Phage Endolysins: Advances in the World of Food Safety
Abstract
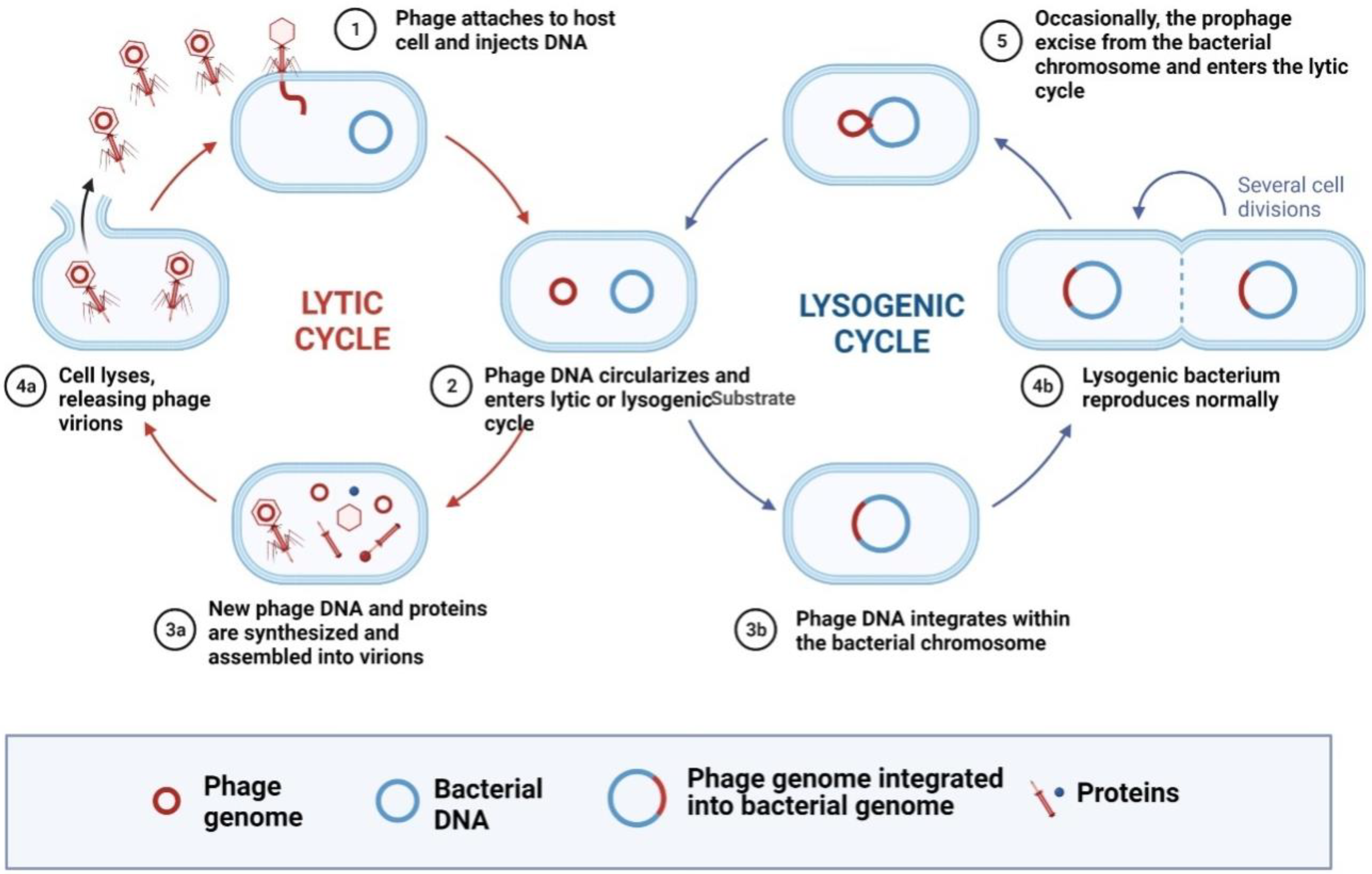
:1. Introduction
2. Primary Features of Endolysins
2.1. Structure and Enzymatic Activity of Endolysins
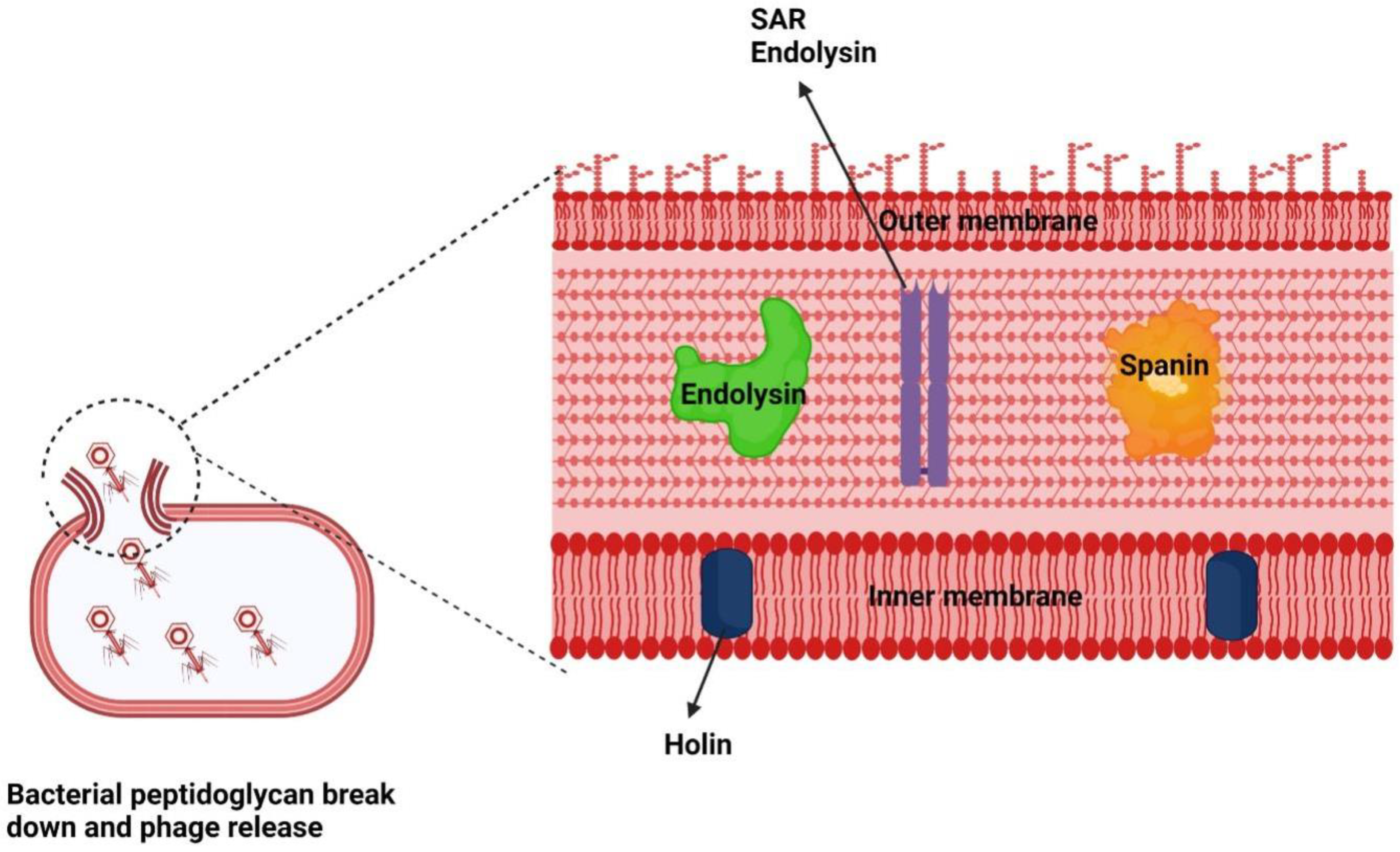
2.2. Endolysins: Mode of Action
3. Impact of Endolysins as Bio-Control Agents in the Food Industry
3.1. Dairy Products
3.2. Fruits and Vegetables
3.3. Meat and Poultry Products
| Host Bacteria | Endolysin | Food | Results | References |
|---|---|---|---|---|
| S. uberis | Ply700 | milk | 81% killing (p < 0.01) was observed when the inoculum was reduced to approximately 600 CFU/mL. | [39] |
| B. cereus | DLn1 LysZC1 | milk food | Viable bacterial counts could be reduced by 4.7-log10 CFU/mL in 24 h. LysZC1 was able to reduce the numbers of logarithmic-phase B. cereus by more than 2-log CFU/mL in 1 h | [56,63] |
| S. typhimurium | LysWL59 | vegetable | Reduction of 93% of S. typhimurium cells on lettucein 1 h when treated with 2.5 μM of lyswl59 and 0.5 mM edta. | [59] |
| LysWL60 | vegetable | |||
| S. aureus | LysH5 | milk | About 8-log CFU/mL reduction at 37 °C in 6 h. | [40,44] |
| Ply187AN-KSH3b | milk | About 3-log CFU/mL reduction at 37 °C immediately. | [65] | |
| λSA2-E-LysO-SH3b, λSA2-E-LysK-SH3b | milk | About 3-log CFU/mL reduction at 37 °C in 3 h. | [35] | |
| HydH5Lyso, HydH5SH3b, CHAPSH3b | milk | About 4-log CFU/mL reduction after CHAPSH3b treatment at 37 °C in 15 min. | [66] | |
| LysSA97 | milk, beef | Beef Synergistic bactericidal effect with carvacrol. | [46] | |
| LysSA11 | milk, ham | About 4-log CFU/cm3 reduction at 25 °C in 15 min. | [47] | |
| Phi11-481 | milk | Showed strong activity at 2–3 mM CaCl2. | [34] | |
| trx-sa1 | meat | Reduction in somatic cells and S. aureus numbers after infusion of 20 mg of trx-sa1 in udder quarters. | [61] | |
| lysRODI | meat | Protective efficacy against mammary infections in mice. | [62] | |
| Clostridial species | Ctp1L | milk | About 1-log CFU/mL reduction in 2 h. | [54] |
| CS74L | cheese | 57.8 ± 1% drop in optical density over 8 min. | [55] | |
| LysCPAS15 | milk | 3-log reduction in 2 h at 37 °C. | [53] | |
| CP25L | meat | 4-log CFU compared to a control even after 43 h of incubation. | [64] | |
| LysCP28 | duck meat | 3.4-log CFUs compared with control samples in duck meat after 72 h. | [51] | |
| Cpp-lys | lettuce | >4-log of bacteria were removed within 15 min. | [58] | |
| C. sakazakii | LysCs4 | powerded milk | 50 μg/mL, a reduction in OD590 was seen over 30 min, demonstrating the peptidoglycan degrading ability of LysCs4. | [52] |
| S. dysgalactiae | λSA2 lysin B30 lysin | milk | Stronger activity with λSA2 lysin (3.5-log CFU/mL reduction at 100 µg/mL) than B30 lysin. | [48] |
| ClyR | milk | More than 2-log CFU/mL reduction within 1 min. | [57] | |
| L. monocytogenes | PlyP825 | mozzarella milk | Synergistic bactericidal effect with high hydrostatic pressure. | [51] |
| PlyP100 | cheese, queso fresco | About 3.5-log CFU/g reduction at 4 °C in 4 weeks. | [50] | |
| Ply500 | iceberg lettuce | About 4-log CFU reduction at 25 °C in 24 h (free or immobilized endolysins). Reduction in viable L. monocytogenes cells in a spiked iceberg. | [27] | |
| Ply511, Ply118 | lettuce | Significant reduction in viable Listeria cells in whole cow milk. | [60] | |
| LysZ5 | soya milk | More than 4-log CFU/mL reduction in 3 h at 4 °C. | [49] |
4. Development of Hybrid Endolysins by Synthetic Engineering to Combat Food-Borne Pathogens
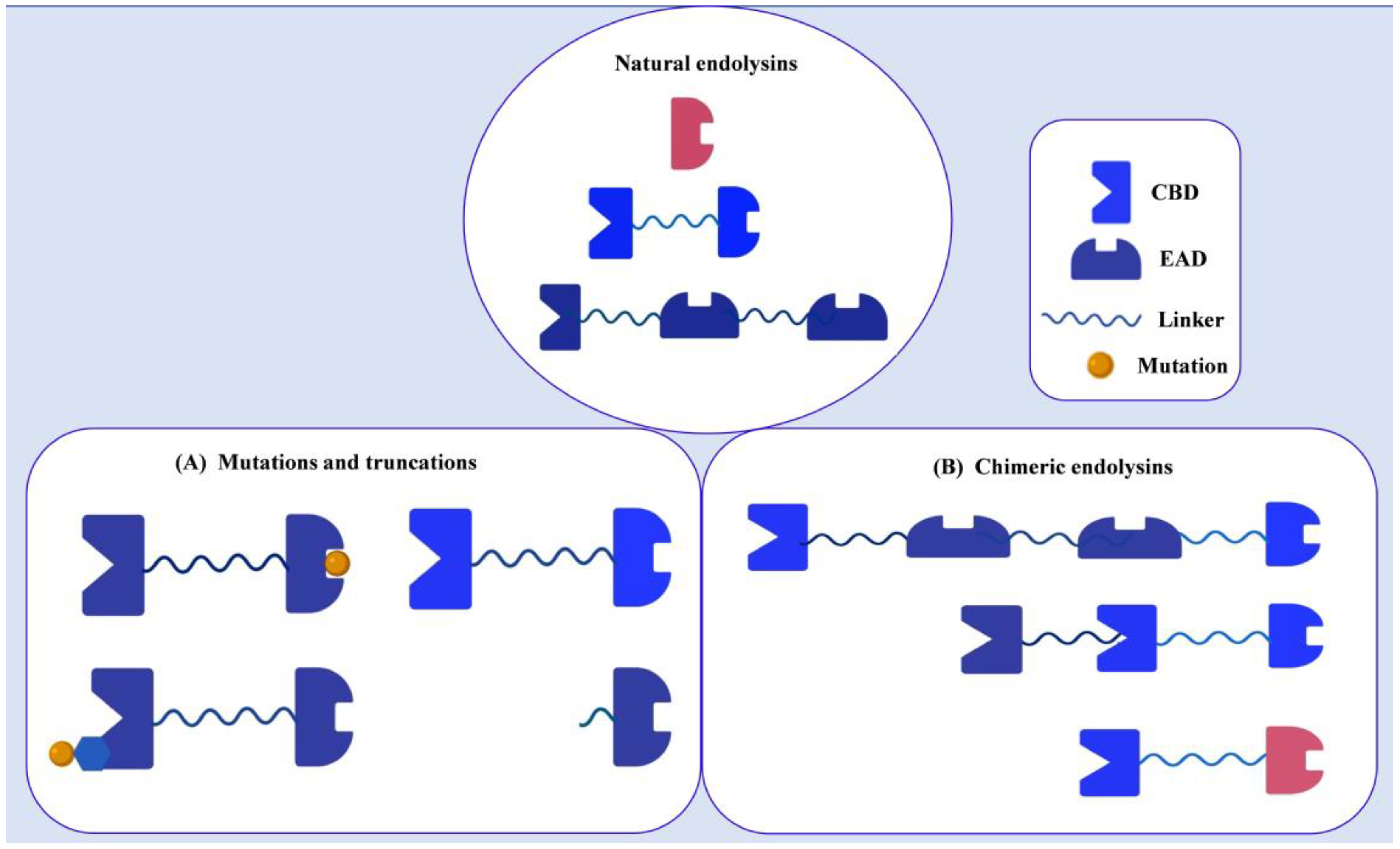
4.1. Endolysins Engineering against Gram-Positive Bacteria
4.1.1. Mutagenesis or Truncations
4.1.2. Chimeric Endolysins
4.2. Engineering of Endolysins against Gram-Negative Bacteria
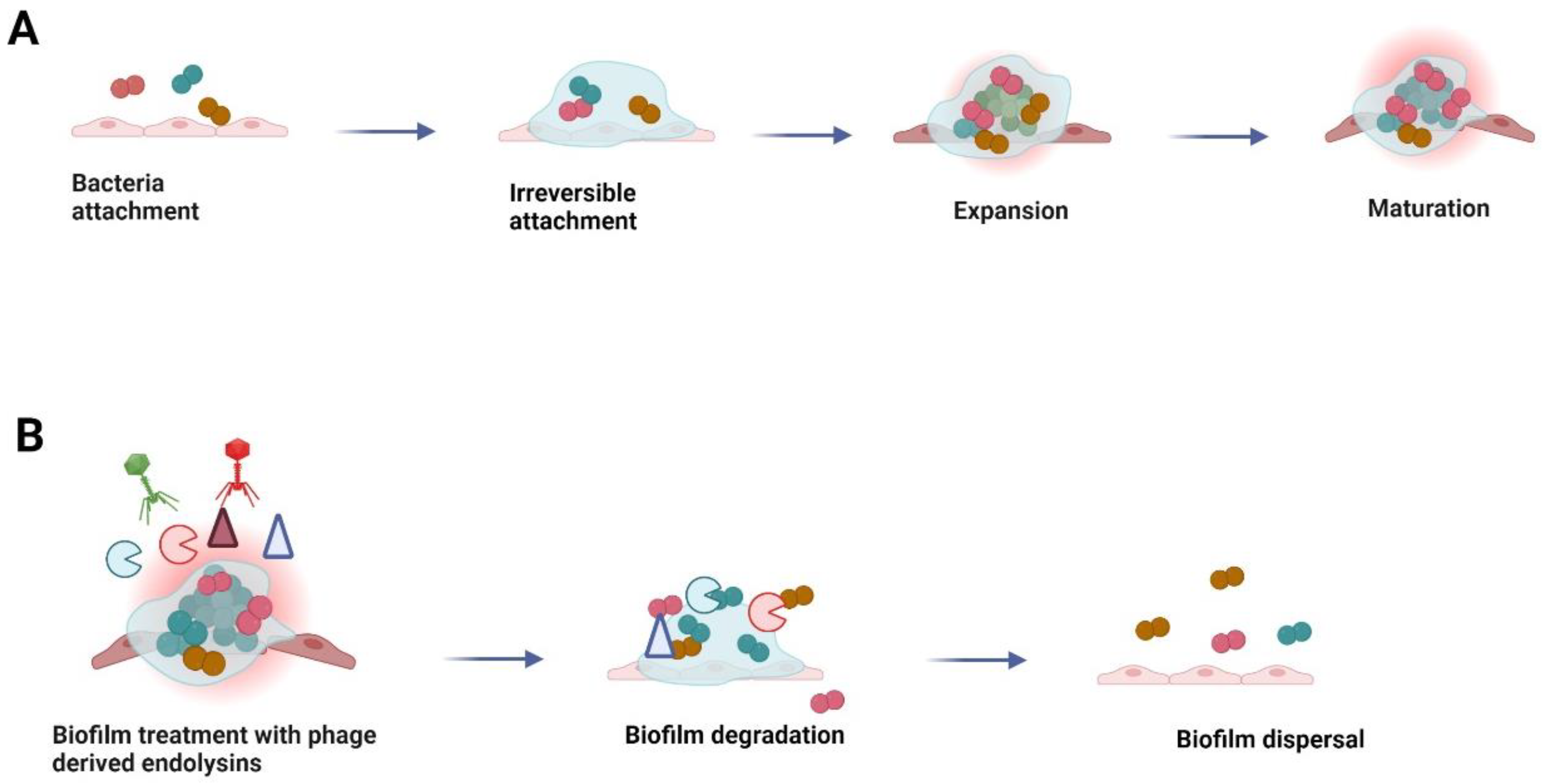
5. Application of Endolysins against Bio-Films
