Effects of Growth Factors on In Vitro Culture of Neonatal Piglet Testicular Tissue Fragments
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Testis Collection and Preparation
2.3. Culture of Tissue Fragments
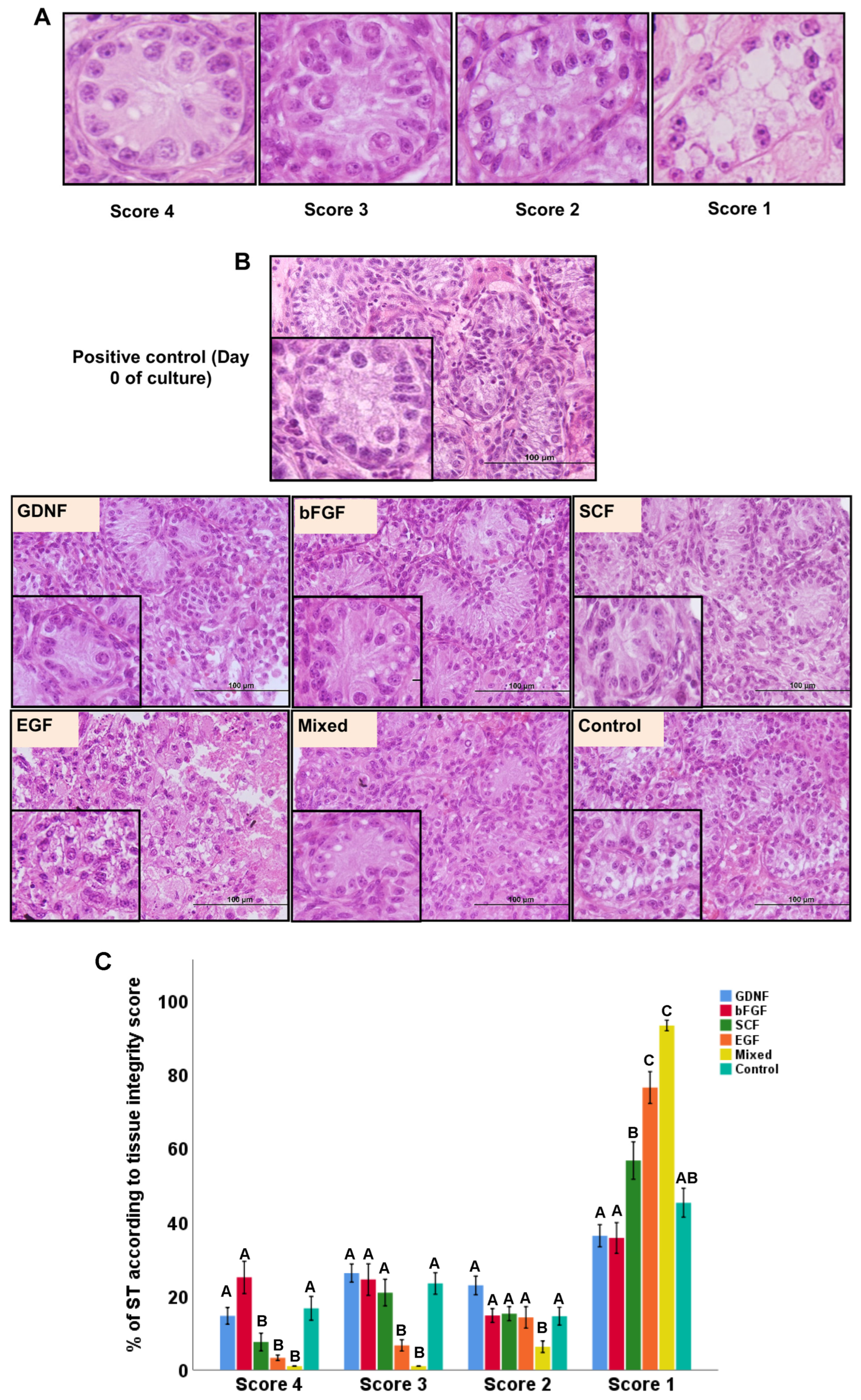
2.4. Histological Examination of Cultured Testicular Tissue Fragments
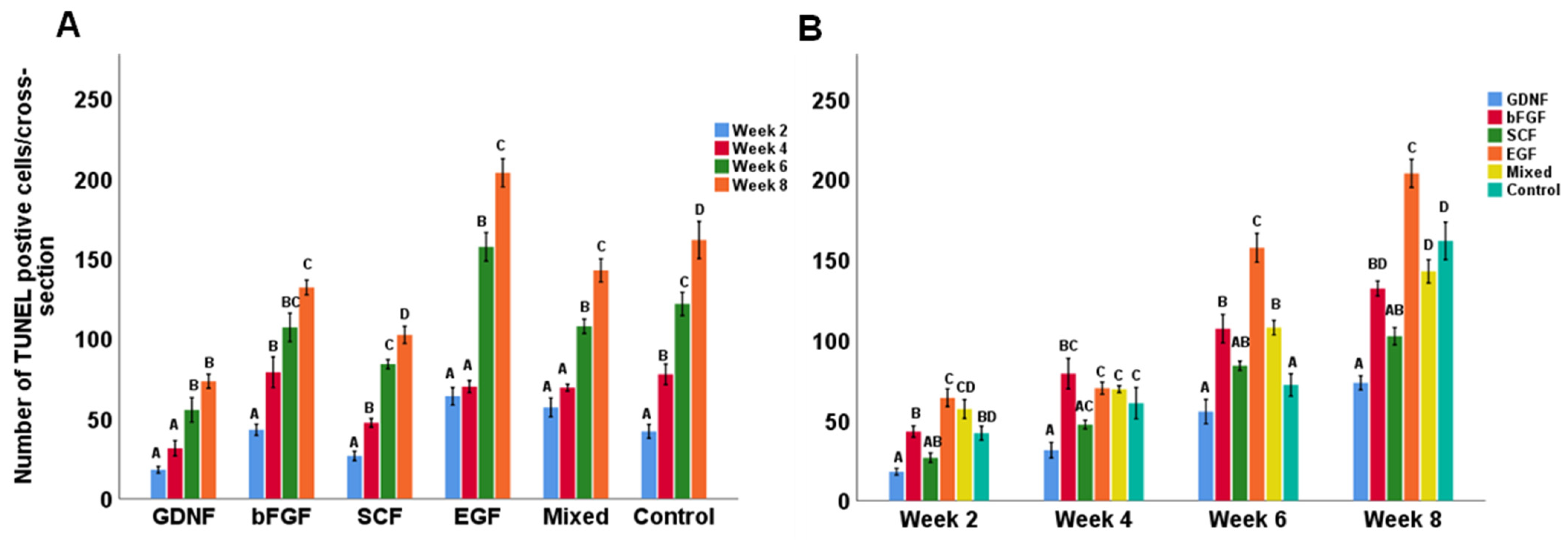
2.5. Quantification of Apoptotic Cells
2.6. Immunofluorescence (IF) and Immunohistochemistry (IHC)
2.7. Analysis of IF and IHC Images
2.8. Statistical Analysis
3. Results
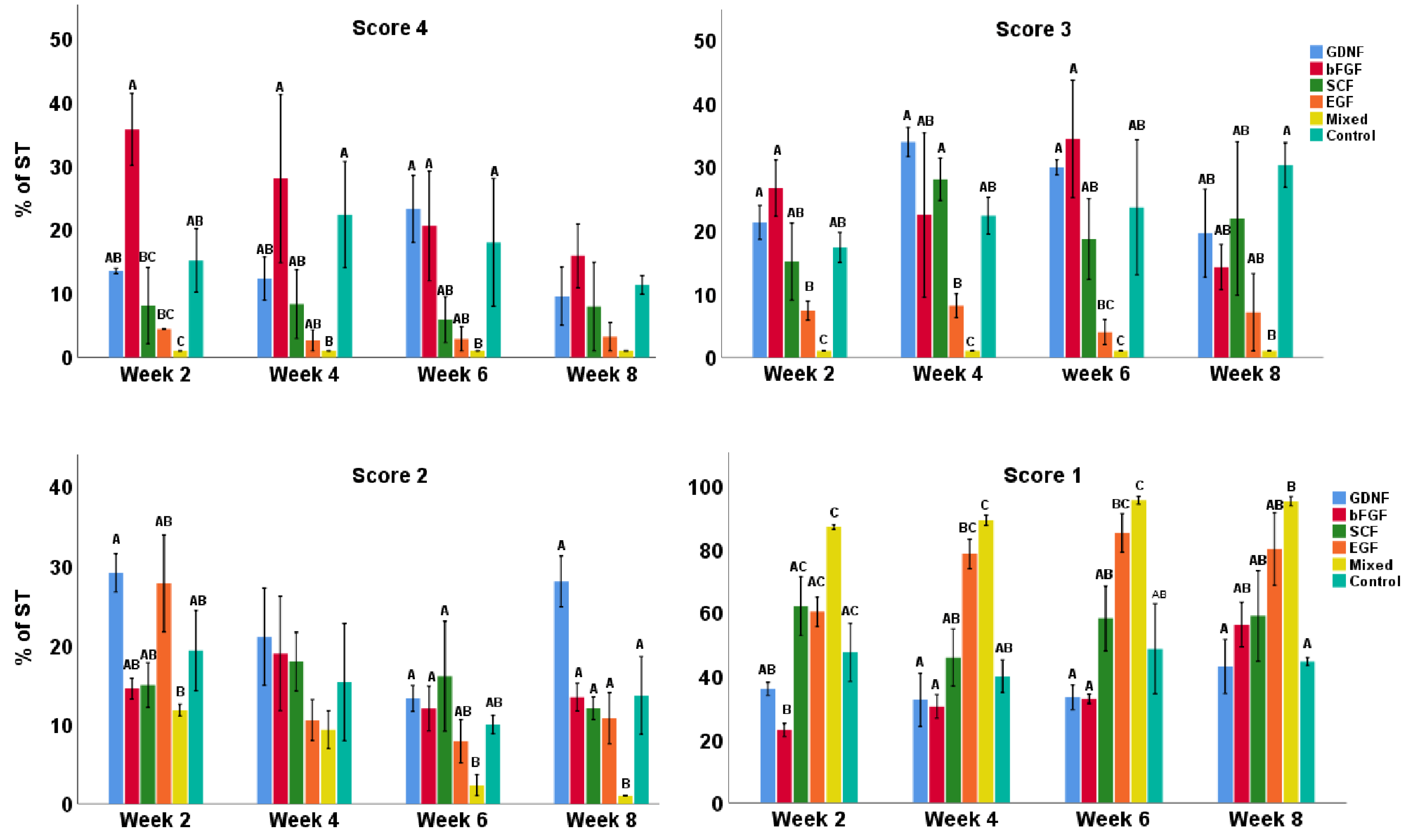
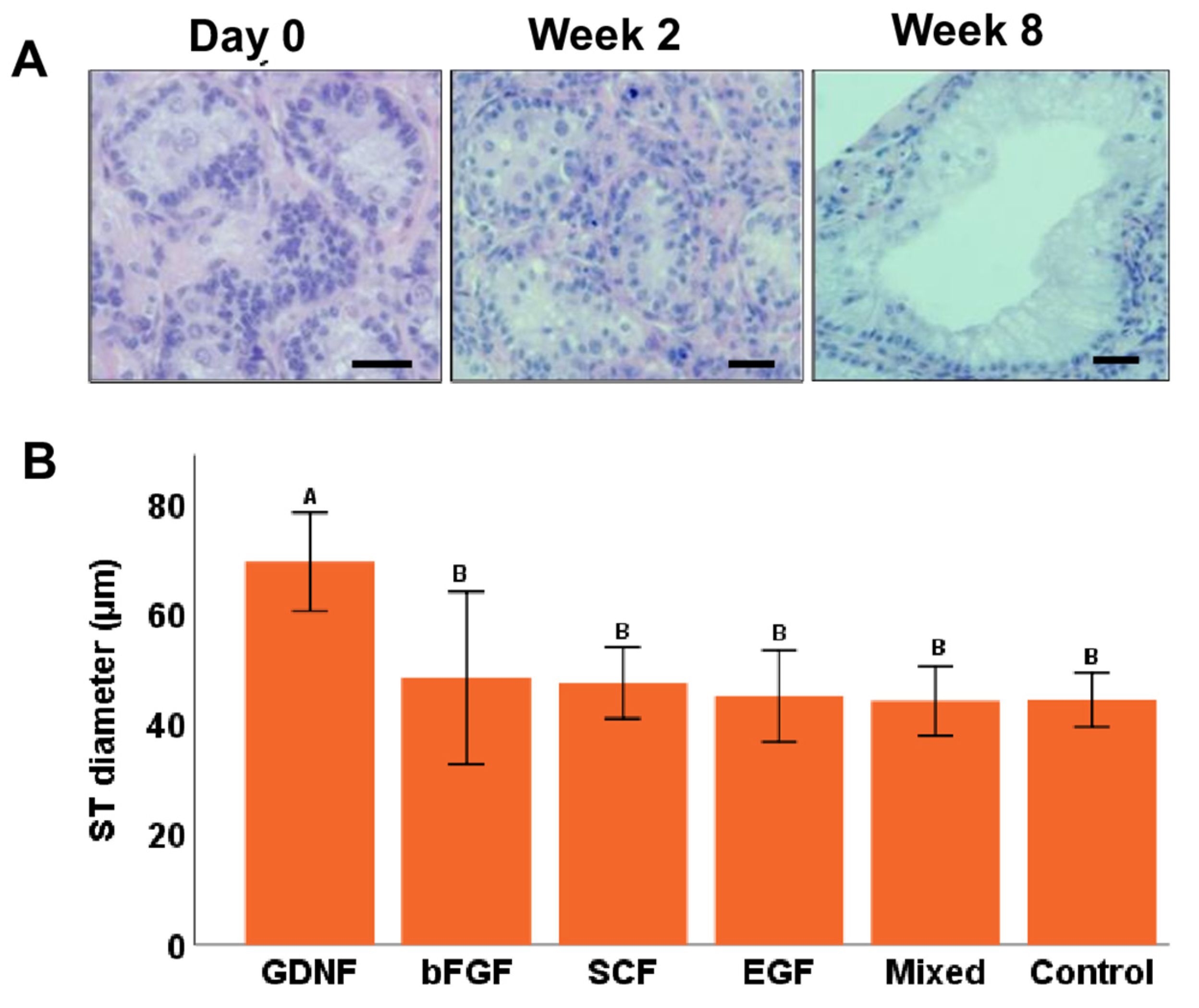
3.1. Tubular Integrity of Testicular Tissue Fragments during Culture
3.2. Cell Apoptosis during Culture of Testicular Tissue Fragments
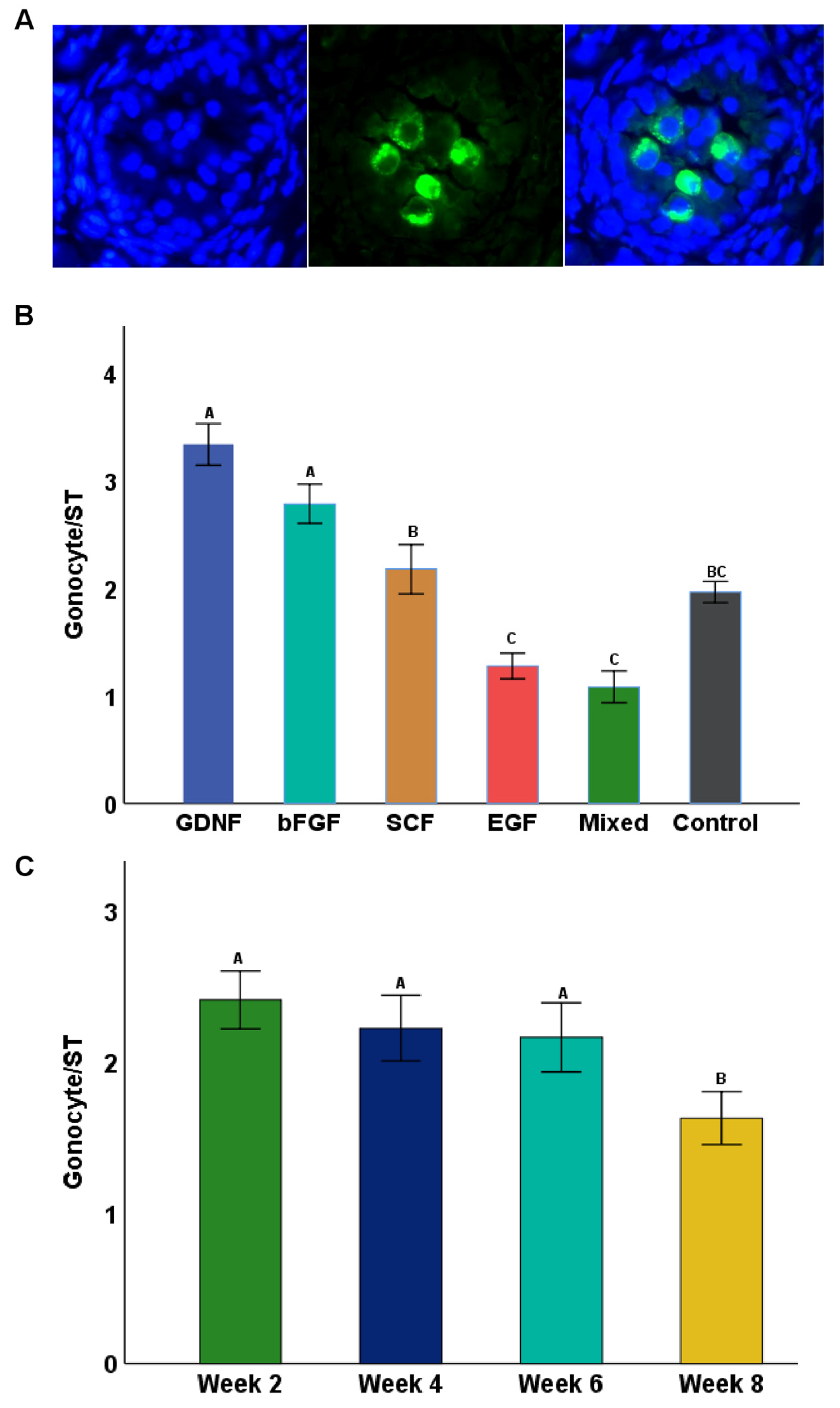
3.3. Seminiferous Tubule Diameters (μm) and Gonocyte Populations during Culture
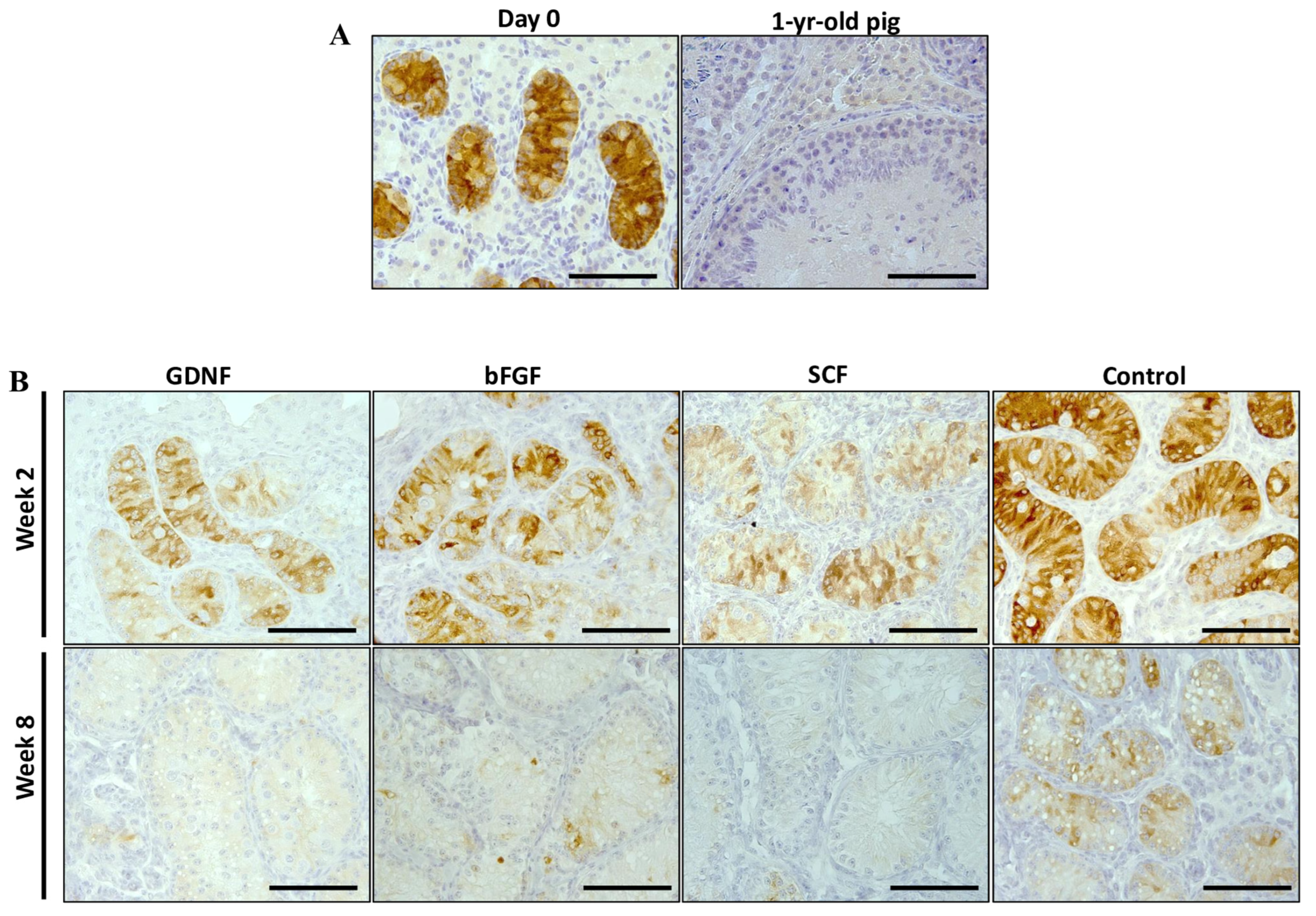
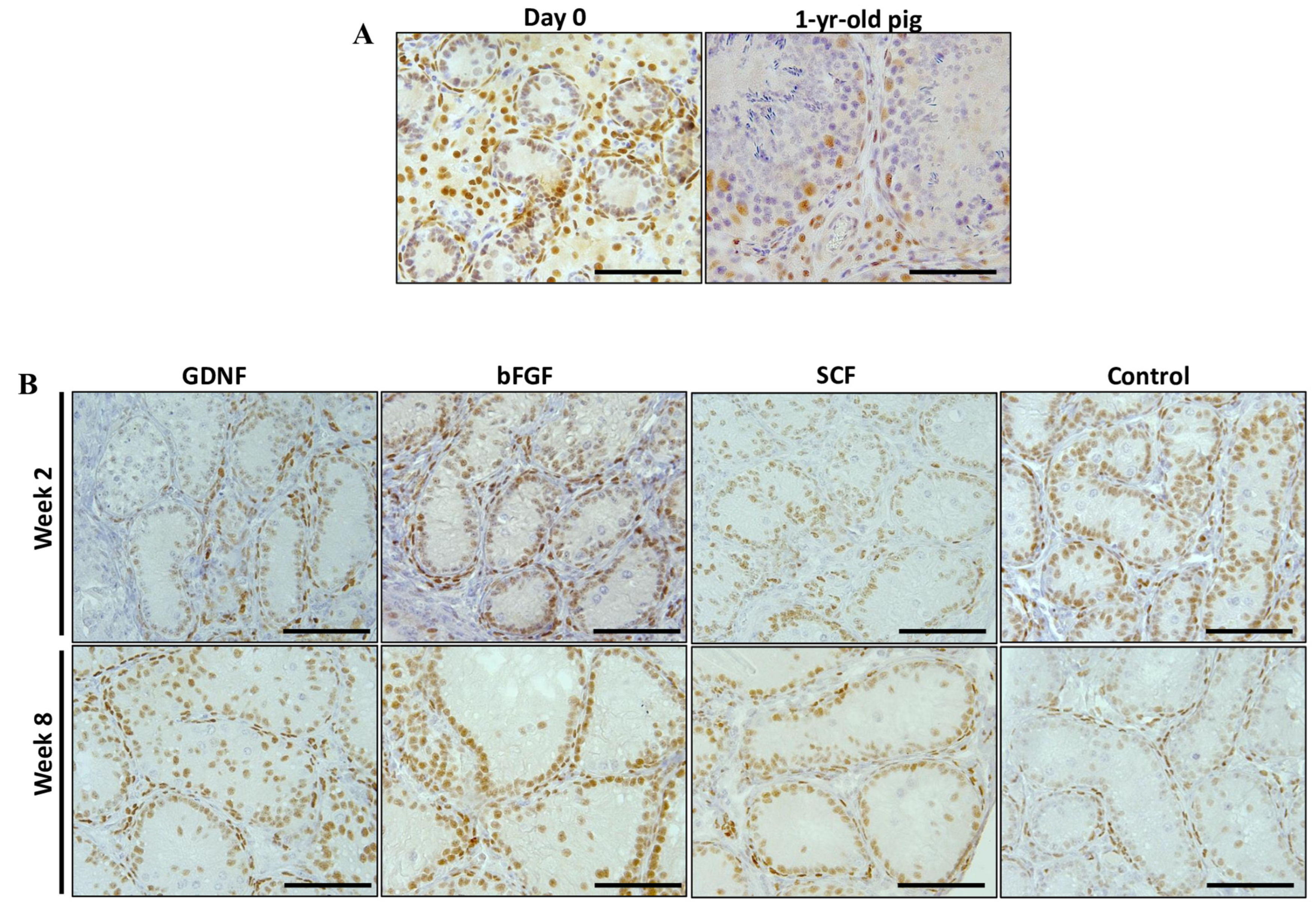
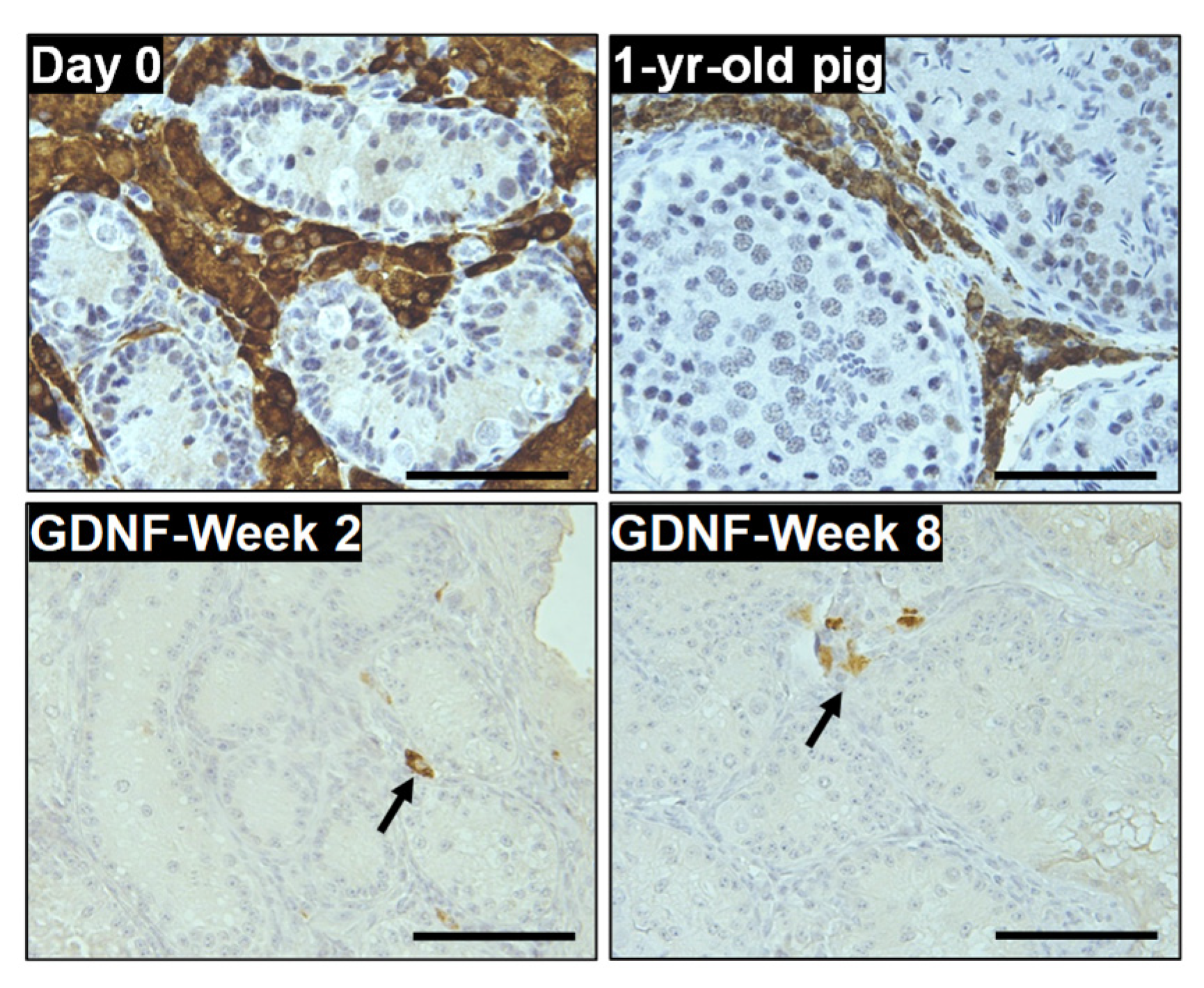
3.4. Maturation of Sertoli Cells
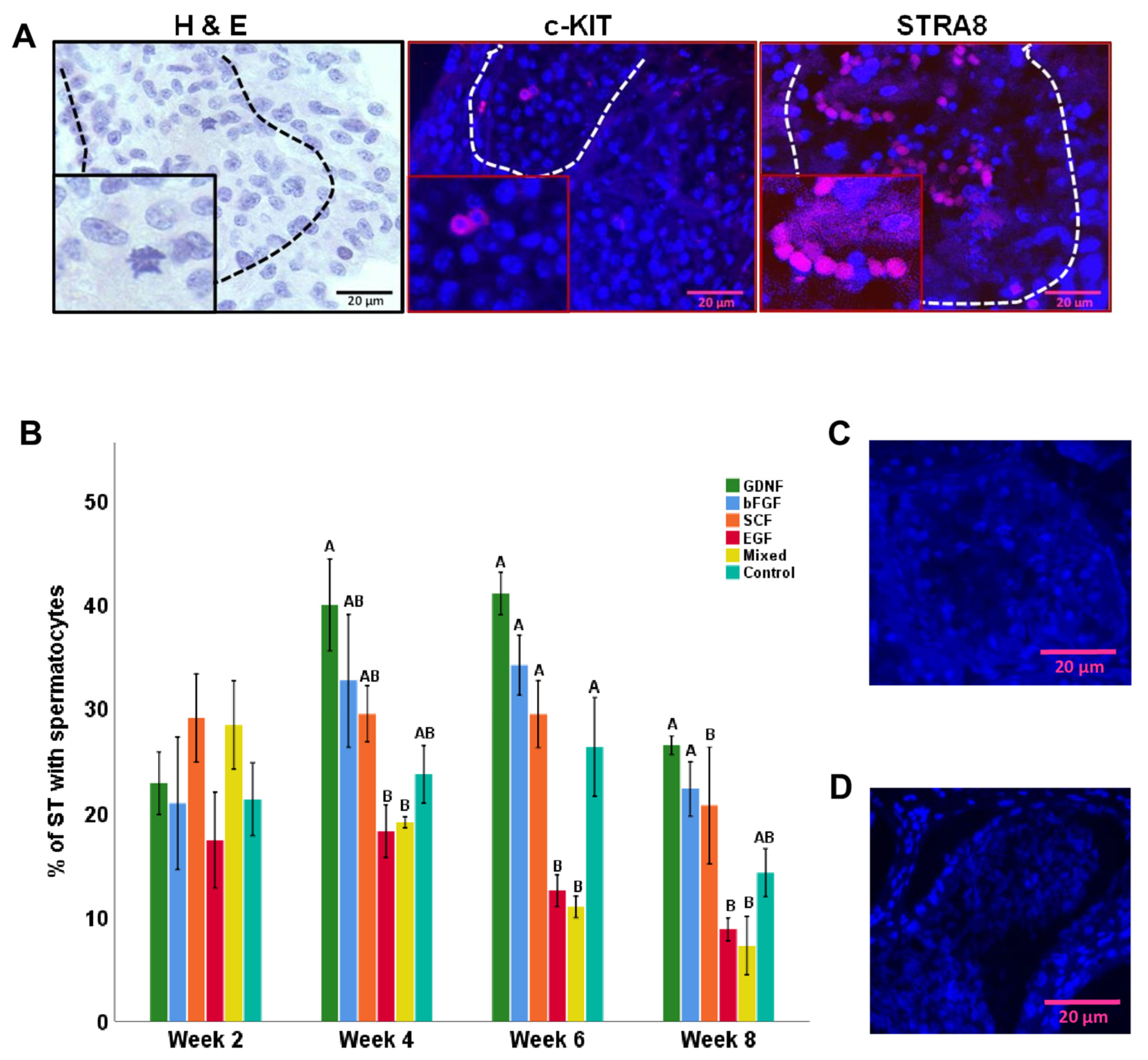
3.5. Differentiation of Germ Cells during Culture of Testicular Tissue Fragments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibtisham, F.; Honaramooz, A. Spermatogonial stem cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef]
- Aoshima, K.; Baba, A.; Makino, Y.; Okada, Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS ONE 2013, 8, e77715. [Google Scholar] [CrossRef] [PubMed]
- Oatley, M.J.; Kaucher, A.V.; Yang, Q.; Waqas, M.S.; Oatley, J.M. Conditions for long-term culture of cattle undifferentiated spermatogonia 1. Biol. Reprod. 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Han, S.Y.; Gupta, M.K.; Uhm, S.J.; Lee, H.T. Isolation and in vitro culture of pig spermatogonial stem cell. Asian-Australas. J. Anim. Sci. 2009, 22, 187–193. [Google Scholar] [CrossRef]
- Fayaz, M.A.; Ibtisham, F.; Cham, T.C.; Honaramooz, A. Culture supplementation of bFGF, GDNF, and LIF alters in vitro proliferation, colony formation, and pluripotency of neonatal porcine germ cells. Cell Tissue Res. 2022, 388, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Cham, T.-C.; Fayaz, M.A.; Honaramooz, A. Long-term in vitro maintenance of piglet testicular tissue: Effects of tissue fragment size, preparation method, and serum source. Animals 2022, 13, 128. [Google Scholar] [CrossRef]
- Fa, R.; Qian, F.; Huaming, X.; Tianyu, F.; Liqiang, W.; Jianhong, H. Platelet-derived growth factor-BB and epidermal growth factor promote dairy goat spermatogonial stem cells proliferation via Ras/ERK1/2 signaling pathway. Theriogenology 2020, 155, 205–212. [Google Scholar]
