Antimycobacterial Activity of Sida hermaphrodita (L.) Rusby (Malvaceae) Seed Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
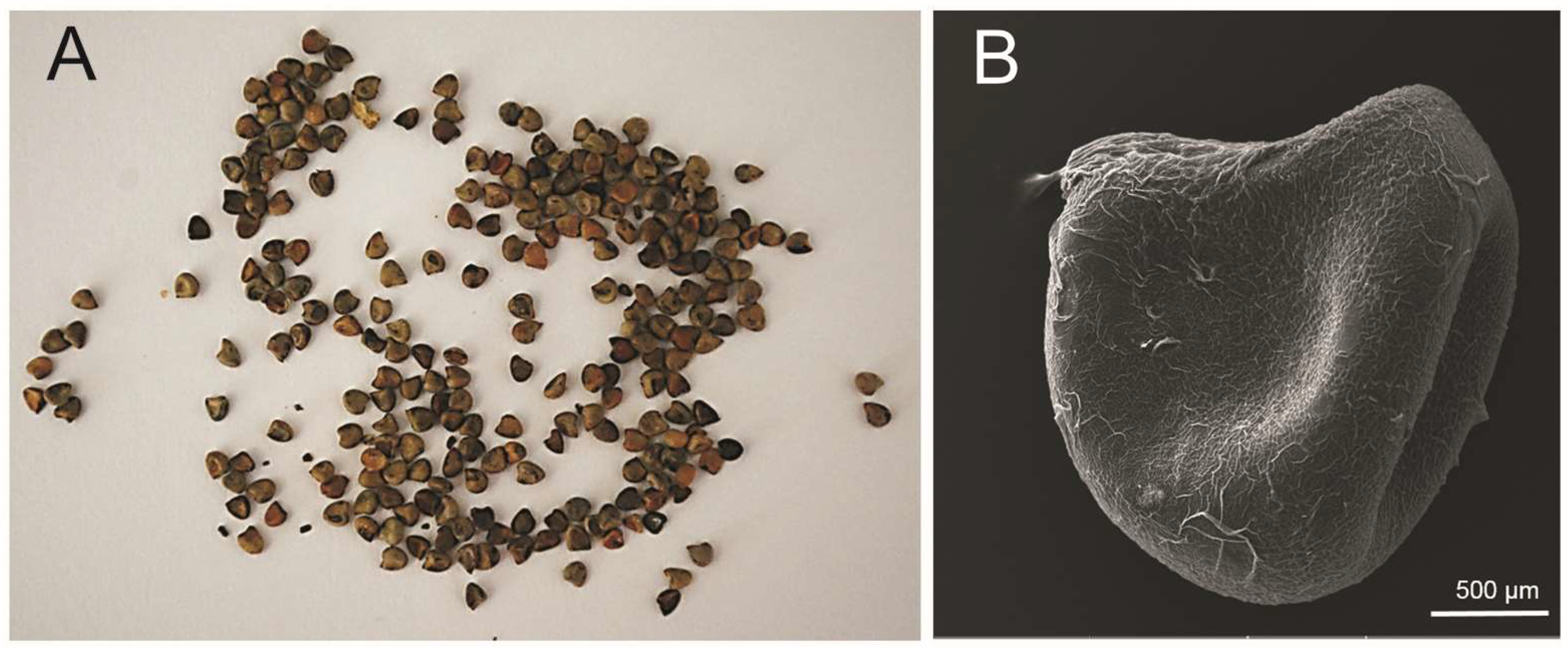
2.2. Plant Material
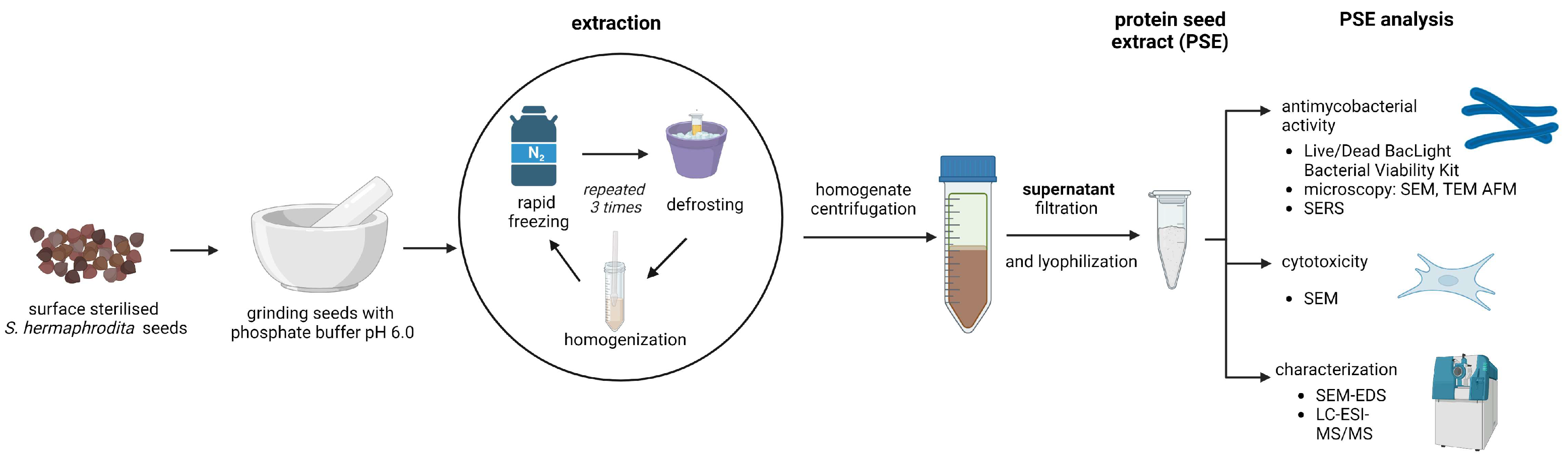
2.3. Preparation of Sida hermaphrodita Seed Extract
2.4. Preparation for Microscopy Techniques
2.5. Determination of Mycobacterium smegmatis Cell Viability
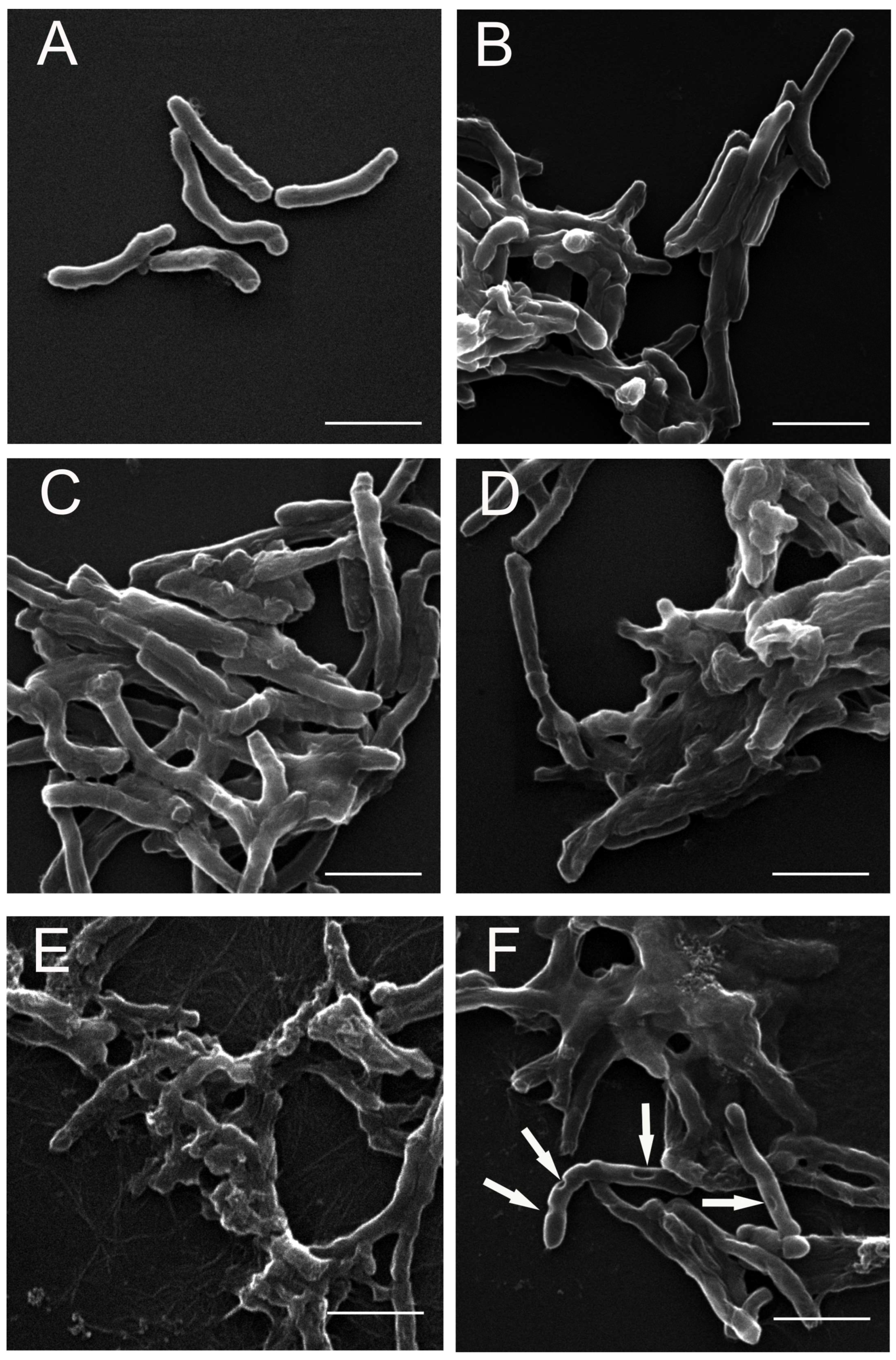
2.6. Scanning Electron Microscopy (SEM)
2.6.1. Mycobacteria
2.6.2. Fibroblasts
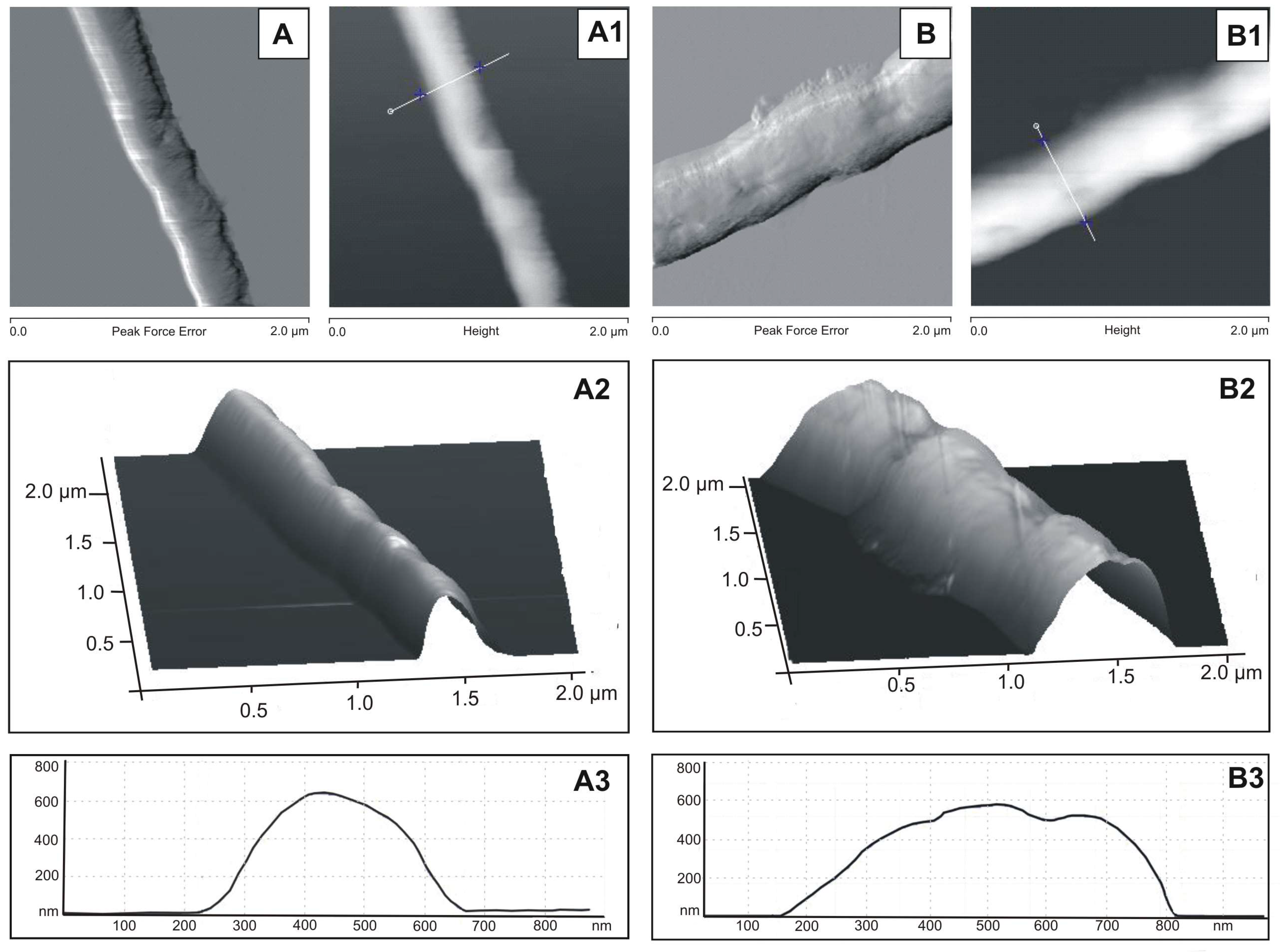
2.7. Atomic Force Microscopy (AFM)
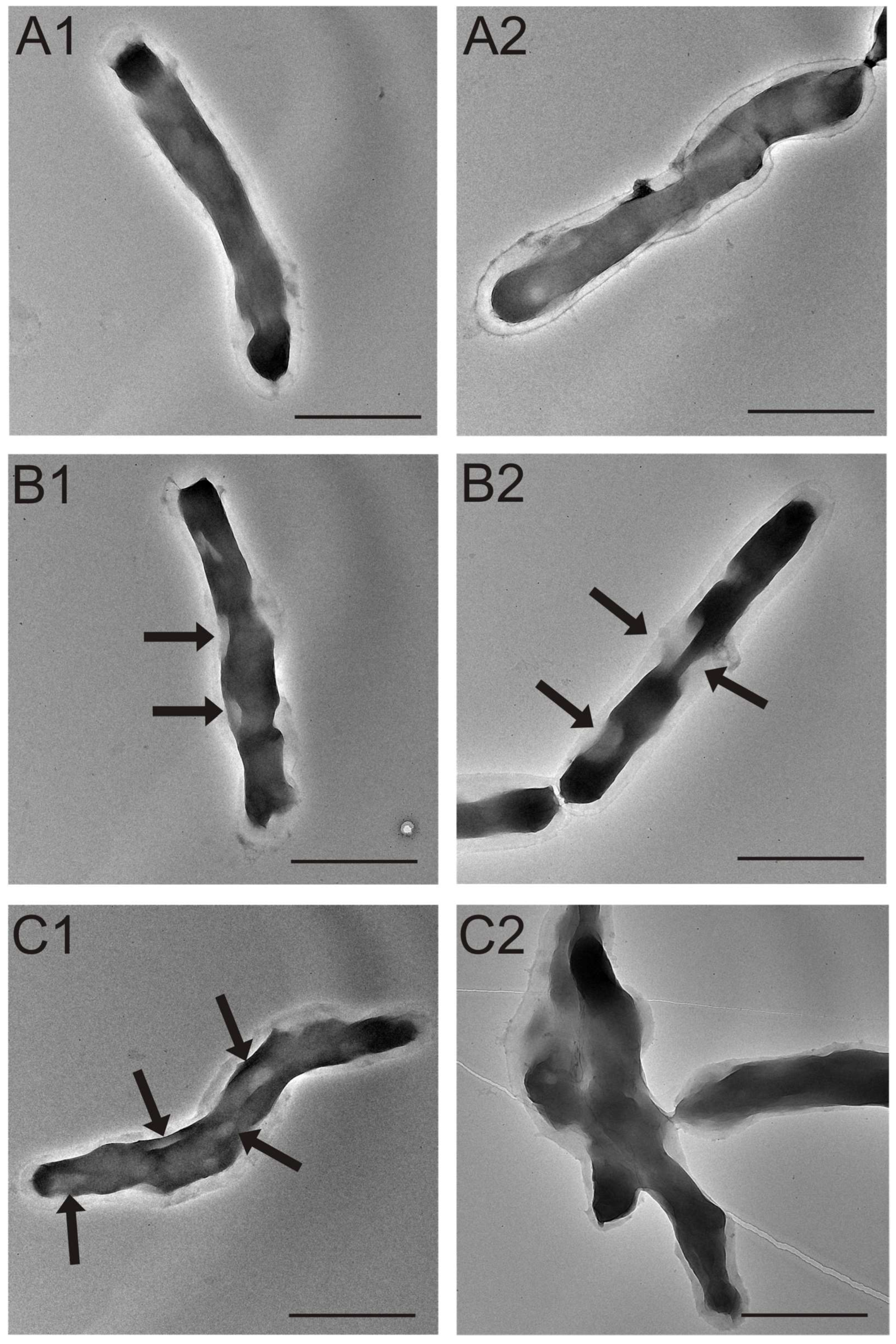
2.8. Transmission Electron Microscopy (TEM)
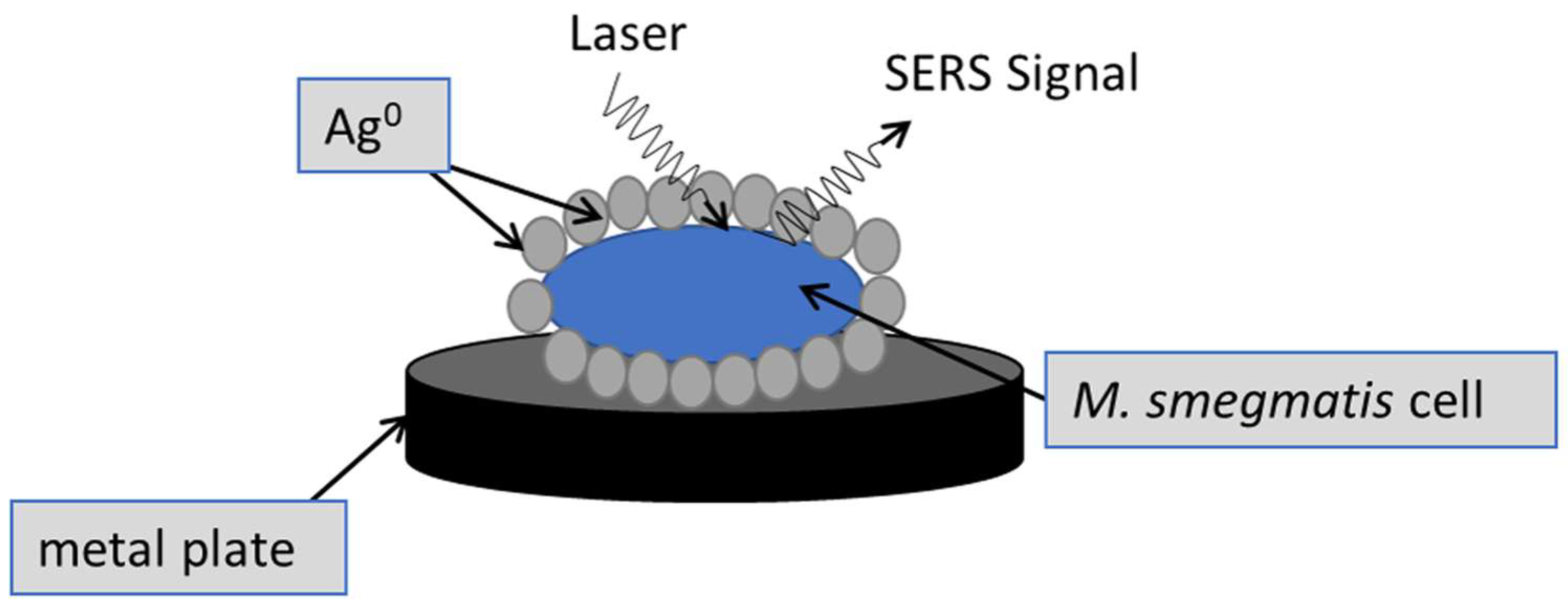
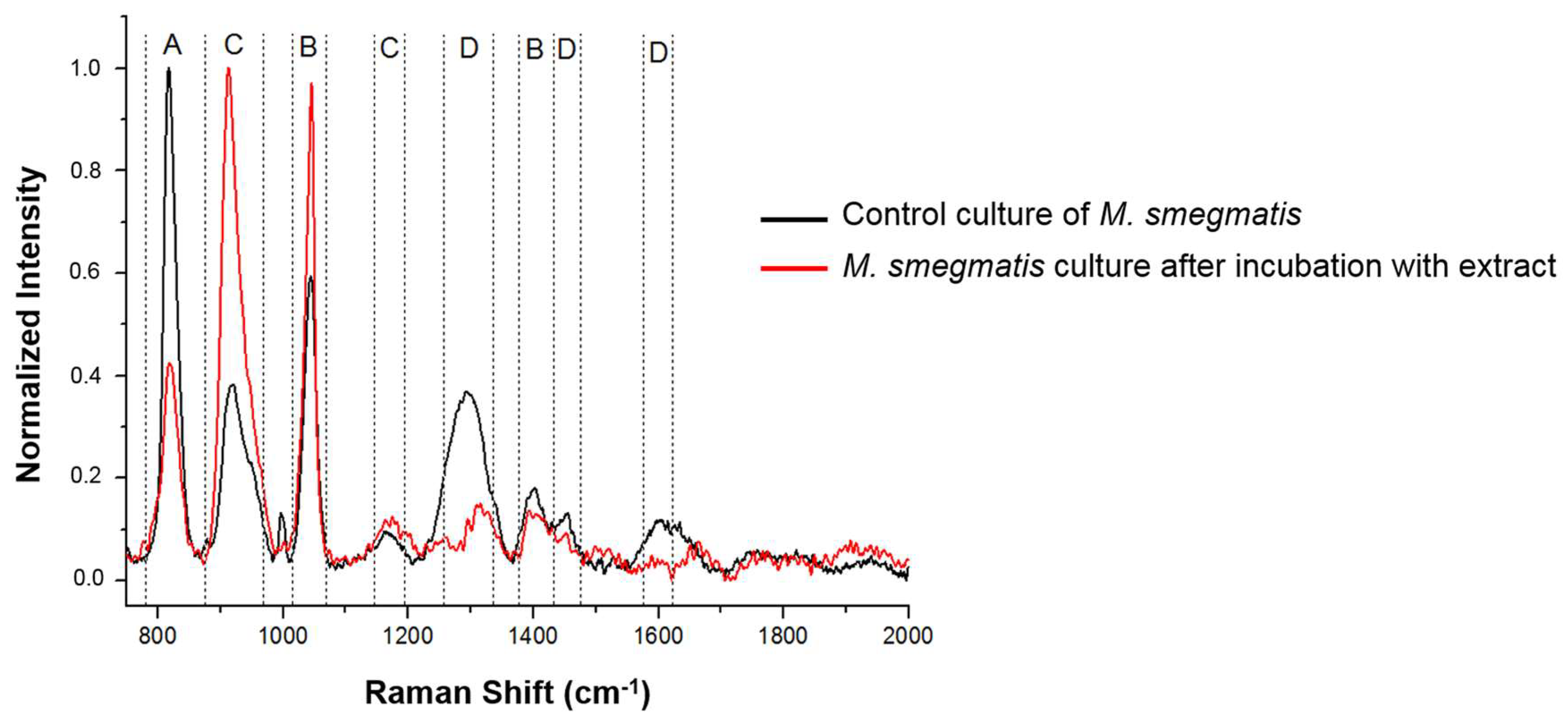
2.9. Surface-Enhanced Raman Spectroscopy (SERS)
2.10. SEM-EDS Analysis of the Microstructure and Elemental Composition of S. hermaphrodita Seed Extract
2.11. LC-ESI-MS/MS Analysis of PSE
2.12. Statistical Analysis
3. Results
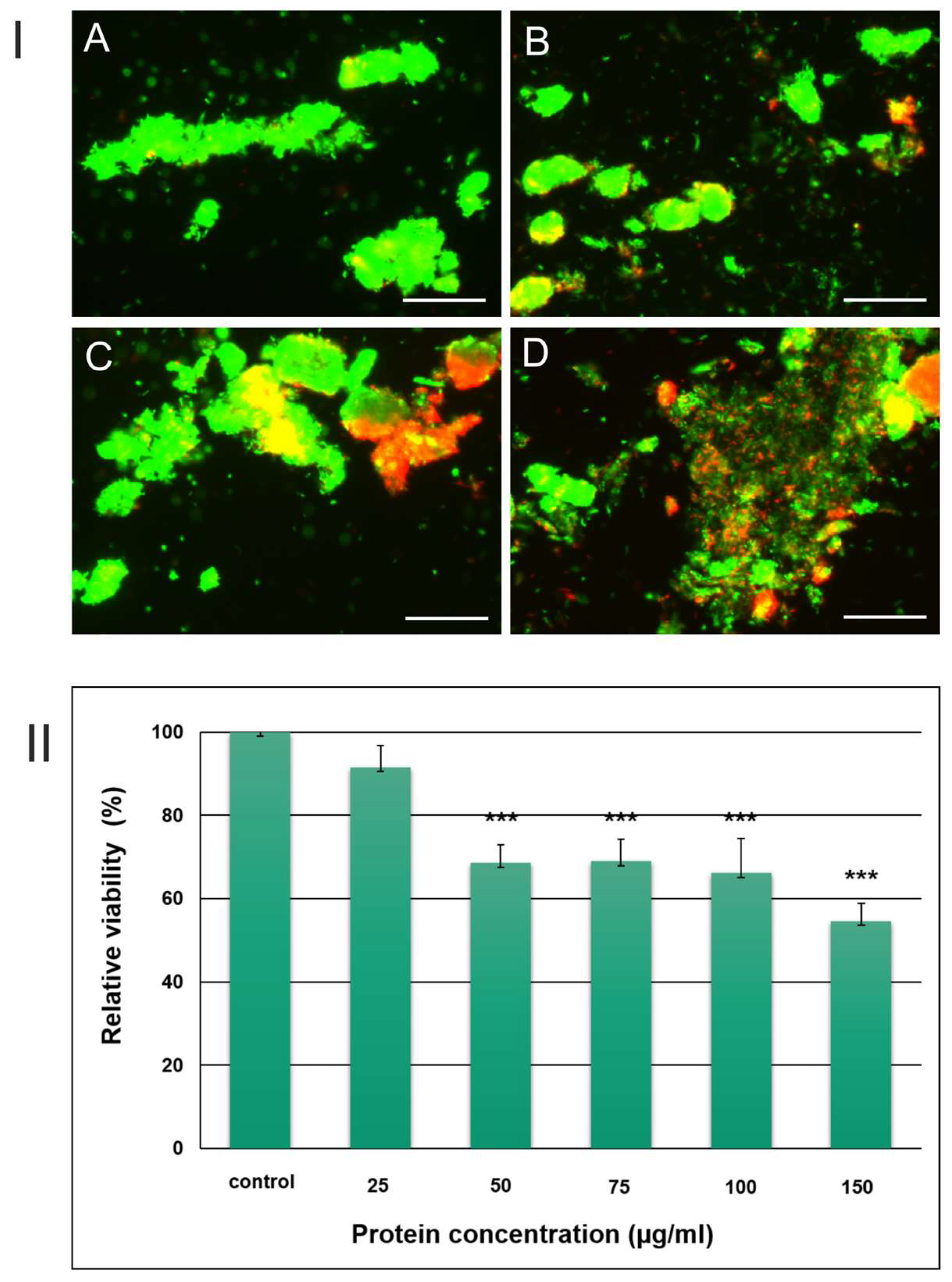
3.1. Quantification of Mycobacterium Viability after Incubation with the S. hermaphrodita Extract
3.2. SEM Analysis of M. smegmatis Cells after the Treatment with the S. hermaphrodita Extract
3.3. AFM Analysis of M. smegmatis Cells after the Treatment with the S. hermaphrodita Extract
3.4. TEM Analysis of M. smegmatis Cells after the Treatment with the S. hermaphrodita Extract
3.5. Analysis of Changes in the Chemical Composition of the M. smegmatis Cell Wall after Exposure to the S. hermaphrodita Extract Using Surface-Enhanced Raman Spectroscopy
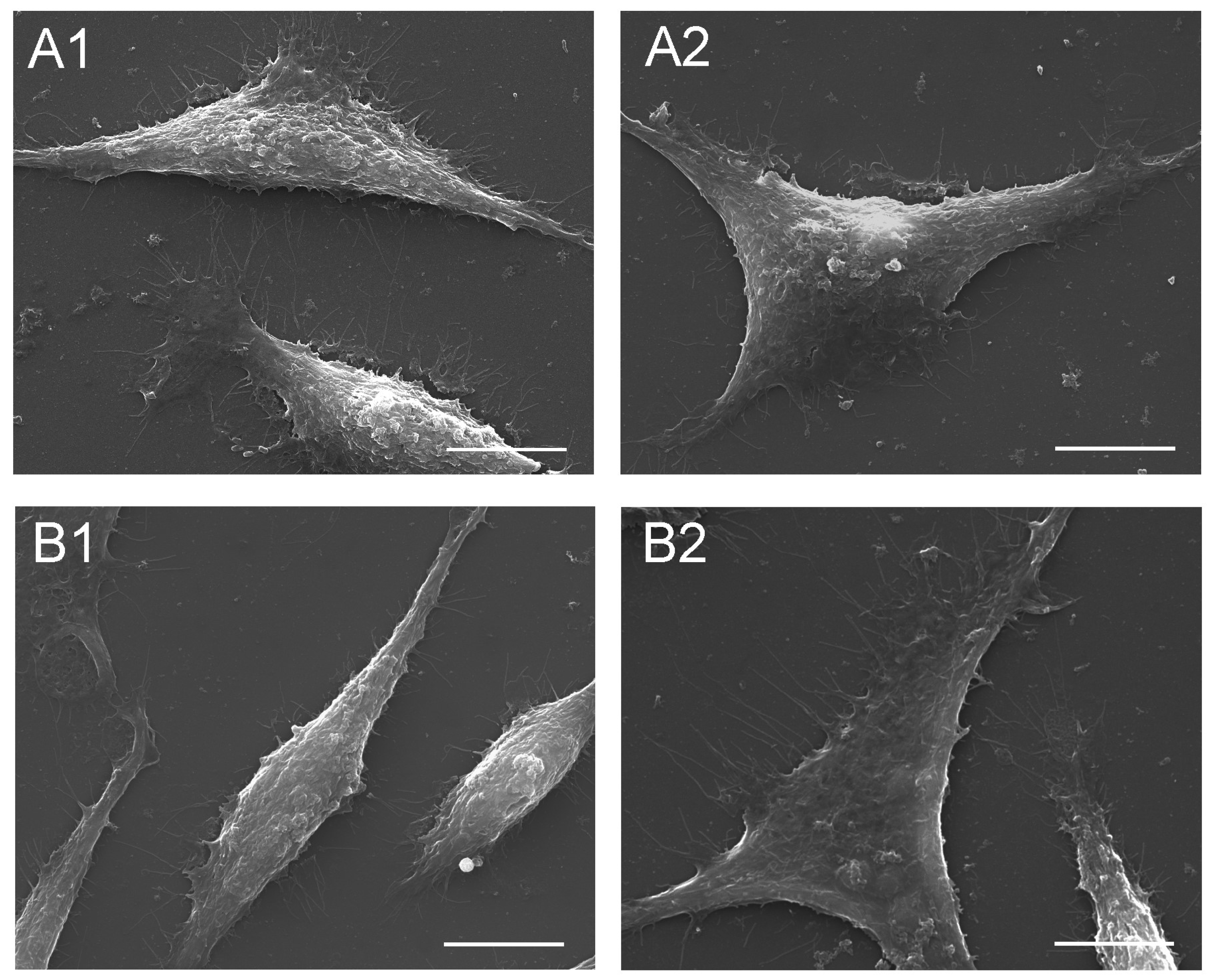
3.6. Effect of the Seed Extract on Fibroblast Cells
3.7. Characterization of S. hermaphrodita Protein Seed Extract (PSE)
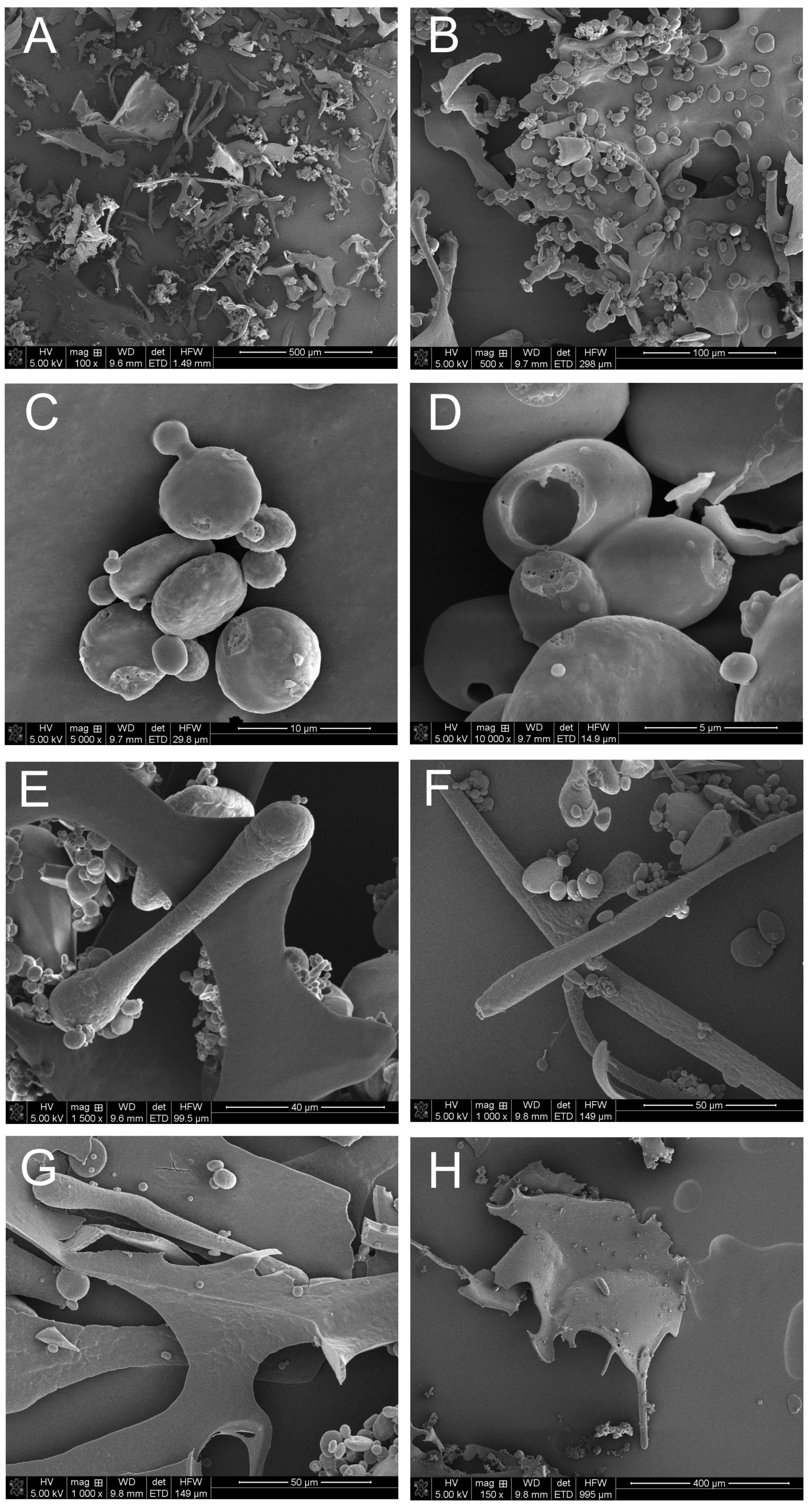
3.7.1. Microanalysis of PSE Using Scanning Electron Microscopy and the X-ray Energy Dispersion Technique