| Host Bacteria | Endolysin | Results | References |
|---|---|---|---|
| S. aureus | lysH5 | Notable staphylococcal biofilm removal activity against persister cells obtained after treatment with rifampicin and ciprofloxacin. | [31] |
| lysCsa13 | Reduction in staphylococcal biofilms mass up to 80–90% on various food utensil surfaces, including polystyrene, stainless steel, and glass. | [103] | |
| S. pyogenes | PlyC | Destruction of biofilm matrixes of S. pyogenes which showed rapid resistance to traditional antibiotics. | [98] |
| Salmonella | lys68 | Synergistic biofilm-reducing effect in combination with malic or citric acid by 1-log CFU. | [100] |
| P. aeruginosa, P. putida | lysPa26 | 2–3 log reduction in viable biofilms of P. aeruginosa 8327 on a polystyrene plate for 48 h. | [101] |
| LysSTG2 | 5 mmol/L EDTA, 100 g/mL LysSTG2 decreased viability by 5.5-log and 3.49-log in 1 h. | [88] | |
| Listeria | PlyLM | Disrupting ability against Listeria biofilms, synergistic effect with a protease. | [102] |
6. Impact of Food Matrix on the Lytic Activity of Endolysin
7. Advantages and Limitations of Endolysins in the Food Industry
8. Fourth Industrial Revolution: Digital Innovations in Food Industry
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAR | Signal-arrest-release |
| EADs | Enzymatically active domains |
| CBDs | Cell-wall binding domains |
| DAP | c-Dglutamyl-m-diaminopimelic acid peptidase |
| CHAP | D-alanyl-glycyl endopeptidase |
| GRAS | Generally consider as safe |
| MRSA | Methicillin resistant termed |
| AI | Artificial intelligence |
| IoT | Internet of things |
| ML | Machine learning |
| FDA | Food and Drug Administration |
| PVPs | Phage virion proteins |
References
- Nazir, A.; Dong, Z.; Liu, J.; Tahir, R.A.; Rasheed, M.; Qing, H.; Peng, D.; Tong, Y. Genomic analysis of bacteriophage Xoo-sp13 infecting Xanthomonas oryzae pv. oryzae. Arch. Virol. 2021, 166, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Dong, Z.; Liu, J.; Zhang, X.; Tahir, R.A.; Ashraf, N.; Qing, H.; Peng, D.; Tong, Y. Sequence analysis of a jumbo bacteriophage, Xoo-sp14, that infects Xanthomonas oryzae pv. oryzae. Microbiol. Resour. Announc. 2020, 9, e01072-20. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Dong, Z.; Liu, J.; Tahir, R.A.; Ashraf, N.; Qing, H.; Peng, D.; Tong, Y. Isolation, Characterization, and Genome Sequence Analysis of a Novel Lytic Phage, Xoo-sp15 Infecting Xanthomonas oryzae pv. oryzae. Curr. Microbiol. 2021, 78, 3192–3200. [Google Scholar] [CrossRef]
- Nazir, A.; Ali, A.; Qing, H.; Tong, Y. Emerging Aspects of Jumbo Bacteriophages. Infect. Drug Resist. 2021, 14, 5041. [Google Scholar] [CrossRef]
- Campbell, A. The future of bacteriophage biology. Nat. Rev. Genet. 2003, 4, 471–477. [Google Scholar] [CrossRef]
- Premaratne, A.; Zhang, H.; Wang, R.; Chinivasagam, N.; Billington, C. Phage biotechnology to mitigate antimicrobial resistance in agriculture. In Sustainable Agriculture Reviews 49: Mitigation of Antimicrobial Resistance Vol 2. Natural and Synthetic Approaches; Springer: Berlin/Heidelberg, Germany, 2021; pp. 313–345. [Google Scholar]
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage–A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef]
- Nazir, A.; Song, J.; Chen, Y.; Liu, Y. Phage-Derived Depolymerase: Its Possible Role for Secondary Bacterial Infections in COVID-19 Patients. Microorganisms 2023, 11, 424. [Google Scholar] [CrossRef]
- Shannon, R.; Radford, D.R.; Balamurugan, S. Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 1631–1640. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E. Phage-encoded endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef]
