Renal Ischemia Tolerance Mediated by eIF5A Hypusination Inhibition Is Regulated by a Specific Modulation of the Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Survival Analysis
2.4. Protein Analysis
2.5. Immunofluorescence
2.6. mRNA Analysis
2.7. Statistical Analysis
3. Results
3.1. GC7 Preconditioning Prevents Anoxia- and Tunicamycin-Induced Cell Death
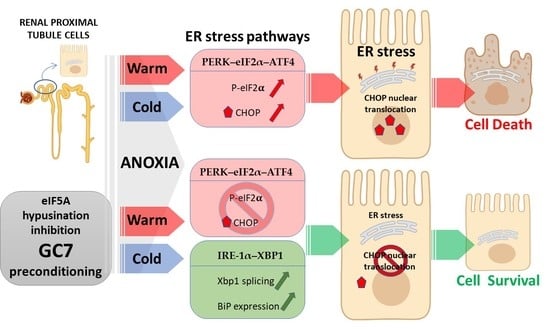
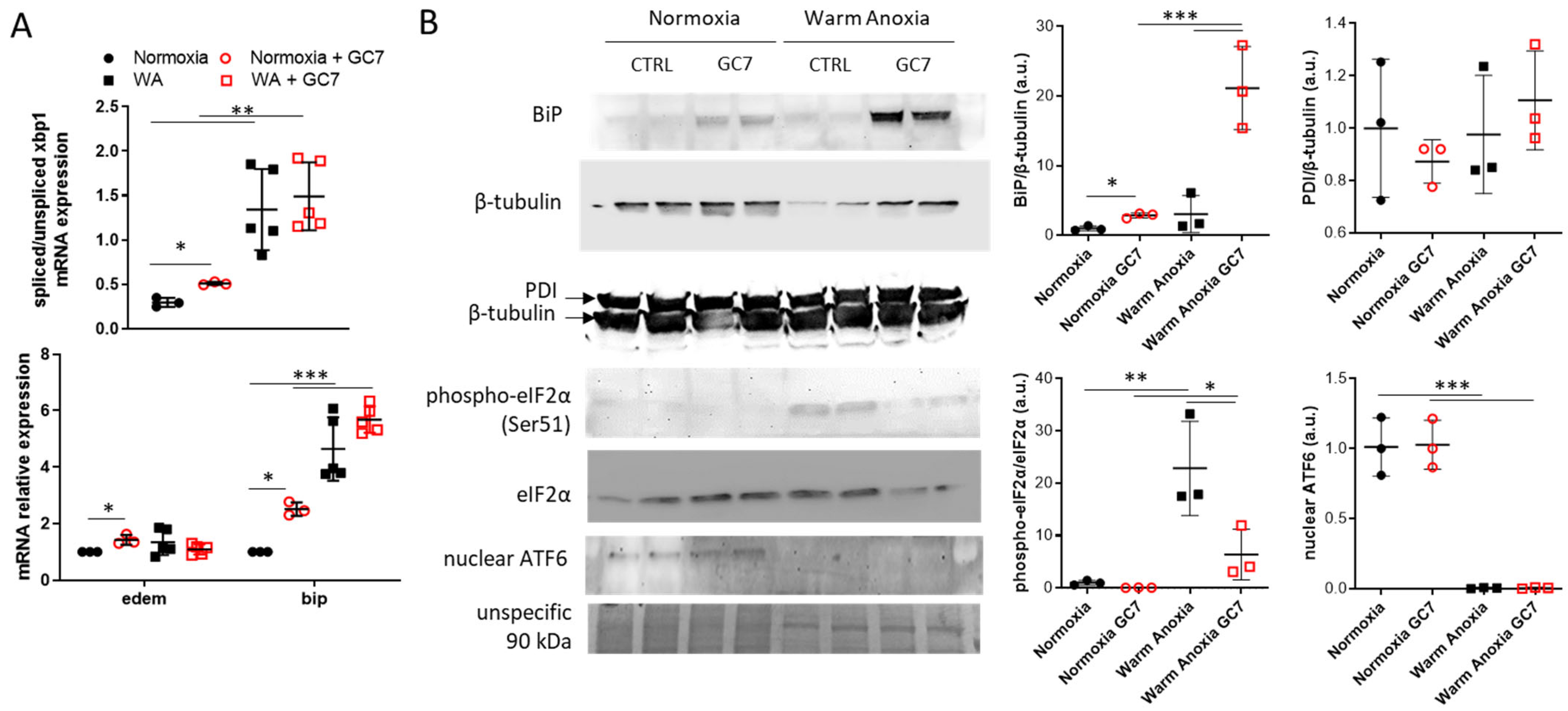
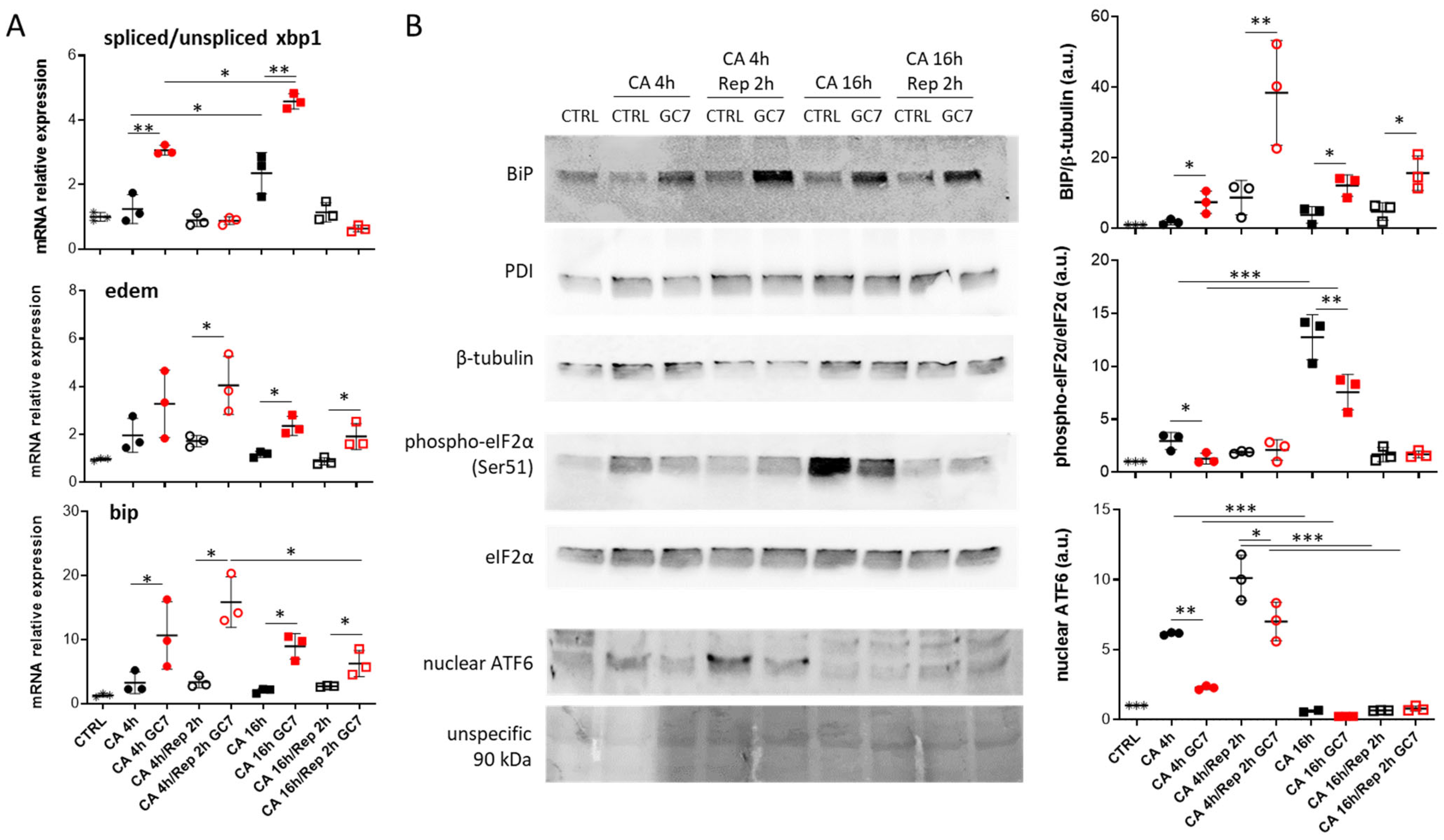
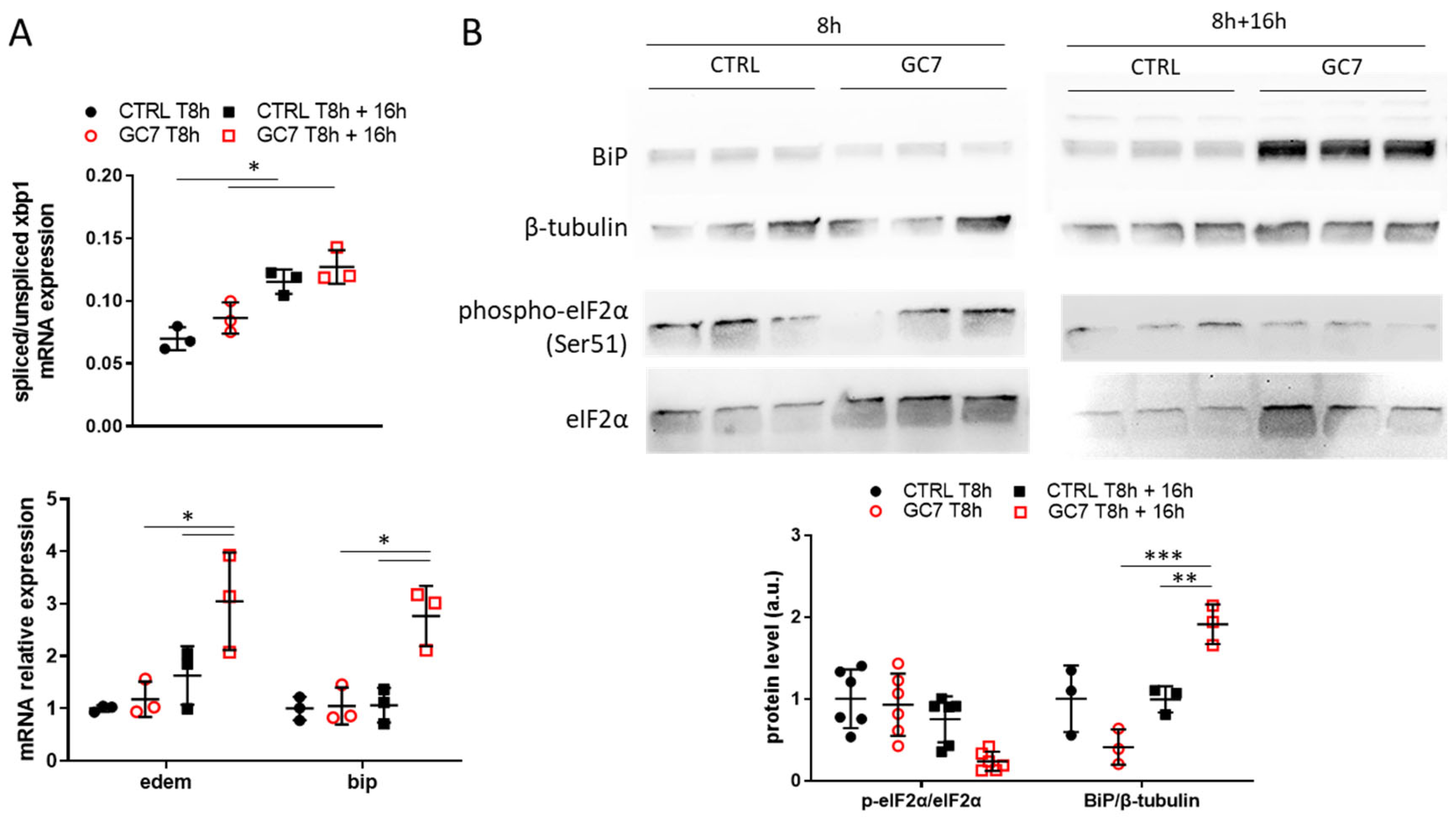
3.2. GC7 Modulates Differentially ER Stress Pathway Activation during Anoxia
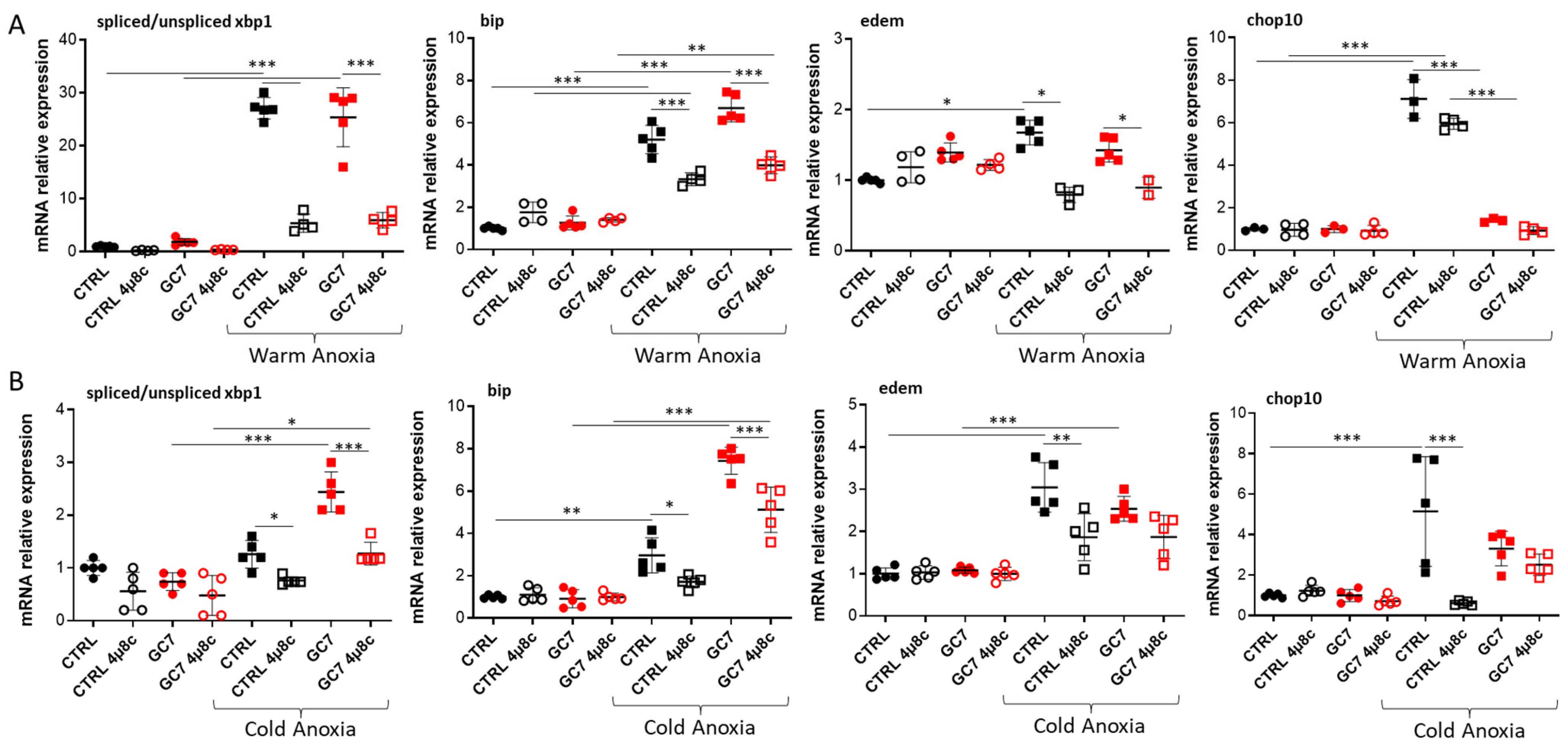
3.3. Involvement of IRE-1α Pathway in GC7 Protective Effect
3.4. Impact of GC7 Preconditioning on ER Stress Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic reticulum and the unfolded protein response: Dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R.; Ishimoto, Y.; Nangaku, M. Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease. Nat. Rev. Nephrol. 2014, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Adachi, Y.; Yamamoto, K.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 2008, 33, 75–89. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Lopez-Neblina, F.; Toledo, A.H.; Toledo-Pereyra, L.H. Molecular biology of apoptosis in ischemia and reperfusion. J. Investig. Surg. 2005, 18, 335–350. [Google Scholar] [CrossRef]