3.7.2. ESI LC MS Analysis of PSE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, L.; de Blanco, E.J.C.; Kinghorn, A.D. Plant-derived natural products as leads for drug discovery. In Plant—Derived Natural Products; Osbourn, A., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 547–567. ISBN 978-0-387-85497-7. [Google Scholar]
- McChesney, J.D.; Venkataraman, S.K.; Henri, J.T. Plant natural products: Back to the future or into extinction? Phytochemistry 2007, 68, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, I.A.; Santos, M.R.V.; Nascimento, N.M.S.; Duarte, J.C. Cardiovascular effects of Sida cordifolia leaves extract in rats. Fitoterapia 2006, 77, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; de Oliveira, A.F.M. The genus Sida L. (Malvaceae): An update of its ethnomedicinal use, pharmacology and phytochemistry. S. Afr. J. Bot. 2020, 132, 432–462. [Google Scholar] [CrossRef]
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998, 62, 183–193. [Google Scholar] [CrossRef]
- Sutradhar, R.K.; Rahman, A.K.M.M.; Ahmad, M.; Bachar, S.C.; Saha, A. Analgesic and anti-inflammatory principle from Sida cordifolia Linn. J. Biol. Sci. 2006, 6, 160–163. [Google Scholar]
- Lewtak, K.; Fiołka, M.J.; Czaplewska, P.; Macur, K.; Kaczyński, Z.; Buchwald, T.; Szczuka, E.; Rzymowska, J. Sida hermaphrodita seeds as the source of anti—Candida albicans activity. Sci. Rep. 2019, 9, 12233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisiuk, W. Possibilities of using Virginia mallow in the power engineering. Agric. Eng. 2005, 6, 105–113. (In Polish) [Google Scholar]
- Nahm, M.; Morhart, C. Virginia mallow (Sida hermaphrodita (L.) Rusby) as perennial multipurpose crop: Biomass yields, energetic valorization, utilization potentials, and management perspectives. GCB Bioenergy 2018, 10, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Wolski, T.; Styk, B. Sida hermaphrodita—A new herbal plant. Herb. News 1989, 6, 1–3. (In Polish) [Google Scholar]