- Chang, Y. Bacteriophage-derived endolysins applied as potent biocontrol agents to enhance food safety. Microorganisms 2020, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Wei, F.; Yu, F.; Zhao, Z. Bactericidal activity of a holin-endolysin system derived from Vibrio alginolyticus phage HH109. Microb. Pathog. 2021, 159, 105135. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, S.; Dou, J.; Xu, X.; Zhi, Y.; Wen, L. Engineered endolysin-based “artilysins” for controlling the gram-negative pathogen Helicobacter pylori. AMB Express 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Z.; Li, M.; Yang, Y.; Lu, S.; Rao, X. Therapeutic potential of bacteriophage endolysins for infections caused by Gram-positive bacteria. J. Biomed. Sci. 2023, 30, 29. [Google Scholar] [CrossRef]
- Oechslin, F.; Zhu, X.; Dion, M.B.; Shi, R.; Moineau, S. Phage endolysins are adapted to specific hosts and are evolutionarily dynamic. PLoS Biol. 2022, 20, e3001740. [Google Scholar] [CrossRef]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The advantages and challenges of using endolysins in a clinical setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Teixeira, P.; Alvarez-Ordóñez, A.; Nevárez-Moorillón, G.V. Editorial: Microbiological Risks in Food Processing. Front. Sustain. Food Syst. 2021, 4, 630598. [Google Scholar] [CrossRef]
- Xu, Y. Phage and phage lysins: New era of bio-preservatives and food safety agents. J. Food Sci. 2021, 86, 3349–3373. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Ho, M.K.Y.; Zhang, P.; Chen, X.; Xia, J.; Leung, S.S.Y. Bacteriophage endolysins against gram-positive bacteria, an overview on the clinical development and recent advances on the delivery and formulation strategies. Crit. Rev. Microbiol. 2022, 48, 303–326. [Google Scholar] [CrossRef]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Wei, J.; Jiang, L.; Jiang, L.; Sun, Y.; Zeng, Z.; Wang, Z. Phage-inspired strategies to combat antibacterial resistance. Crit. Rev. Microbiol. 2023, 1–16. [Google Scholar] [CrossRef]
- Lai, W.C.B.; Chen, X.; Ho, M.K.Y.; Xia, J.; Leung, S.S.Y. Bacteriophage-derived endolysins to target gram-negative bacteria. Int. J. Pharm. 2020, 589, 119833. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.-H.; Hong, H.-W.; Kim, M.S.; Song, M.; Myung, H. Characterization of Three Different Endolysins Effective against Gram-Negative Bacteria. Viruses 2023, 15, 679. [Google Scholar] [CrossRef]
- Kuty, G.F.; Xu, M.; Struck, D.K.; Summer, E.J.; Young, R. Regulation of a phage endolysin by disulfide caging. J. Bacteriol. 2010, 192, 5682–5687. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.; Young, R. Phage lysis: Multiple genes for multiple barriers. Adv. Virus Res. 2019, 103, 33–70. [Google Scholar]
- Oliveira, H.; Azeredo, J.; Lavigne, R.; Kluskens, L.D. Bacteriophage endolysins as a response to emerging foodborne pathogens. Trends Food Sci. Technol. 2012, 28, 103–115. [Google Scholar] [CrossRef]
- Bai, J.; Kim, Y.-T.; Ryu, S.; Lee, J.-H. Biocontrol and rapid detection of food-borne pathogens using bacteriophages and endolysins. Front. Microbiol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Gutierrez, D.; Ruas-Madiedo, P.; Martinez, B.; Rodriguez, A.; Garcia, P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef]
- Ha, E.; Son, B.; Ryu, S. Clostridium perfringens virulent bacteriophage CPS2 and its thermostable endolysin lysCPS2. Viruses 2018, 10, 251. [Google Scholar] [CrossRef]
- Nazir, A.; Zhao, Y.; Li, M.; Manzoor, R.; Tahir, R.A.; Zhang, X.; Qing, H.; Tong, Y. Structural Genomics of repA, repB1-Carrying IncFIB Family pA1705-qnrS, P911021-tetA, and P1642-tetA, Multidrug-Resistant Plasmids from Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 1889. [Google Scholar] [CrossRef]
- Donovan, D.M.; Lardeo, M.; Foster-Frey, J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol. Lett. 2006, 265, 133–139. [Google Scholar] [CrossRef]