- Bahadorani, M.; Hosseini, S.M.; Abedi, P.; Hajian, M.; Hosseini, S.E.; Vahdati, A.; Baharvand, H.; Nasr-Esfahani, M.H. Short-term in-vitro culture of goat enriched spermatogonial stem cells using different serum concentrations. J. Assist. Reprod. Genet. 2012, 29, 39–46. [Google Scholar] [CrossRef]
- Pramod, R.K.; Mitra, A. In vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J. Assist. Reprod. Genet. 2014, 31, 993–1001. [Google Scholar] [CrossRef]
- Bahadorani, M.; Hosseini, S.M.; Abedi, P.; Abbasi, H.; Hosseini, S.M.; Abedi, P.; Abbasi, H.; Bahadorani, M.; Hosseini, S.M.; Abedi, P.; et al. Glial cell line-derived neurotrophic factor in combination with insulin-like growth factor 1 and basic fibroblast growth factor promote in vitro culture of goat spermatogonial stem cells insulin-like growth factor 1 and basic fibroblast growth factor. Growth Factors 2015, 33, 181–191. [Google Scholar] [CrossRef]
- Rafeeqi, T.; Kaul, G. Isolation and enrichment of type A spermatogonia from pre-pubertal buffalo (Bubalus bubalis) testis. Andrologia 2013, 45, 195–203. [Google Scholar] [CrossRef]
- Kala, S.; Kaushik, R.; Singh, K.P.; Kadam, P.H.; Singh, M.K.; Manik, R.S.; Singla, S.K.; Palta, P.; Chauhan, M.S. In vitro culture and morphological characterization of prepubertal buffalo (Bubalus bubalis) putative spermatogonial stem cell. J. Assist. Reprod. Genet. 2012, 29, 1335–1342. [Google Scholar] [CrossRef][Green Version]
- Kadam, P.H.; Kala, S.; Agrawal, H.; Singh, K.P.; Singh, M.K.; Chauhan, M.S.; Palta, P.; Singla, S.K.; Manik, R.S. Effects of glial cell line-derived neurotrophic factor, fibroblast growth factor 2 and epidermal growth factor on proliferation and the expression of some genes in buffalo (Bubalus bubalis) spermatogonial cells. Reprod. Fertil. Dev. 2013, 25, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Qasemi-Panahi, B.; Movahedin, M.; Moghaddam, G.; Tajik, P.; Koruji, M.; Ashrafi-Helan, J.; Rafat, S.A. Isolation and proliferation of spermatogonial cells from ghezel sheep. Avicenna J. Med. Biotechnol. 2018, 10, 93–97. [Google Scholar] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Awang-Junaidi, A.H.; Honaramooz, A. The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res. 2020, 380, 393–414. [Google Scholar] [CrossRef]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef]
- Hofmann, M.-C.; Braydich-Stolle, L.; Dym, M. Isolation of male germ-line stem cells; influence of GDNF. Dev. Biol. 2005, 279, 114–124. [Google Scholar] [CrossRef]