- Hansell, P.; Welch, W.J.; Blantz, R.C.; Palm, F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin. Exp. Pharmacol. Physiol. 2013, 40, 123–137. [Google Scholar] [CrossRef]
- Melis, N.; Thuillier, R.; Steichen, C.; Giraud, S.; Sauvageon, Y.; Kaminski, J.; Pele, T.; Badet, L.; Richer, J.P.; Barrera-Chimal, J.; et al. Emerging therapeutic strategies for transplantation-induced acute kidney injury: Protecting the organelles and the vascular bed. Expert Opin. Ther. Targets 2019, 23, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Raedschelders, K.; Ansley, D.M.; Chen, D.D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012, 133, 230–255. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Kondapalli, J.; Iwase, H.; Chandel, N.S.; Waypa, G.B.; Guzy, R.D.; Vanden Hoek, T.L.; Schumacker, P.T. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim. Biophys. Acta 2011, 1813, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Montie, H.L.; Kayali, F.; Haezebrouck, A.J.; Rossi, N.F.; Degracia, D.J. Renal ischemia and reperfusion activates the eIF 2 alpha kinase PERK. Biochim. Biophys. Acta 2005, 1741, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, M.; Jiang, W.; Dong, H.; Han, Y.; An, X.F.; Zhang, J. Endoplasmic reticulum stress and its effects on renal tubular cells apoptosis in ischemic acute kidney injury. Ren. Fail. 2016, 38, 831–837. [Google Scholar] [CrossRef]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 2018, 37, 269–280. [Google Scholar] [CrossRef]