- Borkowska, H.; Wardzińska, K. Influence of sowing quantity and harvest dates on diversification of Virginia mallow biomass yields. Ann. UMCS Sec. E Agric. 1999, 54, 31–35. (In Polish) [Google Scholar]
- Sharma, S.K.; Upadhyay, V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J. Med. Res. 2020, 152, 185–226. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, J.; Ciura, K.; Rudnicka-Litka, K. Natural anti-tuberculosis drugs. Adv. Phytother. 2016, 17, 320–331. (In Polish) [Google Scholar]
- Shiloh, M.U.; Champion, P.A. To catch a killer. What can mycobacterial models teach us about Mycobacterium tuberculosis pathogenesis? Curr. Opin. Microbiol. 2010, 13, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, N.; Imperiale, B.; Palomino, J.C. New simple decontamination method improves microscopic detection and culture of mycobacteria in clinical practice. Infect. Drug Resist. 2008, 1, 21–26. [Google Scholar] [CrossRef]
- Lahiri, R.; Randhawa, B.; Krahenbuhl, J. Application of viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J. Med. Microbiol. 2005, 54, 235–242. [Google Scholar] [CrossRef]
- Sirgel, F.A.; Wiid, I.J.; van Helden, P.D. Measuring minimum inhibitory concentrations in mycobacteria. In Mycobacteria Protocols. Methods in Molecular Biology; Parish, T., Brown, A., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 465, pp. 173–186. ISBN 978-1-58829-889-8. [Google Scholar]
- Verma, J.; Rohilla, A.; Khuller, G.K. Alterations in macromolecular composition and cell wall integrity by ciprofloxacin in Mycobacterium smegmatis. Lett. Appl. Microbiol. 1999, 29, 113–117. [Google Scholar] [CrossRef]
- Harris, J.R. Negative staining of thinly spread biological samples. Methods Mol. Biol. 2007, 369, 107–142. [Google Scholar] [CrossRef]