- Schmelcher, M.; Powell, A.M.; Becker, S.C.; Camp, M.J.; Donovan, D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 2012, 78, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Misiou, O.; van Nassau, T.J.; Lenz, C.A.; Vogel, R.F. The preservation of Listeria-critical foods by a combination of endolysin and high hydrostatic pressure. Int. J. Food Microbiol. 2018, 266, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Gutiérrez, D.; Martínez, B.; Rodríguez, A.; García, P. Lytic activity of LysH5 endolysin secreted by Lactococcus lactis using the secretion signal sequence of bacteriocin Lcn972. Appl. Environ. Microbiol. 2012, 78, 3469–3472. [Google Scholar] [CrossRef]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Celia, L.K.; Nelson, D.; Kerr, D.E. Characterization of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet. Microbiol. 2008, 130, 107–117. [Google Scholar] [CrossRef]
- Obeso, J.M.; Martínez, B.; Rodríguez, A.; García, P. Lytic activity of the recombinant staphylococcal bacteriophage ΦH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 2008, 128, 212–218. [Google Scholar] [CrossRef]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Choi, Y.-J.; Koh, W.S.; Moon, K.S.; Kang, S.H. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob. Agents Chemother. 2014, 58, 2084–2088. [Google Scholar] [CrossRef]
- Harhala, M.; Nelson, D.C.; Miernikiewicz, P.; Heselpoth, R.D.; Brzezicka, B.; Majewska, J.; Linden, S.B.; Shang, X.; Szymczak, A.; Lecion, D. Safety studies of pneumococcal endolysins Cpl-1 and Pal. Viruses 2018, 10, 638. [Google Scholar] [CrossRef]
- Rashel, M.; Uchiyama, J.; Ujihara, T.; Uehara, Y.; Kuramoto, S.; Sugihara, S.; Yagyu, K.; Muraoka, A.; Sugai, M.; Hiramatsu, K.; et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J. Infect. Dis. 2007, 196, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Martínez, B.; Rodríguez, L.; Rodríguez, A. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food Microbiol. 2010, 141, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Donovan, D.M.; García, P. Enhanced staphylolytic activity of the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 HydH5 virion-associated peptidoglycan hydrolase: Fusions, deletions, and synergy with LysH5. Appl. Environ. Microbiol. 2012, 78, 2241–2248. [Google Scholar] [CrossRef]
- Chang, Y.; Yoon, H.; Kang, D.-H.; Chang, P.-S.; Ryu, S. Endolysin LysSA97 is synergistic with carvacrol in controlling Staphylococcus aureus in foods. Int. J. Food Microbiol. 2017, 244, 19–26. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, M.; Ryu, S. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiol. 2017, 68, 112–120. [Google Scholar] [CrossRef]
- Schmelcher, M.; Powell, A.M.; Camp, M.J.; Pohl, C.S.; Donovan, D.M. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl. Microbiol. Biotechnol. 2015, 99, 8475–8486. [Google Scholar] [CrossRef]
- Zhang, H.; Bao, H.; Billington, C.; Hudson, J.A.; Wang, R. Isolation and lytic activity of the Listeria bacteriophage endolysin LysZ5 against Listeria monocytogenes in soya milk. Food Microbiol. 2012, 31, 133–136. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; Van Tassell, M.L.; Miller, M.J. Antimicrobial behavior of phage endolysin PlyP100 and its synergy with nisin to control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018, 72, 128–134. [Google Scholar] [CrossRef]
- Lu, R.; Liu, B.; Wu, L.; Bao, H.; García, P.; Wang, Y.; Zhou, Y.; Zhang, H. A Broad-Spectrum Phage Endolysin (LysCP28) Able to Remove Biofilms and Inactivate Clostridium perfringens Strains. Foods 2023, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Endersen, L.; Coffey, A.; Ross, R.P.; McAuliffe, O.; Hill, C.; O’Mahony, J. Characterisation of the antibacterial properties of a bacterial derived peptidoglycan hydrolase (LysCs4), active against C. sakazakii and other Gram-negative food-related pathogens. Int. J. Food Microbiol. 2015, 215, 79–85. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kwon, J.-G.; O’Sullivan, D.J.; Ryu, S.; Lee, J.-H. Development of an endolysin enzyme and its cell wall–binding domain protein and their applications for biocontrol and rapid detection of Clostridium perfringens in food. Food Chem. 2021, 345, 128562. [Google Scholar] [CrossRef]