- Grgic, I.; Campanholle, G.; Bijol, V.; Wang, C.; Sabbisetti, V.S.; Ichimura, T.; Humphreys, B.D.; Bonventre, J.V. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012, 82, 172–183. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Fan, Y.; Xiao, W.; Lee, K.; Salem, F.; Wen, J.; He, L.; Zhang, J.; Fei, Y.; Cheng, D.; Bao, H.; et al. Inhibition of Reticulon-1A-Mediated Endoplasmic Reticulum Stress in Early AKI Attenuates Renal Fibrosis Development. J. Am. Soc. Nephrol. 2017, 28, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ding, W.; Wang, Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 5828120. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.R.; Kim, J.I.; Han, S.J.; Lee, T.J.; Park, K.M. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim. Biophys. Acta 2015, 1852, 1895–1901. [Google Scholar] [CrossRef]
- Le Pape, S.; Pasini-Chabot, O.; Couturier, P.; Delpech, P.O.; Volmer, R.; Quellard, N.; Ploeg, R.; Hauet, T.; Thuillier, R. Decoding cold ischaemia time impact on kidney graft: The kinetics of the unfolded protein response pathways. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S873–S885. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 2006, 139, 161–169. [Google Scholar] [CrossRef]
- Saini, P.; Eyler, D.E.; Green, R.; Dever, T.E. Hypusine-containing protein eIF5A promotes translation elongation. Nature 2009, 459, 118–121. [Google Scholar] [CrossRef]