- Efrima, S.; Zeiri, L. Understanding SERS of bacteria. J. Raman Spectrosc. 2009, 40, 277–288. [Google Scholar] [CrossRef]
- Hamasha, K.; Sahana, M.B.; Jani, C.; Nyayapathy, S.; Kang, C.M.; Rehse, S.J. The effect of Wag31 phosphorylation on the cells and the cell envelope fraction of wild-type and conditional mutants of Mycobacterium smegmatis studied by visible-wavelength Raman spectroscopy. Biochem. Biophys. Res. Commun. 2010, 391, 664–668. [Google Scholar] [CrossRef]
- Gundry, R.L.; White, M.Y.; Murray, C.I.; Kane, L.A.; Fu, Q.; Stanley, B.A.; Van Eyk, J.E. Preparation of proteins and peptides for mass spectrometry analysis in a bottom-up proteomics workflow. Curr. Protoc. Mol. Biol. 2009, 88, 10.25.1–10.25.23. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, A.E.; Macur, K.; Czaplewska, P.; Liss, J.; Łukaszuk, K.; Ołdziej, S. Qualitative and quantitative analysis of proteome and peptidome of human follicular fluid using multiple samples from single donor with LC-MS and SWATH methodology. J. Proteome Res. 2017, 16, 3053–3067. [Google Scholar] [CrossRef] [PubMed]
- Cordone, A.; Audrain, B.; Calabrese, I.; Euphrasie, D.; Reyrat, J.M. Characterization of a Mycobacterium smegmatis uvrA mutant impaired in dormancy induced by hypoxia and low carbon concentration. BMC Microbiol. 2011, 11, 231. [Google Scholar] [CrossRef] [Green Version]
- Lelovic, N.; Mitachi, K.; Yang, J.; Lemieux, M.R.; Ji, Y.; Kurosu, M. Application of Mycobacterium smegmatis as a surrogate to evaluate drug leads against Mycobacterium tuberculosis. J. Antibiot. 2020, 73, 780–789. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 14, 223–237. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Dwivedi, N.; Tripathi, R.P.; Sinha, S. Evaluation of Mycobacterium smegmatis as a possible surrogate screen for selecting molecules active against multi-drug resistant Mycobacterium tuberculosis. J. Gen. Appl. Microbiol. 