I.; Bezrukov, N.A.; Novikov, V.V. S. aureus and E. coli change the force and work of adhesion between P- and E-selectins of endothelial cells and ligands of neutrophil granulocytes. Micron 2021, 150, 103139. [Google Scholar] [CrossRef]
- Martinelli, R.; Zeiger, A.S.; Whitfield, M.; Sciuto, T.E.; Dvorak, A.; Van Vliet, K.J.; Greenwood, J.; Carman, C.V. Probing the biomechanical contribution of the endothelium to lymphocyte migration: Diapedesis by the path of least resistance. J. Cell Sci. 2014, 127, 3720–3734. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Bezrukov, N.A.; Gorshkova, E.N.; Bobyk, S.Z.; Lazarenko, E.V. Study of EA.hy 926 endothelial cells by atomic force and scanning ion-conductance microscopy. Tsitologiya 2023, 5, 441–449. [Google Scholar]
- Marin, V.; Kaplanski, G.; Grès, S.; Farnarier, C.; Bongrand, P. Endothelial cell culture: Protocol to obtain and cultivate human umbilical endothelial cells. J. Immunol. Methods 2001, 254, 183–190. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Kriukov, R.N.; Gorshkova, E.N.; Boryakov, A.V. Characteristics of quantum dots phagocytosis by neutrophil granulocytes. Heliyon 2019, 5, e01439. [Google Scholar] [CrossRef] [PubMed]
- Pleskova, S.N.; Gorshkova, E.N.; Kriukov, R.N. Dynamics of formation and morphological features of neutrophil extracellular traps formed under the influence of opsonized Staphylococcus aureus. J. Mol. Recognit. 2018, 31, e2707. [Google Scholar] [CrossRef] [PubMed]
- Niethamer, T.K.; Stabler, C.T.; Leach, J.P.; Zepp, J.A.; Morley, M.P.; Babu, A.; Zhou, S.; Morrisey, E.E. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife 2020, 9, e53072. [Google Scholar] [CrossRef] [PubMed]
- Townsley, M.I. Structure and composition of pulmonary arteries, capillaries, and veins. Compr. Physiol. 2012, 2, 675–709. [Google Scholar]
- Crosara-Alberto, D.P.; Darini, A.L.; Inoue, R.Y.; Silva, J.S.; Ferreira, S.H.; Cunha, F.Q. Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br. J. Pharmacol. 2002, 136, 645–658. [Google Scholar] [CrossRef]
- Kienle, K.; Glaser, K.M.; Eickhoff, S.; Mihlan, M.; Knöpper, K.; Reátegui, E.; Epple, M.W.; Gunzer, M.; Baumeister, R.; Tarrant, T.K.; et al. Neutrophils self-limit swarming to contain bacterial growth in vivo. Science 2021, 372, eabe7729. [Google Scholar] [CrossRef]
- Krewson, E.A.; Sanderlin, E.J.; Marie, M.A.; Akhtar, S.N.; Velcicky, J.; Loetscher, P.; Yang, L.V. The proton-sensing GPR4 receptor regulates paracellular gap formation and permeability of vascular endothelial cells. iScience 2020, 23, 100848. [Google Scholar] [CrossRef]
- Schattner, M. Platelet TLR4 at the crossroads of thrombosis and the innate immune response. J. Leukoc. Biol. 2019, 105, 873–880. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Qu, M.; Nan, K.; Cao, H.; Cata, J.P.; Chen, W.; Miao, C. Review: The emerging role of neutrophil extracellular traps in sepsis and sepsis-associated thrombosis. Front. Cell Infect. Microbiol. 2021, 11, 653228. [Google Scholar] [CrossRef]
- Yu, X.; Diamond, S.L. Fibrin modulates shear-induced netosis in sterile occlusive thrombi formed under haemodynamic flow. Thromb. Haemost. 2019, 119, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lelliott, P.M.; Nishide, M.; Pavillon, N.; Okita, Y.; Shibahara, T.; Mizuno, Y.; Yoshimura, H.; Obata, S.; Kumanogoh, A.; Smith, N.I. Cellular adhesion is a controlling factor in neutrophil extracellular trap formation induced by anti-neutrophil cytoplasmic antibodies. Immunohorizons 2022, 6, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Joshi, M.B.; Philippova, M.; Erne, P.; Hasler, P.; Hahn, S.; Resink, T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010, 584, 3193–3197. [Google Scholar] [CrossRef]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin g. Arter. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef]
- McDonald, A.I.; Shirali, A.S.; Aragon, R.; Ma, F.; Hernandez, G.; Vaughn, D.A.; Mack, J.J.; Lim, T.Y.; Sunshine, H.; Zhao, P.; et al. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell 2018, 23, 210–225. [Google Scholar] [CrossRef]
- Itoh, Y.; Toriumi, H.; Yamada, S.; Hoshino, H.; Suzuki, N. Resident endothelial cells surrounding damaged arterial endothelium reendothelialize the lesion. Arter. Thromb. Vasc. Biol. 2010, 30, 1725–1732. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Marsboom, G.; Jambusaria, A.; Xiong, S.; Toth, P.T.; Benevolenskaya, E.V.; Rehman, J.; Malik, A.B. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat. Commun. 2019, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Iruela-Arispe, M.L.; Zhao, Y.Y. Mechanisms of Endothelial Regeneration and Vascular Repair and Their Application to Regenerative Medicine. Am. J. Pathol. 2021, 191, 52–65. [Google Scholar] [CrossRef]

| Characteristics | Before Migration | After Migration |
|---|---|---|
| Cell projection area, μm2 | 422.3 ± 116.7 | 354.6 ± 105.2 1 |
| Cell volume, μm3 | 1620.8 ± 580.2 | 1697.2 ± 663.3 |
| Young’s modulus, Pa | 758.5 ± 158.1 | 711.9 ± 129.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleskova, S.N.; Bezrukov, N.A.; Gorshkova, E.N.; Bobyk, S.Z.; Lazarenko, E.V. Exploring the Process of Neutrophil Transendothelial Migration Using Scanning Ion-Conductance Microscopy. Cells 2023, 12, 1806. https://doi.org/10.3390/cells12131806
Pleskova SN, Bezrukov NA, Gorshkova EN, Bobyk SZ, Lazarenko EV. Exploring the Process of Neutrophil Transendothelial Migration Using Scanning Ion-Conductance Microscopy. Cells. 2023; 12(13):1806. https://doi.org/10.3390/cells12131806
Chicago/Turabian StylePleskova, Svetlana N., Nikolay A. Bezrukov, Ekaterina N. Gorshkova, Sergey Z. Bobyk, and Ekaterina V. Lazarenko. 2023. "Exploring the Process of Neutrophil Transendothelial Migration Using Scanning Ion-Conductance Microscopy" Cells 12, no. 13: 1806. https://doi.org/10.3390/cells12131806
APA StylePleskova, S. N., Bezrukov, N. A., Gorshkova, E. N., Bobyk, S. Z., & Lazarenko, E. V. (2023). Exploring the Process of Neutrophil Transendothelial Migration Using Scanning Ion-Conductance Microscopy. Cells, 12(13), 1806. https://doi.org/10.3390/cells12131806