- Meng, X.; Lindahl, M.; Hyvönen, M.E.; Parvinen, M.; de Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M.; et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493. [Google Scholar] [CrossRef]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Suter-Crazzolara, C.; Unsicker, K. GDNF is expressed in two forms in many tissues outside the CNS. Neuroreport 1994, 5, 2486–2488. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C. GDNF stimulates the proliferation of cultured mouse immature Sertoli cells via its receptor subunit NCAM and ERK1/2 signaling pathway. BMC Cell Biol. 2010, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.-C. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol. Cell Endocrinol. 2008, 288, 95–103. [Google Scholar] [CrossRef]
- Sharma, M.; Braun, R.E. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. Development 2018, 145, dev.151555. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.; Nolan, C.; Dym, M.; Marie-Claude, H. Role of glial cell line-derived neurotrophic factor in germ-line stem cell fate. Ann. N. Y Acad. Sci. 2005, 1061, 94–99. [Google Scholar] [CrossRef]
- Simon, L.; Ekman, G.C.; Tyagi, G.; Hess, R.A.; Murphy, K.M.; Cooke, P.S. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp. Cell Res. 2007, 313, 3090–3099. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Muneto, T.; Lee, J.; Takenaka, M.; Chuma, S.; Nakatsuji, N.; Horiuchi, T.; Shinohara, T. Long-term culture of male germline stem cells from hamster testes. Biol. Reprod. 2008, 78, 611–617. [Google Scholar] [CrossRef]
- Moghal, N.; Sternberg, P.W. Multiple positive and negative regulators of signaling by the EGF-receptor. Curr. Opin. Cell Biol. 1999, 11, 190–196. [Google Scholar] [CrossRef]
- Yan, Y.C.; Sun, Y.P.; Zhang, M.L.; Koide, S.S. Testis epidermal growth factor and spermatogenesis. Arch. Androl. 1998, 40, 133–146. [Google Scholar] [CrossRef]
- Lee, R.; Park, H.-J.; Lee, W.-Y.; Han, M.-G.; Park, J.H.; Moon, J.; Kwon, D.A.; Song, H. Effect of epidermal growth factor on the colony-formation ability of porcine spermatogonial germ cells. Biotechnol. Bioprocess Eng. 2021, 26, 677–687. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Oke, B.O.; Papadopoulos, V.; DiAugustine, R.P.; Suarez-Quian, C.A. Characterization of epidermal growth factor in mouse testis. Endocrinology 1992, 131, 3091–3099. [Google Scholar] [CrossRef]