- Mayer, M.J.; Payne, J.; Gasson, M.J.; Narbad, A. Genomic sequence and characterization of the virulent bacteriophage ΦCTP1 from Clostridium tyrobutyricum and heterologous expression of its endolysin. Appl. Environ. Microbiol. 2010, 76, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Gasson, M.J.; Narbad, A. Genomic sequence of bacteriophage ATCC 8074-B1 and activity of its endolysin and engineered variants against Clostridium sporogenes. Appl. Environ. Microbiol. 2012, 78, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yuan, X.; Li, C.; Chen, N.; Wang, J.; Chen, B.; Yu, S.; Yu, P.; Zhang, J.; Zeng, H. A novel Bacillus cereus bacteriophage DLn1 and its endolysin as biocontrol agents against Bacillus cereus in milk. Int. J. Food Microbiol. 2022, 369, 109615. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Linden, S.B.; Wang, J.; Yu, J.; Nelson, D.C.; Wei, H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Zhang, Q.; Li, M.; Hu, M.; Luo, Y.; Xu, X.; Chen, Y.; Liu, Y. Characterization of the Clostridium perfringens phage endolysin cpp-lys and its application on lettuce. Int. J. Food Microbiol. 2023, 405, 110343. [Google Scholar] [CrossRef]
- Liu, A.; Wang, Y.; Cai, X.; Jiang, S.; Cai, X.; Shen, L.; Liu, Y.; Han, G.; Chen, S.; Wang, J. Characterization of endolysins from bacteriophage LPST10 and evaluation of their potential for controlling Salmonella Typhimurium on lettuce. LWT 2019, 114, 108372. [Google Scholar] [CrossRef]
- Schmelcher, M.; Waldherr, F.; Loessner, M.J. Listeria bacteriophage peptidoglycan hydrolases feature high thermoresistance and reveal increased activity after divalent metal cation substitution. Appl. Microbiol. Biotechnol. 2012, 93, 633–643. [Google Scholar] [CrossRef]
- Fan, J.; Zeng, Z.; Mai, K.; Yang, Y.; Feng, J.; Bai, Y.; Sun, B.; Xie, Q.; Tong, Y.; Ma, J. Preliminary treatment of bovine mastitis caused by Staphylococcus aureus, with trx-SA1, recombinant endolysin of S. aureus bacteriophage IME-SA1. Vet. Microbiol. 2016, 191, 65–71. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Garrido, V.; Fernández, L.; Portilla, S.; Rodríguez, A.; Grilló, M.J.; García, P. Phage lytic protein LysRODI prevents staphylococcal mastitis in mice. Front. Microbiol. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.; Gangakhedkar, R.; Nair, G.; El-Didamony, G.; Askora, A.; Jain, V.; El-Shibiny, A. Pseudomonas Phage ZCPS1 Endolysin as a Potential Therapeutic Agent. Viruses 2023, 15, 520. [Google Scholar] [CrossRef] [PubMed]
- Kazanavičiūtė, V.; Misiūnas, A.; Gleba, Y.; Giritch, A.; Ražanskienė, A. Plant-expressed bacteriophage lysins control pathogenic strains of Clostridium perfringens. Sci. Rep. 2018, 8, 10589. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Schmelcher, M.; Harty, W.J.; Foster-Frey, J.; Donovan, D.M. Chimeric Ply187 endolysin kills Staphylococcus aureus more effectively than the parental enzyme. FEMS Microbiol. Lett. 2013, 342, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Martínez, B.; Donovan, D.M.; García, P.; Rodríguez, A. Potential of the virion-associated peptidoglycan hydrolase HydH5 and its derivative fusion proteins in milk biopreservation. PLoS ONE 2013, 8, e54828. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Ryu, S. Bacteriophage and endolysin engineering for biocontrol of food pathogens/pathogens in the food: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2022, 9, 1–20. [Google Scholar] [CrossRef]