2007, 53, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Pfyffer, G.E. Mycobacterium: General characteristics, laboratory detection, and staining procedures. In Manual of Clinical Microbiology; Jorgensen, J.H., Carroll, K.C., Funke, G., Pfaller, M.A., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; American Society for Microbiology Press: Washington, DC, USA, 2015; pp. 543–572. ISBN 978-155-581-983-5. [Google Scholar]
- Bagieńska, M.; Rzewuska, M. Occurrence of mycobacteria from the Mycobacterium tuberculosis complex in animals—Transmission of selected species between humans and animals. Vet. Life 2010, 85, 742–746. (In Polish) [Google Scholar]
- Sundarsingh, T.J.A.; Ranjitha, J.; Rajan, A.; Shankar, V. Features of the biochemistry of Mycobacterium smegmatis, as a possible model for Mycobacterium tuberculosis. J. Infect. Public Health 2020, 13, 1255–1264. [Google Scholar]
- Li, X.; Mei, H.; Chen, F.; Tang, Q.; Yu, Z.; Cao, X.; Andongma, B.T.; Chou, S.H.; He, J. Transcriptome Landscape of Mycobacterium smegmatis. Front. Microbiol. 2017, 18, 2505. [Google Scholar] [CrossRef] [Green Version]
- Kazemian, H.; Heidari, H.; Yamchi, J.K.; Zandi, H.; Taji, A.; Yazdani, F.; Hamzehloo, G.; Ghanavati, R.; Rahdar, H.A.; Feizabadi, M.M. In vitro anti-mycobacterial activity of three medicinal plants of Lamiaceae family. Recent Pat. Antiinfect. Drug Discov. 2018, 13, 240–245. [Google Scholar] [CrossRef]
- El Omari, K.; Hamze, M.; Alwan, S.; Osman, M.; Jama, C.; Chihib, N.E. In-vitro evaluation of the antibacterial activity of the essential oils of Micromeria barbata, Eucalyptus globulus and Juniperus excelsa against strains of Mycobacterium tuberculosis (including MDR), Mycobacterium kansasii and Mycobacterium gordonae. J. Infect. Public Health 2019, 12, 615–618. [Google Scholar] [CrossRef]