- Hakovirta, H.; Yan, W.; Kaleva, M.; Zhang, F.; Vänttinen, K.; Morris, P.L.; Söder, M.; Parvinen, M.; Toppari, J. Function of stem cell factor as a survival factor of spermatogonia and localization of messenger ribonucleic acid in the rat seminiferous epithelium. Endocrinology 1999, 140, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Nishikawa, S.; Ogawa, M.; Hayashi, S.; Kunisada, T.; Fujimoto, T.; Nishikawa, S. Role of c-kit in mouse spermatogenesis: Identification of spermatogonia as a specific site of c-kit expression and function. Development 1991, 113, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Kero, J.; Huhtaniemi, I.; Toppari, J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: Implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev. Biol. 2000, 227, 169–182. [Google Scholar] [CrossRef][Green Version]
- Azizi, H.; Conrad, S.; Hinz, U.; Asgari, B.; Nanus, D.; Peterziel, H.; Hajizadeh Moghaddam, A.; Baharvand, H.; Skutella, T. Derivation of Pluripotent Cells from Mouse SSCs Seems to Be Age Dependent. Stem Cells Int. 2016, 2016, 8216312. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Farrace, M.G.; Piacentini, M.; Dolci, S.; De Felici, M.; DeChiara, T.M.; Yancopoulous, G.D. Stem cell factor and leukemia inhibitory factor promote primordial germ cell survival by suppressing programmed cell death (apoptosis). Development 1993, 118, 1089–1094. [Google Scholar] [CrossRef]
- Packer, A.I.; Besmer, P.; Bachvarova, R.F. Kit ligand mediates survival of type A spermatogonia and dividing spermatocytes in postnatal mouse testes. Mol. Reprod. Dev. 1995, 42, 303–310. [Google Scholar] [CrossRef]
- Awang-Junaidi, A.H.; Fayaz, M.A.; Goldstein, S.; Honaramooz, A. Brief exposure of neonatal testis cells to EGF or GDNF alters the regenerated tissue. Reprod. Fertil. 2022, 3, 39–56. [Google Scholar] [CrossRef]
- Awang-Junaidi, A.H.; Fayaz, M.A.; Goldstein, S.; Honaramooz, A. Using a testis regeneration model, FGF9, LIF, and SCF improve testis cord formation while RA enhances gonocyte survival. Cell Tissue Res. 2022, 389, 351–370. [Google Scholar] [CrossRef]
- Wittenburg, G.; Volkel, C.; Mai, R.; Lauer, G. Immunohistochemical comparison of differentiation markers on paraffin and plastic embedded human bone samples. J. Physiol. Pharmacol. 2009, 60 (Suppl. S8), 43–49. [Google Scholar]
- Polkowska, J.; Wańkowska, M.; Wójcik-Gładysz, A. Expression of NPY-immunoreactive neurons in the hypothalamus of the cycling ewe. Folia Histochem. Cytobiol. 2006, 44, 13–16. [Google Scholar] [PubMed]
- Ibtisham, F.; Zhao, Y.; Nawab, A.; Wu, J.; Mei, X.; Honaramooz, A.; An, L. In vitro production of haploid germ cells from murine spermatogonial stem cells using a two-dimensional cell culture system. Theriogenology 2021, 162, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Nie, J.; Zhu, X.; Lu, Y.; Liang, X.; Xu, H.; Yang, X.; Zhang, Y.; Lu, K.; Lu, S. In vitro differentiation of spermatogonial stem cells using testicular cells from Guangxi Bama mini-pig. J. Vet. Sci. 2018, 19, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, Y.; Zhang, B.L.; Wu, H.Y.; Jiang, W.Q.; Wang, S.T.; Han, D.P.; Liu, Y.X.; Lian, Z.X.; Deng, S.L. In-vitro differentiation of early pig spermatogenic cells to haploid germ cells. Mol. Hum. Reprod. 2019, 25, 507–518. [Google Scholar] [CrossRef]