- Cheng, Q.; Fischetti, V.A. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 2007, 74, 1284–1291. [Google Scholar] [CrossRef]
- Horgan, M.; O’Flynn, G.; Garry, J.; Cooney, J.; Coffey, A.; Fitzgerald, G.F.; Ross, R.P.; McAuliffe, O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 2009, 75, 872–874. [Google Scholar] [CrossRef]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011, 286, 34391–34403. [Google Scholar] [CrossRef]
- Mayer, M.J.; Garefalaki, V.; Spoerl, R.; Narbad, A.; Meijers, R. Structure-based modification of a Clostridium difficile-targeting endolysin affects activity and host range. J. Bacteriol. 2011, 193, 5477–5486. [Google Scholar] [CrossRef]
- Díez-Martínez, R.; de Paz, H.; Bustamante, N.; García, E.; Menéndez, M.; García, P. Improving the lethal effect of Cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef]
- Love, M.J.; Coombes, D.; Manners, S.H.; Abeysekera, G.S.; Billington, C.; Dobson, R.C. The molecular basis for Escherichia coli O157: H7 phage FAHEc1 endolysin function and protein engineering to increase thermal stability. Viruses 2021, 13, 1101. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Bao, H.; Wang, X.; Wang, R. The lytic activity of recombinant phage lysin LysKΔamidase against staphylococcal strains associated with bovine and human infections in the Jiangsu province of China. Res. Vet. Sci. 2017, 111, 113–119. [Google Scholar] [CrossRef]
- Donovan, D.M.; Rubio, L.R.; Fernandez, B.M.; Rodriguez, A.; Suarez, P.G. Enhanced Staphylolytic Activity of the Staphylococcus Aureus Bacteriophage vB—SauS-philPLA88 Virion-Associated Peptidoglycan Hydrolase HydH5: Fusions, Deletions and Synergy with LysH5. Patent No. US 8,986,695 B2, 24 March 2015. [Google Scholar]
- Swift, S.M.; Seal, B.S.; Garrish, J.K.; Oakley, B.B.; Hiett, K.; Yeh, H.-Y.; Woolsey, R.; Schegg, K.M.; Line, J.E.; Donovan, D.M. A thermophilic phage endolysin fusion to a Clostridium perfringens-specific cell wall binding domain creates an anti-Clostridium antimicrobial with improved thermostability. Viruses 2015, 7, 3019–3034. [Google Scholar] [CrossRef]
- Son, B.; Kong, M.; Lee, Y.; Ryu, S. Development of a novel chimeric endolysin, Lys109 with enhanced lytic activity against Staphylococcus aureus. Front. Microbiol. 2021, 11, 615887. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.; Son, B.; Ryu, S. Development of advanced chimeric endolysin to control multidrug-resistant Staphylococcus aureus through domain shuffling. ACS Infect. Dis. 2021, 7, 2081–2092. [Google Scholar] [CrossRef]
- Endersen, L.; Guinane, C.M.; Johnston, C.; Neve, H.; Coffey, A.; Ross, R.P.; McAuliffe, O.; O’Mahony, J. Genome analysis of Cronobacter phage vB_CsaP_Ss1 reveals an endolysin with potential for biocontrol of Gram-negative bacterial pathogens. J. Gen. Virol. 2015, 96, 463–477. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P. Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. MBio 2014, 5, e01314–e01379. [Google Scholar] [CrossRef]
- Zampara, A.; Sørensen, M.C.H.; Grimon, D.; Antenucci, F.; Vitt, A.R.; Bortolaia, V.; Briers, Y.; Brøndsted, L. Exploiting phage receptor binding proteins to enable endolysins to kill Gram-negative bacteria. Sci. Rep. 2020, 10, 12087. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yang, E.; Chang, P.-S.; Ryu, S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzym. Microb. Technol. 2019, 128, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Novel biocontrol methods for Listeria monocytogenes biofilms in food production facilities. Front. Microbiol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Ling, N.; Forsythe, S.; Wu, Q.; Ding, Y.; Zhang, J.; Zeng, H. Insights into Cronobacter sakazakii biofilm formation and control strategies in the food industry. Engineering 2020, 6, 393–405. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Abee, T.; Kovács, Á.T.; Kuipers, O.P.; Van der Veen, S. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 2011, 22, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, H.-H.; Duc, H.M.; Masuda, Y.; Honjoh, K.-i.; Miyamoto, T. Application of endolysin LysSTG2 as a potential biocontrol agent against planktonic and biofilm cells of Pseudomonas on various food and food contact surfaces. Food Control 2022, 131, 108460. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.-H.; Duc, H.M.; Masuda, Y.; Honjoh, K.-i.; Miyamoto, T. Endolysin LysSTG2: Characterization and application to control Salmonella Typhimurium biofilm alone and in combination with slightly acidic hypochlorous water. Food Microbiol. 2021, 98, 103791. [Google Scholar] [CrossRef]
- Cooper, I. A review of the potential for bacteriophages to effect anti-biofilm activity, using selected examples. J. Appl. Microbiol. 2022, 134, lxac056. [Google Scholar] [CrossRef]
- Sass, P.; Bierbaum, G. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007, 73, 347–352. [Google Scholar] [CrossRef]
- Son, J.-S.; Lee, S.-J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.-J. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl. Microbiol. Biotechnol. 2010, 86, 1439–1449. [Google Scholar] [CrossRef]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Oh, M.-D.; Choi, Y.-J.; Lee, W.J.; Kong, J.-C.; Seol, J.G.; Kang, S.H. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int. J. Antimicrob. Agents 2013, 41, 156–161. [Google Scholar] [CrossRef]
- Linden, S.B.; Zhang, H.; Heselpoth, R.D.; Shen, Y.; Schmelcher, M.; Eichenseher, F.; Nelson, D.C. Biochemical and biophysical characterization of PlyGRCS, a bacteriophage endolysin active against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2015, 99, 741–752. [Google Scholar] [CrossRef]
- Fenton, M.; Keary, R.; McAuliffe, O.; Ross, R.P.; O’Mahony, J.; Coffey, A. Bacteriophage-derived peptidase eliminates and prevents Staphylococcal biofilms. Int. J. Microbiol. 2013, 2013, 625341. [Google Scholar] [CrossRef]
- Cha, Y.; Son, B.; Ryu, S. Effective removal of staphylococcal biofilms on various food contact surfaces by Staphylococcus aureus phage endolysin LysCSA13. Food Microbiol. 2019, 84, 103245. [Google Scholar] [CrossRef]
- Meng, X.; Shi, Y.; Ji, W.; Meng, X.; Zhang, J.; Wang, H.; Lu, C.; Sun, J.; Yan, Y. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011, 77, 8272–8279. [Google Scholar] [CrossRef]
- Shen, Y.; Köller, T.; Kreikemeyer, B.; Nelson, D.C. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother. 2013, 68, 1818–1824. [Google Scholar] [CrossRef]
- Pennone, V.; Sanz-Gaitero, M.; O’Connor, P.; Coffey, A.; Jordan, K.; van Raaij, M.J.; McAuliffe, O. Inhibition of L. monocytogenes biofilm formation by the amidase domain of the phage vB_LmoS_293 endolysin. Viruses 2019, 11, 722. [Google Scholar] [CrossRef]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A novel antimicrobial endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Simmons, M.; Morales, C.A.; Oakley, B.B.; Seal, B.S. Recombinant expression of a putative amidase cloned from the genome of Listeria monocytogenes that lyses the bacterium and its monolayer in conjunction with a protease. Probiotics Antimicrob. Proteins 2012, 4, 1–10. [Google Scholar] [CrossRef]