- Tuyiringire, N.; Mugisha, I.T.; Tusubira, D.; Munyampundu, J.P.; Muvunyi, C.M.; Vander, Y. In vitro antimycobacterial activity of medicinal plants Lantana camara, Cryptolepis sanguinolenta, and Zanthoxylum leprieurii. J. Clin. Tuberc. Other Mycobact. Dis. 2022, 27, 100307. [Google Scholar] [CrossRef]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef]
- Vieira Bard, G.C.; Nascimento, V.V.; Oliveira, A.E.; Rodrigues, R.; Da Cunha, M.; Dias, G.B.; Vasconcelos, I.M.; Carvalho, A.O.; Gomes, V.M. Vicilin-like peptides from Capsicum baccatum L. seeds are α-amylase inhibitors and exhibit antifungal activity against important yeasts in medical mycology. Biopolymers 2014, 102, 335–343. [Google Scholar] [CrossRef]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Peptides of the innate immune system of plants. Part I. Structure, biological activity, and mechanisms of action. Russ. J. Bioorg. Chem. 2019, 44, 573–585. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Rogozhin, E.A. Isolation of antimicrobial peptides from different plant sources: Does a general extraction method exist? Plant Methods 2020, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Odeku, O. Potentials of tropical starches as pharmaceutical excipients: A review. Starch/Stärke 2013, 65, 89–106. [Google Scholar] [CrossRef]
- Miranda, J.; de Carvalho, L.M.J.; Castro, A.V.I. Scanning electron microscopy and crystallinity of starches granules from cowpea, black and carioca beans in raw and cooked forms. Food Sci. Technol. 2019, 39, 2. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Gill, B.S.; Karwasra, B.L. In vitro digestibility, pasting, and structural properties of starches from different cereals. Int. J. Food Prop. 2018, 21, 70–85. [Google Scholar] [CrossRef] [Green Version]