- Jaillard, C.; Chatelain, P.G.; Saez, J.M. In vitro regulation of pig Sertoli cell growth and function: Effects of fibroblast growth factor and somatomedin-C. Biol. Reprod. 1987, 37, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Laslett, A.L.; McFarlane, J.R.; Risbridger, G.P. Developmental response by Leydig cells to acidic and basic fibroblast growth factor. J. Steroid Biochem. Mol. Biol. 1997, 60, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.Y.; Cheng, C.Y. Extracellular matrix and its role in spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 74–91. [Google Scholar]
- O’Shaughnessy, P.J.; Morris, I.D.; Huhtaniemi, I.; Baker, P.J.; Abel, M.H. Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: Data from mutant and genetically modified mice. Mol. Cell Endocrinol. 2009, 306, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Vaahtokari, A.; Aberg, T.; Thesleff, I. Apoptosis in the developing tooth: Association with an embryonic signaling center and suppression by EGF and FGF-4. Development 1996, 122, 121–129. [Google Scholar] [CrossRef]
- Wagle, A.; Singh, J.P. Fibroblast growth factor protects nitric oxide-induced apoptosis in neuronal SHSY-5Y cells. J. Pharmacol. Exp. Ther. 2000, 295, 889–895. [Google Scholar] [PubMed]
- Hirai, K.; Sasaki, H.; Yamamoto, H.; Sakamoto, H.; Kubota, Y.; Kakizoe, T.; Terada, M.; Ochiya, T. HST-1/FGF-4 protects male germ cells from apoptosis under heat-stress condition. Exp. Cell Res. 2004, 294, 77–85. [Google Scholar] [CrossRef]
- Clarkson, E.D.; Zawada, W.M.; Freed, C.R. GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res. 1997, 289, 207–210. [Google Scholar] [CrossRef]
- Yan, W.; Suominen, J.; Toppari, J. Stem cell factor protects germ cells from apoptosis in vitro. J. Cell Sci. 2000, 113 Pt 1, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Cotton, L.M.; O’Bryan, M.K.; Hinton, B.T. Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr. Rev. 2008, 29, 193–216. [Google Scholar] [CrossRef]
- Gantner, C.W.; de Luzy, I.R.; Kauhausen, J.A.; Moriarty, N.; Niclis, J.C.; Bye, C.R.; Penna, V.; Hunt, C.P.J.; Ermine, C.M.; Pouton, C.W.; et al. Viral delivery of GDNF promotes functional integration of human stem cell grafts in parkinson’s disease. Cell Stem Cell 2020, 26, 511–526.e5. [Google Scholar] [CrossRef]