- Wang, F.W.; Guan, X.Y.; Xie, D. Roles of eukaryotic initiation factor 5A2 in human cancer. Int. J. Biol. Sci. 2013, 9, 1013–1020. [Google Scholar] [CrossRef]
- Bourourou, M.; Gouix, E.; Melis, N.; Friard, J.; Heurteaux, C.; Tauc, M.; Blondeau, N. Inhibition of eIF5A hypusination pathway as a new pharmacological target for stroke therapy. J. Cereb. Blood Flow Metab. 2020, 41, 1080–1090. [Google Scholar] [CrossRef]
- Giraud, S.; Kerforne, T.; Zely, J.; Ameteau, V.; Couturier, P.; Tauc, M.; Hauet, T. The inhibition of eIF5A hypusination by GC7, a preconditioning protocol to prevent brain death-induced renal injuries in a preclinical porcine kidney transplantation model. Am. J. Transplant. 2020, 20, 3326–3340. [Google Scholar] [CrossRef]
- Melis, N.; Rubera, I.; Cougnon, M.; Giraud, S.; Mograbi, B.; Belaid, A.; Pisani, D.F.; Huber, S.M.; Lacas-Gervais, S.; Fragaki, K.; et al. Targeting eIF5A Hypusination Prevents Anoxic Cell Death through Mitochondrial Silencing and Improves Kidney Transplant Outcome. J. Am. Soc. Nephrol. 2017, 28, 811–822. [Google Scholar] [CrossRef]
- Cougnon, M.; Carcy, R.; Melis, N.; Rubera, I.; Duranton, C.; Dumas, K.; Tanti, J.F.; Pons, C.; Soubeiran, N.; Shkreli, M.; et al. Inhibition of eIF5A hypusination reprogrammes metabolism and glucose handling in mouse kidney. Cell Death Dis. 2021, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Jasiulionis, M.G.; Luchessi, A.D.; Moreira, A.G.; Souza, P.P.; Suenaga, A.P.; Correa, M.; Costa, C.A.; Curi, R.; Costa-Neto, C.M. Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination impairs melanoma growth. Cell Biochem. Funct. 2007, 25, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Barriere, H.; Belfodil, R.; Rubera, I.; Tauc, M.; Poujeol, C.; Bidet, M.; Poujeol, P. CFTR null mutation altered cAMP-sensitive and swelling-activated Cl- currents in primary cultures of mouse nephron. Am. J. Physiol. Renal Physiol. 2003, 284, F796–F811. [Google Scholar] [CrossRef] [PubMed]
- Barriere, H.; Rubera, I.; Belfodil, R.; Tauc, M.; Tonnerieux, N.; Poujeol, C.; Barhanin, J.; Poujeol, P. Swelling-activated chloride and potassium conductance in primary cultures of mouse proximal tubules. Implication of KCNE1 protein. J. Membr. Biol. 2003, 193, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Komura, E.; Chagraoui, H.; Mansat de Mas, V.; Blanchet, B.; de Sepulveda, P.; Larbret, F.; Larghero, J.; Tulliez, M.; Debili, N.; Vainchenker, W.; et al. Spontaneous STAT5 activation induces growth factor independence in idiopathic myelofibrosis: Possible relationship with FKBP51 overexpression. Exp. Hematol. 2003, 31, 622–630. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Carcy, R.; Cougnon, M.; Poet, M.; Durandy, M.; Sicard, A.; Counillon, L.; Blondeau, N.; Hauet, T.; Tauc, M.; D, F.P. Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Free Radic. Biol. Med. 2021, 169, 258–270. [Google Scholar] [CrossRef]