- Leemud, P.; Karrila, S.; Kaewmanee, T.; Karrila, T. Functional and physicochemical properties of Durian seed flour blended with cassava starch. J. Food Meas. Charact. 2020, 14, 388–400. [Google Scholar] [CrossRef]
- Bard, G.C.V.; Zottich, U.; Souza, T.A.M.; Ribeiro, S.F.F.; Dias, G.B.; Pireda, S.; Da Cunha, M.; Rodrigues, R.; Pereira, L.S.; Machado, O.L.; et al. Purification, biochemical characterization, and antimicrobial activity of a new lipid transfer protein from Coffea canephora seeds. Genet. Mol. Res. 2016, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.E.; Darwish, N.I.; Garcia-Sanchez, J.; Tyagi, N.; Trick, H.N.; McCormick, S.; Dill-Macky, R.; Tumer, N.E. A lipid transfer protein has antifungal and antioxidant activity and suppresses fusarium head blight disease and DON accumulation in transgenic wheat. Phytopathology 2021, 111, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Abedinzadeh, M.; Gaeini, M.; Sardari, S. Natural antimicrobial peptides against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2015, 70, 1285–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiołka, M.J.; Grzywnowicz, K.; Mendyk, E.; Zagaja, M.; Szewczyk, R.; Rawski, M.; Keller, R.; Rzymowska, J.; Wydrych, J. Antimycobacterial action of a new glycolipid-peptide complex obtained from extracellular metabolites of Raoultella ornithinolytica. APMIS 2015, 123, 1069–1080. [Google Scholar] [CrossRef]
- Maria Curie-Skłodowska University. Virginia Mallow (Sida hermaphrodita) Seed Extract for Use in Combating Infections Caused by the Candida albicans Fungus. Patent PL 231698, 2 April 2019. [Google Scholar]
- Sher, A. Antimicrobial activity of natural products from medicinal plants. Gomal J. Med. Sci. 2009, 7, 72–76. [Google Scholar]
| Element | Wt% | At% |
|---|---|---|
| C | 48.40 (±2.45) | 56.15 (±1.74) |
| N | 15.47 (±2.01) | 15.39 (±1.55) |
| O | 27.47 (±1.98) | 23.93 (±0.90) |
| Na | 0.58 (±0.04) | 0.35 (±0.02) |