- Benson, D.M.J.; Yu, J.; Becknell, B.; Wei, M.; Freud, A.G.; Ferketich, A.K.; Trotta, R.; Perrotti, D.; Briesewitz, R.; Caligiuri, M.A. Stem cell factor and interleukin-2/15 combine to enhance MAPK-mediated proliferation of human natural killer cells. Blood 2009, 113, 2706–2714. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhu, Q.; Yuan, K.; Su, Z.; Ge, F.; Ge, R.S.; Huang, Y. Epidermal growth factor regulates the development of stem and progenitor Leydig cells in rats. J. Cell Mol. Med. 2020, 24, 7313. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kojima, Y.; Mizuno, K.; Nakane, A.; Hayashi, Y.; Kohri, K. Effect of epidermal growth factors on spermatogenesis in the cryptorchid rat. J. Urol. 2005, 174, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Do TUNEL and other apoptosis assays detect cell death in preclinical studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef]
- Kanoh, M.; Takemura, G.; Misao, J.; Hayakawa, Y.; Aoyama, T.; Nishigaki, K.; Noda, T.; Fujiwara, T.; Fukuda, K.; Minatoguchi, S.; et al. Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: Not apoptosis but DNA repair. Circulation 1999, 99, 2757–2764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baima, B.; Sticherling, M. How specific is the TUNEL reaction? An account of a histochemical study on human skin. Am. J. Dermatopathol. 2002, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Josso, N.; Lamarre, I.; Picard, J.Y.; Berta, P.; Davies, N.; Morichon, N.; Peschanski, M.; Jeny, R. Anti-müllerian hormone in early human development. Early Hum. Dev. 1993, 33, 91–99. [Google Scholar] [CrossRef]
- Tran, D.; Muesy-Dessole, N.; Josso, N. Anti-Müllerian hormone is a functional marker of foetal Sertoli cells. Nature 1977, 269, 411–412. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Valeri, C.; Lovaisa, M.M.; Schteingart, H.F.; Rey, R.A. AMH regulation by steroids in the mammalian testis: Underlying mechanisms and clinical implications. Front. Endocrinol. 2022, 13, 906381. [Google Scholar] [CrossRef]
- Hirobe, S.; He, W.W.; Lee, M.M.; Donahoe, P.K. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology 1992, 131, 854–862. [Google Scholar]
- Al-Attar, L.; Noël, K.; Dutertre, M.; Belville, C.; Forest, M.G.; Burgoyne, P.S.; Josso, N.; Rey, R. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J. Clin. Investig. 1997, 100, 1335–1343. [Google Scholar] [CrossRef]