- Robbins, R.D.; Tersey, S.A.; Ogihara, T.; Gupta, D.; Farb, T.B.; Ficorilli, J.; Bokvist, K.; Maier, B.; Mirmira, R.G. Inhibition of deoxyhypusine synthase enhances islet {beta} cell function and survival in the setting of endoplasmic reticulum stress and type 2 diabetes. J. Biol. Chem. 2010, 285, 39943–39952. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, D.; Zhu, J.; Wang, M.; Zhang, Y.; Fu, Q.; Zhang, S.; Lin, H. Oxygen level regulates N-terminal translation elongation of selected proteins through deoxyhypusine hydroxylation. Cell Rep. 2022, 39, 110855. [Google Scholar] [CrossRef]
- Barba-Aliaga, M.; Alepuz, P. Role of eIF5A in Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 1284. [Google Scholar] [CrossRef]
- Puleston, D.J.; Baixauli, F.; Sanin, D.E.; Edwards-Hicks, J.; Villa, M.; Kabat, A.M.; Kaminski, M.M.; Stanckzak, M.; Weiss, H.J.; Grzes, K.M.; et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 2021, 184, 4186–4202.e20. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef] [PubMed]
- Tauc, M.; Cougnon, M.; Carcy, R.; Melis, N.; Hauet, T.; Pellerin, L.; Blondeau, N.; Pisani, D.F. The eukaryotic initiation factor 5A (eIF5A1), the molecule, mechanisms and recent insights into the pathophysiological roles. Cell Biosci. 