Ca2+-Driven Selectivity of the Effect of the Cardiotonic Steroid Marinobufagenin on Rabbit Sinoatrial Node Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Use
2.2. SAN Cell Isolation and Culture
2.3. Ca2+ Imaging and Measurements
2.4. Drugs
2.5. Statistics
3. Results
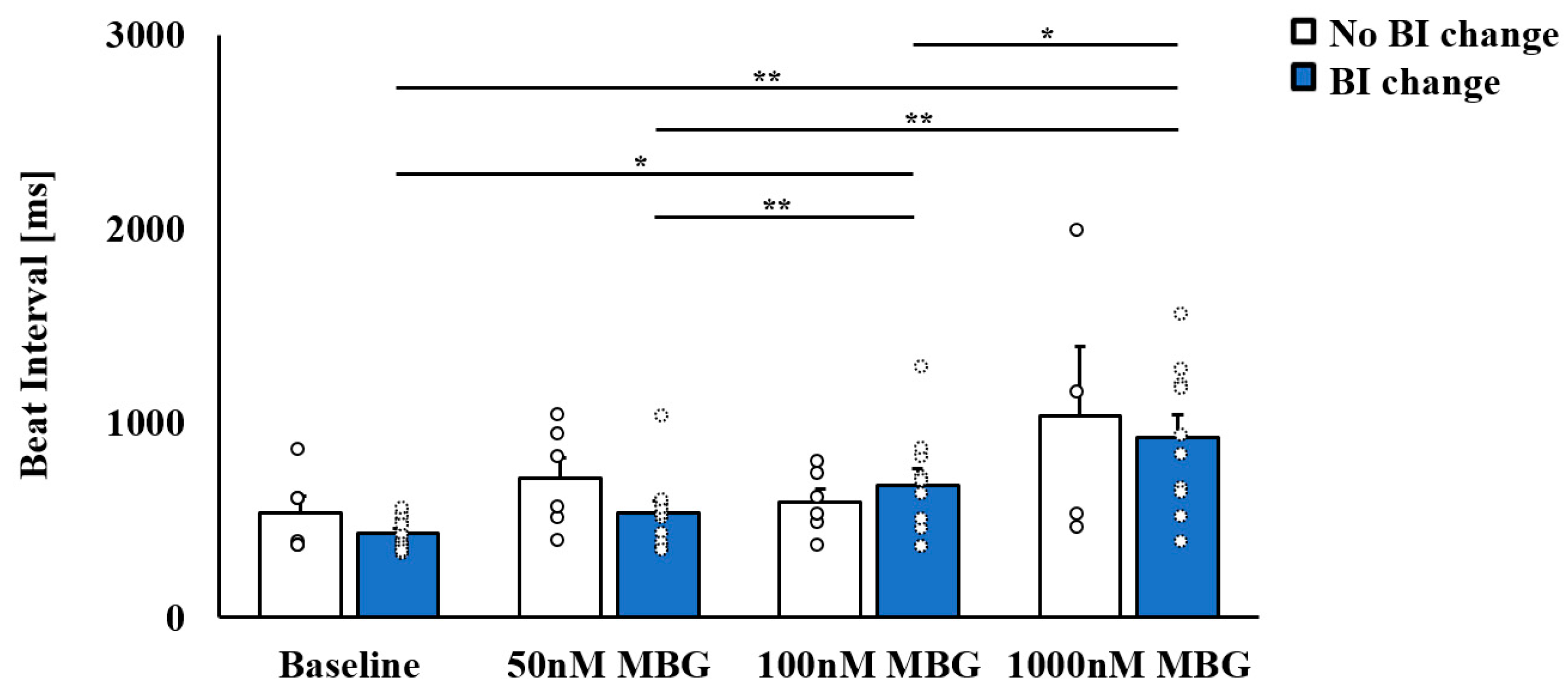
3.1. Selective Effect of MBG on Rabbit SANC Beat Interval
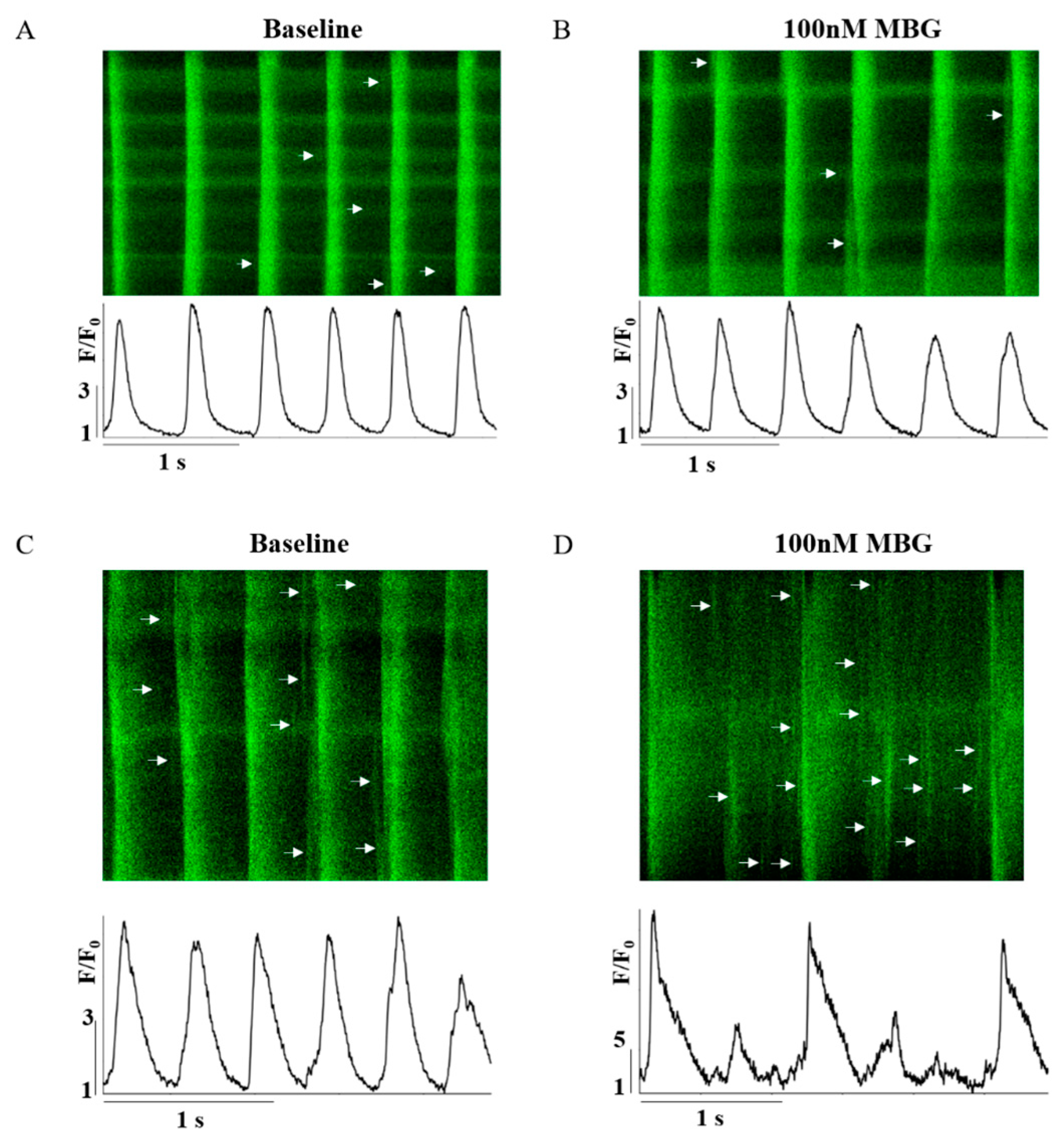
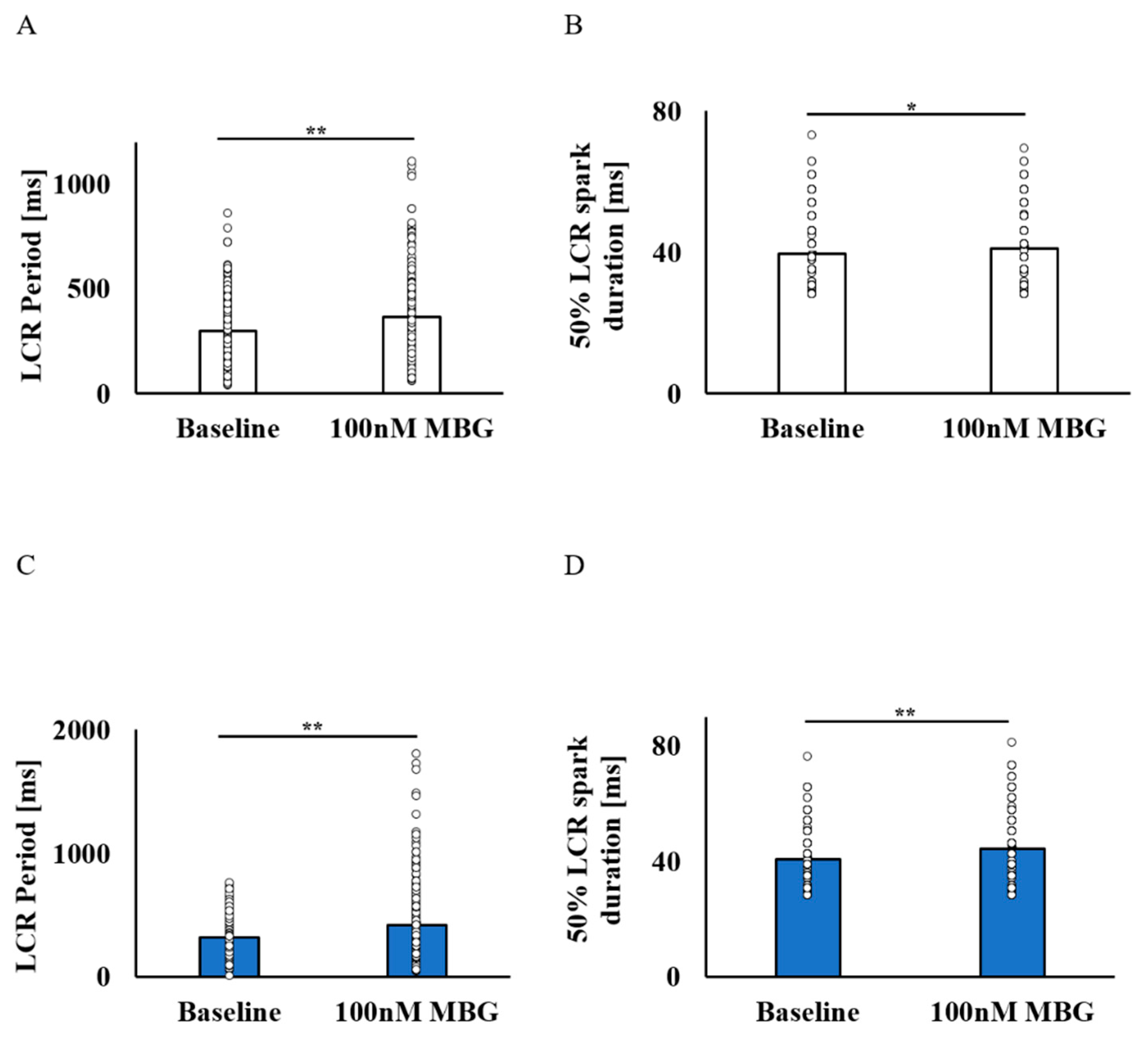
3.2. Selective Effect of MBG on Global and Local Ca2+ Parameters of Rabbit SANCs
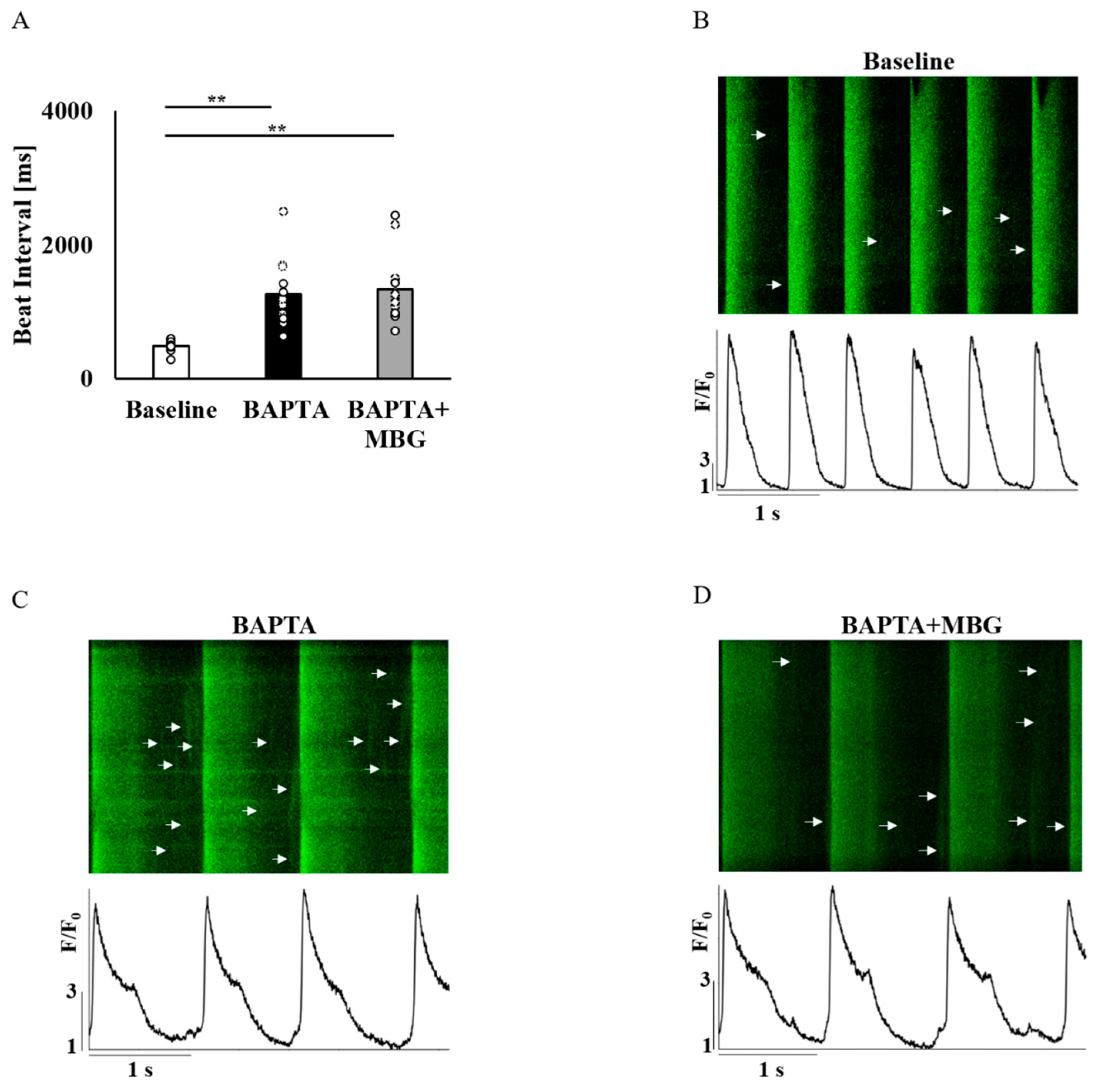
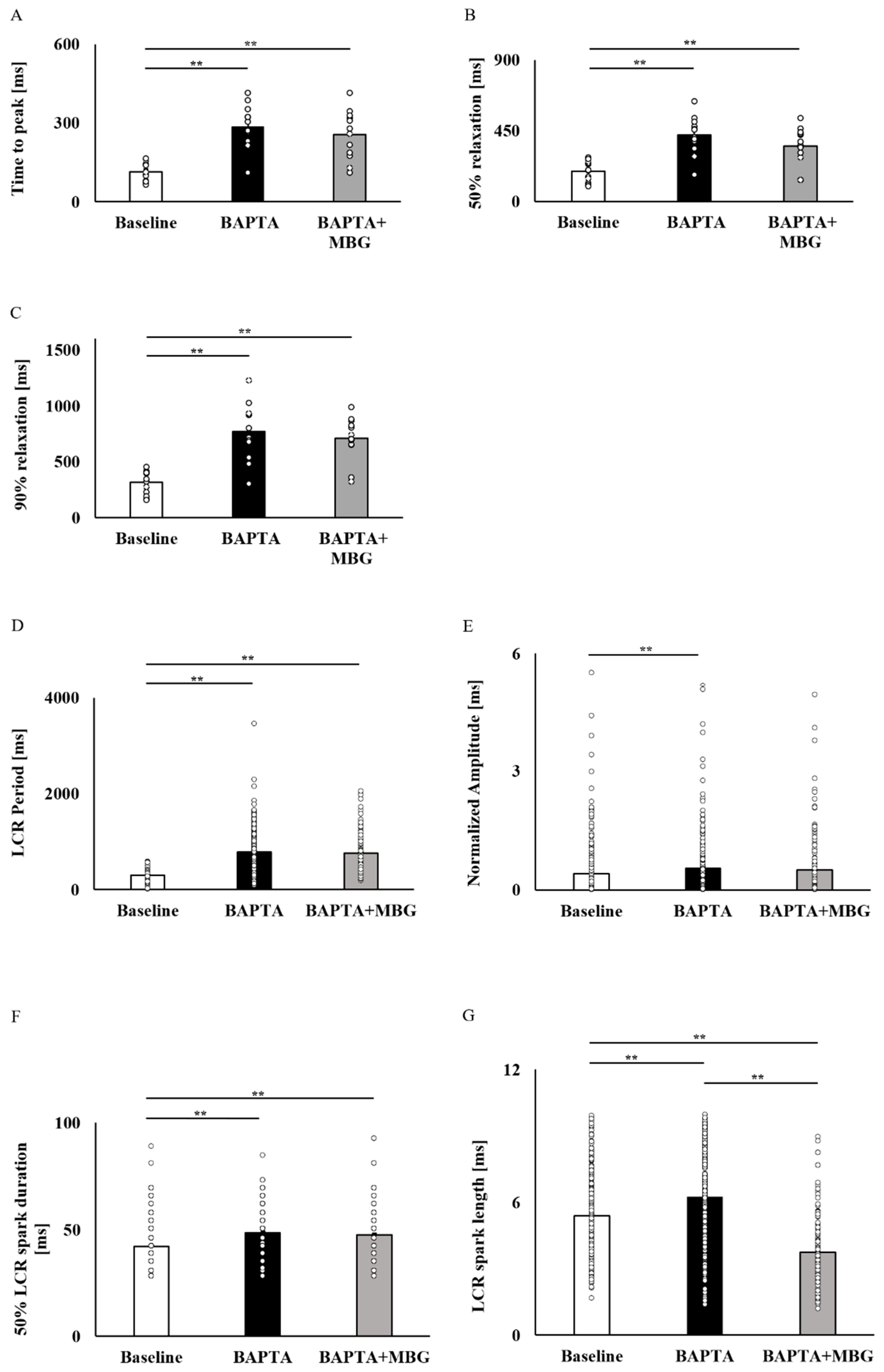
3.3. Ca2+ Chelation Abolishes the Selective Effect of MBG
4. Discussion
Study Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A Coupled SYSTEM of Intracellular Ca2+ Clocks and Surface Membrane Voltage Clocks Controls the Timekeeping Mechanism of the Heart’s Pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From Two Competing Oscillators to One Coupled-Clock Pacemaker Cell System. Front. Physiol. 