- Cha, Y.; Chun, J.; Son, B.; Ryu, S. Characterization and genome analysis of Staphylococcus aureus podovirus CSA13 and its anti-biofilm capacity. Viruses 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Tsoukleris, D.; Panagou, E.; Falaras, P.; Nychas, G.-J. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiol. 2011, 28, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Tchang, V.S.; Loessner, M.J. Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb. Biotechnol. 2011, 4, 651–662. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Ibarra-Sánchez, L.A.; Hoepker, G.P.; Miller, M.J. Hot topic: Antilisterial activity by endolysin PlyP100 in fresh cheese. J. Dairy Sci. 2017, 100, 2482–2487. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.; Wang, J.; Li, M. Characteristics of two lysis-related proteins from a Shewanella putrefaciens phage with high lytic activity and wide Spectrum. J. Food Prot. 2017, 81, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Llull, D.; López, R.; García, E. Skl, a novel choline-binding N-acetylmuramoyl-L-alanine amidase of Streptococcus mitis SK137 containing a CHAP domain. FEBS Lett. 2006, 580, 1959–1964. [Google Scholar] [CrossRef]
- Kwok, R. Five hard truths for synthetic biology: Can engineering approaches tame the complexity of living systems? Roberta Kwok explores five challenges for the field and how they might be resolved. Nature 2010, 463, 288–291. [Google Scholar] [CrossRef]
- Wittmann, J.; Brancato, C.; Berendzen, K.; Dreiseikelmann, B. Development of a tomato plant resistant to Clavibacter michiganensis using the endolysin gene of bacteriophage CMP1 as a transgene. Plant Pathol. 2016, 65, 496–502. [Google Scholar] [CrossRef]
- Hassoun, A.; Aït-Kaddour, A.; Abu-Mahfouz, A.M.; Rathod, N.B.; Bader, F.; Barba, F.J.; Biancolillo, A.; Cropotova, J.; Galanakis, C.M.; Jambrak, A.R. The fourth industrial revolution in the food industry—Part I: Industry 4.0 technologies. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Saha, D.; Manickavasagan, A. Machine learning techniques for analysis of hyperspectral images to determine quality of food products: A review. Curr. Res. Food Sci. 2021, 4, 28–44. [Google Scholar] [CrossRef]
- Macdonald, G.J. Stars in Alignment for Artificial Intelligence in Bioprocessing: Sensor data, mathematical models, process analytical technology, and regulatory initiatives are in place to bring about an AI revolution. Genet. Eng. Biotechnol. News 2021, 41, 40–42, 44. [Google Scholar] [CrossRef]
- Ren, Q.-S.; Fang, K.; Yang, X.-T.; Han, J.-W. Ensuring the quality of meat in cold chain logistics: A comprehensive review. Trends Food Sci. Technol. 2022, 119, 133–151. [Google Scholar] [CrossRef]
- Kayikci, Y.; Subramanian, N.; Dora, M.; Bhatia, M.S. Food supply chain in the era of Industry 4.0: Blockchain technology implementation opportunities and impediments from the perspective of people, process, performance, and technology. Prod. Plan. Control 2022, 33, 301–321. [Google Scholar] [CrossRef]
- Abeysekera, G.S.; Love, M.J.; Manners, S.H.; Billington, C.; Dobson, R.C. Bacteriophage-encoded lethal membrane disruptors: Advances in understanding and potential applications. Front. Microbiol. 2022, 13, 1044143. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zeng, X.; Cao, J.; Jiang, W. Application of blockchain technology in food safety control: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 2800–2819. [Google Scholar] [CrossRef]
- Barman, R.K.; Chakrabarti, A.K.; Dutta, S. Prediction of Phage Virion Proteins Using Machine Learning Methods. Molecules 2023, 28, 2238. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, A.; Xu, X.; Liu, Y.; Chen, Y. Phage Endolysins: Advances in the World of Food Safety. Cells 2023, 12, 2169. https://doi.org/10.3390/cells12172169
Nazir A, Xu X, Liu Y, Chen Y. Phage Endolysins: Advances in the World of Food Safety. Cells. 2023; 12(17):2169. https://doi.org/10.3390/cells12172169
Chicago/Turabian StyleNazir, Amina, Xiaohui Xu, Yuqing Liu, and Yibao Chen. 2023. "Phage Endolysins: Advances in the World of Food Safety" Cells 12, no. 17: 2169. https://doi.org/10.3390/cells12172169
APA StyleNazir, A., Xu, X., Liu, Y., & Chen, Y. (2023). Phage Endolysins: Advances in the World of Food Safety. Cells, 12(17), 2169. https://doi.org/10.3390/cells12172169