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, N.; Rubera, I.; Giraud, S.; Cougnon, M.; Duranton, C.; Poet, M.; Jarretou, G.; Thuillier, R.; Counillon, L.; Hauet, T.; et al. Renal Ischemia Tolerance Mediated by eIF5A Hypusination Inhibition Is Regulated by a Specific Modulation of the Endoplasmic Reticulum Stress. Cells 2023, 12, 409. https://doi.org/10.3390/cells12030409
Melis N, Rubera I, Giraud S, Cougnon M, Duranton C, Poet M, Jarretou G, Thuillier R, Counillon L, Hauet T, et al. Renal Ischemia Tolerance Mediated by eIF5A Hypusination Inhibition Is Regulated by a Specific Modulation of the Endoplasmic Reticulum Stress. Cells. 2023; 12(3):409. https://doi.org/10.3390/cells12030409
Chicago/Turabian StyleMelis, Nicolas, Isabelle Rubera, Sebastien Giraud, Marc Cougnon, Christophe Duranton, Mallorie Poet, Gisèle Jarretou, Raphaël Thuillier, Laurent Counillon, Thierry Hauet, and et al. 2023. "Renal Ischemia Tolerance Mediated by eIF5A Hypusination Inhibition Is Regulated by a Specific Modulation of the Endoplasmic Reticulum Stress" Cells 12, no. 3: 409. https://doi.org/10.3390/cells12030409
APA StyleMelis, N., Rubera, I., Giraud, S., Cougnon, M., Duranton, C., Poet, M., Jarretou, G., Thuillier, R., Counillon, L., Hauet, T., Pellerin, L., Tauc, M., & Pisani, D. F. (2023). Renal Ischemia Tolerance Mediated by eIF5A Hypusination Inhibition Is Regulated by a Specific Modulation of the Endoplasmic Reticulum Stress. Cells, 12(3), 409. https://doi.org/10.3390/cells12030409