| Mg | 5.39 (±0.15) | 3.09 (±0.24) |
| P | 1.03 (±0.01) | 0.46 (±0.02) |
| S | 0.50 (±0.03) | 0.22 (±0.008) |
| Cl | 0.23 (±0.005) | 0.09 (±0.003) |
| Ca | 0.92 (±0.01) | 0.32 (±0.019) |
| Total | 100.00 | 100.00 |
| N. | Name | Accession | Species | Peptides (95%) | % Cov |
|---|---|---|---|---|---|
| 1. | uncharacterized protein LOC107929052 similar to Vicilin GC72-A GOSHI | A0A1U8LMX7 | GOSHI | 47 | 29.1 |
| 2. | vicilin-like antimicrobial peptides 2-2 | A0A0B0PUP3 | GOSAR | 31 | 44.9 |
| 3. | vicilin-like seed storage protein At2g28490 | A0A1U8P4G8 | GOSHI | 28 | 26.8 |
| 4. | vicilin-like seed storage protein At2g18540 | A0A1U8K3C6 | GOSHI | 8 | 24.2 |
| 5. | vicilin GC72-A | A0A1U8LQ34 | GOSHI | 35 | 34.0 |
| 6. | non-specific lipid-transfer protein | U5HUL1 | GOSRA | 1 | 22.5 |
| 7. | uncharacterized protein similar to non-specific lipid-transfer protein-like protein At5g64080 GOSHI | A0A0D2SK78 | GOSRA | 2 | 10.5 |
| 8 | non-specific lipid-transfer protein-like protein At5g64080 | A0A1U8MVT2 | GOSHI | 1 | 18.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewtak, K.; Czaplewska, P.; Wydrych, J.; Keller, R.; Nowicka, A.; Skrzypiec, K.; Fiołka, M.J. Antimycobacterial Activity of Sida hermaphrodita (L.) Rusby (Malvaceae) Seed Extract. Cells 2023, 12, 397. https://doi.org/10.3390/cells12030397
Lewtak K, Czaplewska P, Wydrych J, Keller R, Nowicka A, Skrzypiec K, Fiołka MJ. Antimycobacterial Activity of Sida hermaphrodita (L.) Rusby (Malvaceae) Seed Extract. Cells. 2023; 12(3):397. https://doi.org/10.3390/cells12030397
Chicago/Turabian StyleLewtak, Kinga, Paulina Czaplewska, Jerzy Wydrych, Radosław Keller, Aldona Nowicka, Krzysztof Skrzypiec, and Marta Julia Fiołka. 2023. "Antimycobacterial Activity of Sida hermaphrodita (L.) Rusby (Malvaceae) Seed Extract" Cells 12, no. 3: 397. https://doi.org/10.3390/cells12030397