2015, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeck, G.; Bonke, F.I.M.; Allessie, M.A.; Lammers, W.J.E.P. The Effect of Ouabain on the Isolated Sinus Node Preparation of the Rabbit Studied with Microelectrodes. Circ. Res. 1980, 46, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, K.; Jalife, J. Effects of Digitalis Intoxication on Pacemaker Rhythm and Synchronization in Rabbit Sinus Node. Am. J. Physiol. 1986, 250, H567–H578. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, S.G.; Maltsev, V.A.; Yaniv, Y.; Bychkov, R.; Yaeger, D.; Vinogradova, T.; Spurgeon, H.A.; Lakatta, E.G. Electrochemical Na+ and Ca2+ Gradients Drive Coupled-Clock Regulation of Automaticity of Isolated Rabbit Sinoatrial Nodal Pacemaker Cells. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H251–H267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, M.; Smith, W.; Fedorova, O.V.; Schutte, A.E. The Na+K+-ATPase Inhibitor Marinobufagenin and Early Cardiovascular Risk in Humans: A Review of Recent Evidence. Curr. Hypertens. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Segal, S.; Arbel-Ganon, L.; Mazgaoker, S.; Davoodi, M.; Yaniv, Y. Increase in Ca2+-Activated CAMP/PKA Signaling Prevents Hydroxychloroquine-Induced Bradycardia of the Cardiac Pacemaker. Front. Physiol. 2022, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Segal, S.; Kirschner Peretz, N.; Arbel-Ganon, L.; Liang, J.; Li, L.; Marbach, D.; Yang, D.; Wang, S.Q.; Yaniv, Y. Eliminating Contraction during Culture Maintains Global and Local Ca2+ Dynamics in Cultured Rabbit Pacemaker Cells. Cell Calcium 2019, 78, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kirschner Peretz, N.; Segal, S.; Yaniv, Y. May the Force Not Be with You during Culture: Eliminating Mechano-Associated Feedback during Culture Preserves Cultured Atrial and Pacemaker