- Tran, D.; Meusy-Dessolle, N.; Josso, N. Waning of anti-mullerian activity: An early sign of sertoli cell maturation in the developing pig. Biol. Reprod. 1981, 24, 923–931. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Q.; Li, T.; Liu, R.; Cheng, Z.; Guo, M.; Xiao, J.; Wu, D.; Zeng, W. Sertoli cell and spermatogonial development in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Yimpring, N.; Teankum, K.; Srisuwatanasagul, S.; Kunnasut, N.; Am-in, N.; Suriyaphol, G. Alteration of androgen receptor expression, apoptosis and cell proliferation in cryptorchid suckling, nursery and growing-finishing pigs. Theriogenology 2019, 127, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lindzey, J.; Kumar, M.V.; Grossman, M.; Young, C.; Tindall, D.J. Molecular mechanisms of androgen action. Vitam. Horm. 1994, 49, 383–432. [Google Scholar] [PubMed]
- Mendis-Handagama, S.M. Luteinizing hormone on Leydig cell structure and function. Histol. Histopathol. 1997, 12, 869–882. [Google Scholar]
- Cham, T.C.; Ibtisham, F.; Fayaz, M.A.; Honaramooz, A. Generation of a highly biomimetic organoid, including vasculature, resembling the native immature testis tissue. Cells 2021, 10, 1696. [Google Scholar] [CrossRef]
- Wang, P.; Suo, L.J.; Wang, Y.F.; Shang, H.; Li, G.X.; Hu, J.H.; Li, Q.W. Effects of GDNF and LIF on mouse spermatogonial stem cells proliferation in vitro. Cytotechnology 2014, 66, 309–316. [Google Scholar] [CrossRef]
- Aponte, P.M.; Soda, T.; Teerds, K.J.; Mizrak, S.C.; van de Kant, H.J.G.; de Rooij, D.G. Propagation of bovine spermatogonial stem cells in vitro. Reproduction 2008, 136, 543–557. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Mizrak, S.C.; van Daalen, S.K.; Korver, C.M.; Roepers-Gajadien, H.L.; Koruji, M.; Hovingh, S.; de Reijke, T.M.; de la Rosette, J.J.; van der Veen, F.; et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009, 302, 2127–2134. [Google Scholar] [CrossRef]
- Viglietto, G.; Dolci, S.; Bruni, P.; Baldassarre, G.; Chiariotti, L.; Melillo, R.M.; Salvatore, G.; Chiappetta, G.; Sferratore, F.; Fusco, A.; et al. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int. J. Oncol. 2000, 16, 689–694. [Google Scholar] [CrossRef]
- Van Dissel-Emiliani, F.M.; De Boer-Brouwer, M.; De Rooij, D.G. Effect of fibroblast growth factor-2 on Sertoli cells and gonocytes in coculture during the perinatal period. Endocrinology 1996, 137, 647–654. [Google Scholar] [CrossRef][Green Version]
- Piravar, Z.; Jeddi-Tehrani, M.; Sadeghi, M.R.; Mohazzab, A.; Eidi, A.; Akhondi, M.M. In vitro culture of human testicular stem cells on feeder-free condition. J. Reprod. Infertil. 2013, 14, 17–22. [Google Scholar] [PubMed]
- Wahab-Wahlgren, A.; Martinelle, N.; Holst, M.; Jahnukainen, K.; Parvinen, M.; Söder, O. EGF stimulates rat spermatogonial DNA synthesis in seminiferous tubule segments in vitro. Mol. Cell Endocrinol. 2003, 201, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Kojima, K.; Komeya, M.; Yao, M.; Ogawa, T. In vitro spermatogenesis in explanted adult mouse testis tissues. PLoS ONE 2015, 10, e0130171. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Kubota, Y.; Ogawa, T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat. Protoc. 2013, 8, 2098–2104. [Google Scholar] [CrossRef]
- Yokonishi, T.; Sato, T.; Komeya, M.; Katagiri, K.; Kubota, Y.; Nakabayashi, K.; Hata, K.; Inoue, K.; Ogonuki, N.; Ogura, A.; et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun. 2014, 5, 4320. [Google Scholar] [CrossRef]
- de Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017, 32, 32–45. [Google Scholar] [CrossRef]
- Schrans-Stassen, B.H.; van de Kant, H.J.; de Rooij, D.G.; van Pelt, A.M. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; de Rooij, D.G.; Page, D.C. Periodic retinoic acid–STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef]
- West, F.D.; Machacek, D.W.; Boyd, N.L.; Pandiyan, K.; Robbins, K.R.; Stice, S.L. Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells 2008, 26, 2768–2776. [Google Scholar] [CrossRef]
- Mauduit, C.; Hamamah, S.; Benahmed, M. Stem cell factor/c-kit system in spermatogenesis. Hum. Reprod. Update 1999, 5, 535–545. [Google Scholar] [CrossRef]
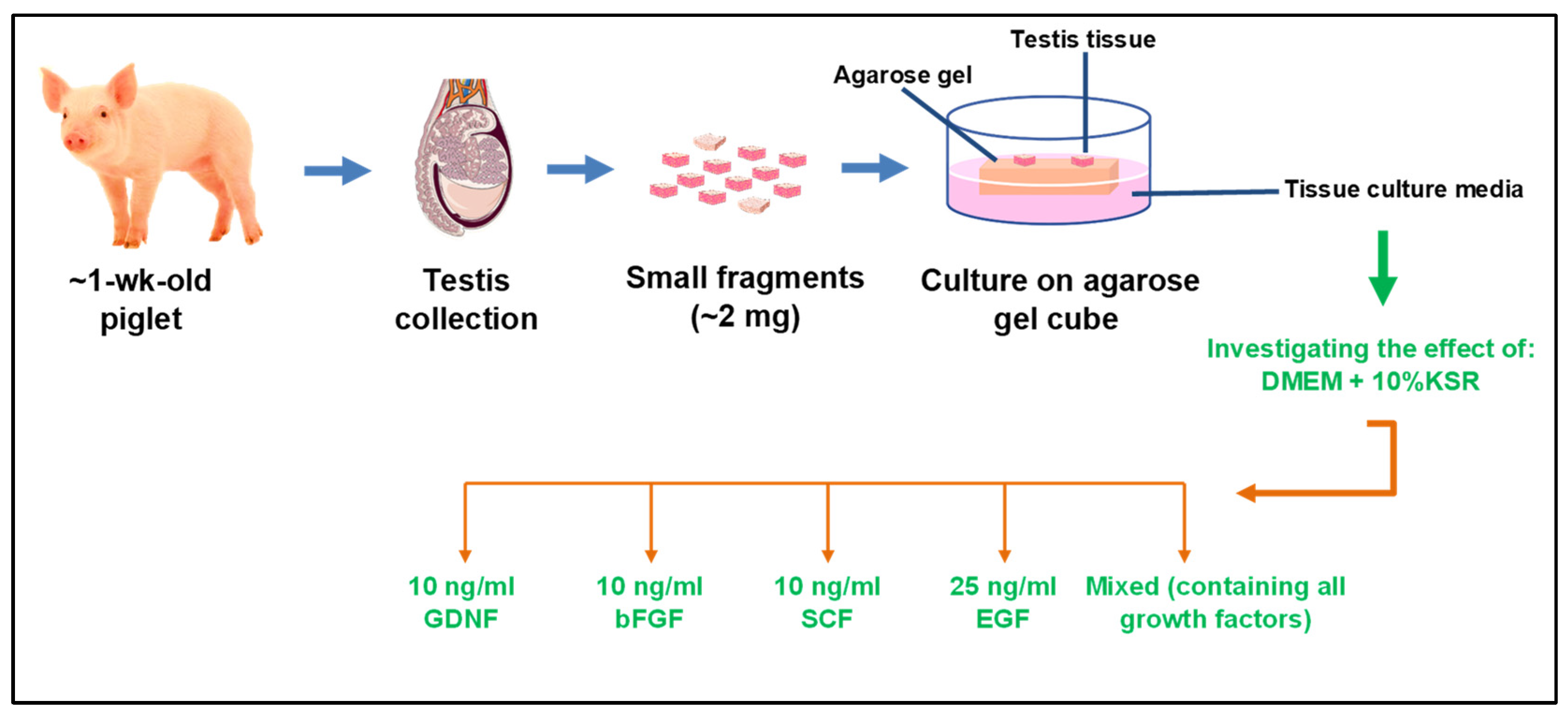


| Groups | Culture Media |
|---|---|
| 1 | DMEM + 10% KSR + 10 ng/mL GDNF |
| 2 | DMEM + 10% KSR + 10 ng/mL bFGF |
| 3 | DMEM + 10% KSR + 10 ng/mL SCF |
| 4 | DMEM + 10% KSR + 25 ng/mL EGF |
| 5 | DMEM + 10% KSR + 10 ng/mL GDNF + 10 ng/mL bFGF + 10 ng/mL SCF + 25 ng/mL EGF |
| 6 (Control) | DMEM + 10% KSR (No growth factors) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibtisham, F.; Cham, T.-C.; Fayaz, M.A.; Honaramooz, A. Effects of Growth Factors on In Vitro Culture of Neonatal Piglet Testicular Tissue Fragments. Cells 2023, 12, 2234. https://doi.org/10.3390/cells12182234
Ibtisham F, Cham T-C, Fayaz MA, Honaramooz A. Effects of Growth Factors on In Vitro Culture of Neonatal Piglet Testicular Tissue Fragments. Cells. 2023; 12(18):2234. https://doi.org/10.3390/cells12182234
Chicago/Turabian StyleIbtisham, Fahar, Tat-Chuan Cham, Mohammad Amin Fayaz, and Ali Honaramooz. 2023. "Effects of Growth Factors on In Vitro Culture of Neonatal Piglet Testicular Tissue Fragments" Cells 12, no. 18: 2234. https://doi.org/10.3390/cells12182234
APA StyleIbtisham, F., Cham, T.-C., Fayaz, M. A., & Honaramooz, A. (2023). Effects of Growth Factors on In Vitro Culture of Neonatal Piglet Testicular Tissue Fragments. Cells, 12(18), 2234. https://doi.org/10.3390/cells12182234