Cell Functions. Front. Physiol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoodi, M.; Segal, S.; Kirschner Peretz, N.; Kamoun, D.; Yaniv, Y. Semi-Automated Program for Analysis of Local Ca2+ Spark Release with Application for Classification of Heart Cell Type. Cell Calcium 2017, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mattick, P.; Parrington, J.; Odia, E.; Simpson, A.; Collins, T.; Terrar, D. Ca2+-Stimulated Adenylyl Cyclase Isoform AC1 Is Preferentially Expressed in Guinea-Pig Sino-Atrial Node Cells and Modulates the I(f) Pacemaker Current. J. Physiol. 2007, 582, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Lyashkov, A.E.; Graham, D.; Sheydina, A.; Volkova, M.V.; Mitsak, M.; Vinogradova, T.M.; Lukyanenko, Y.O.; Li, Y.; Ruknudin, A.M.; et al. Ca2+-Stimulated Basal Adenylyl Cyclase Activity Localization in Membrane Lipid Microdomains of Cardiac Sinoatrial Nodal Pacemaker Cells. J. Biol. Chem. 2008, 283, 14461–14468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.Y.; Xie, Z.J. Binding of Src to Na+/K+-ATPase Forms a Functional Signaling Complex. Mol. Biol. Cell 2006, 17, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Lin, Y.C.; Hileman, S.; Martin, K.H.; Hull, R.; Yu, H.G. PP2 Prevents Isoproterenol Stimulation of Cardiac Pacemaker Activity. J. Cardiovasc. Pharmacol. 2015, 65, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorova, Y.N.; Juhasz, O.; Long, J.M.; Zernetkina, V.I.; Hall, M.L.; Wei, W.; Morrell, C.H.; Petrashevskaya, N.; Morrow, A.; Lanasa, K.H.; et al. Effect of Cardiotonic Steroid Marinobufagenin on Vascular Remodeling and Cognitive Impairment in Young Dahl-S Rats. Int. J. Mol. Sci. 2022, 23, 4563. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Haller, S.; Periyasamy, S.; Brewster, P.; Zhang, H.; Adlakha, S.; Fedorova, O.V.; Xie, Z.J.; Bagrov, A.Y.; Shapiro, J.I.; et al. Renal Ischemia Regulates Marinobufagenin Release in Humans. Hypertension (1979) 2010, 56, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segal, S.; Yaniv, Y. Ca2+-Driven Selectivity of the Effect of the Cardiotonic Steroid Marinobufagenin on Rabbit Sinoatrial Node Function. Cells 2023, 12, 1881. https://doi.org/10.3390/cells12141881
Segal S, Yaniv Y. Ca2+-Driven Selectivity of the Effect of the Cardiotonic Steroid Marinobufagenin on Rabbit Sinoatrial Node Function. Cells. 2023; 12(14):1881. https://doi.org/10.3390/cells12141881
Chicago/Turabian StyleSegal, Sofia, and Yael Yaniv. 2023. "Ca2+-Driven Selectivity of the Effect of the Cardiotonic Steroid Marinobufagenin on Rabbit Sinoatrial Node Function" Cells 12, no. 14: 1881. https://doi.org/10.3390/cells12141881
APA StyleSegal, S., & Yaniv, Y. (2023). Ca2+-Driven Selectivity of the Effect of the Cardiotonic Steroid Marinobufagenin on Rabbit Sinoatrial Node Function. Cells, 12(14), 1881. https://doi.org/10.3390/cells12141881






