Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil
Abstract
1. Introduction
2. Phosphorus Nutrition and Al Toxicity Stress
2.1. Uptake, Transport and Distribution of Al in Plants
2.2. Aluminum Resistance in Plants
2.3. Phosphorus–Aluminum Interactions in Soils and Plants
3. Phosphorus Nutrition and Mn Toxicity Stress
3.1. Uptake, Transport and Redistribution of Mn in Plants
3.2. Manganese Resistance in Plants
3.3. Phosphorus–Manganese Interactions in Soils and Plants
4. Phosphorus and Cd Toxicity Stress
4.1. Uptake, Transport and Redistribution of Cd by Plants
4.2. Cadmium Resistance in Plants
4.3. Phosphorus–Cadmium Interaction in Soils and Plants
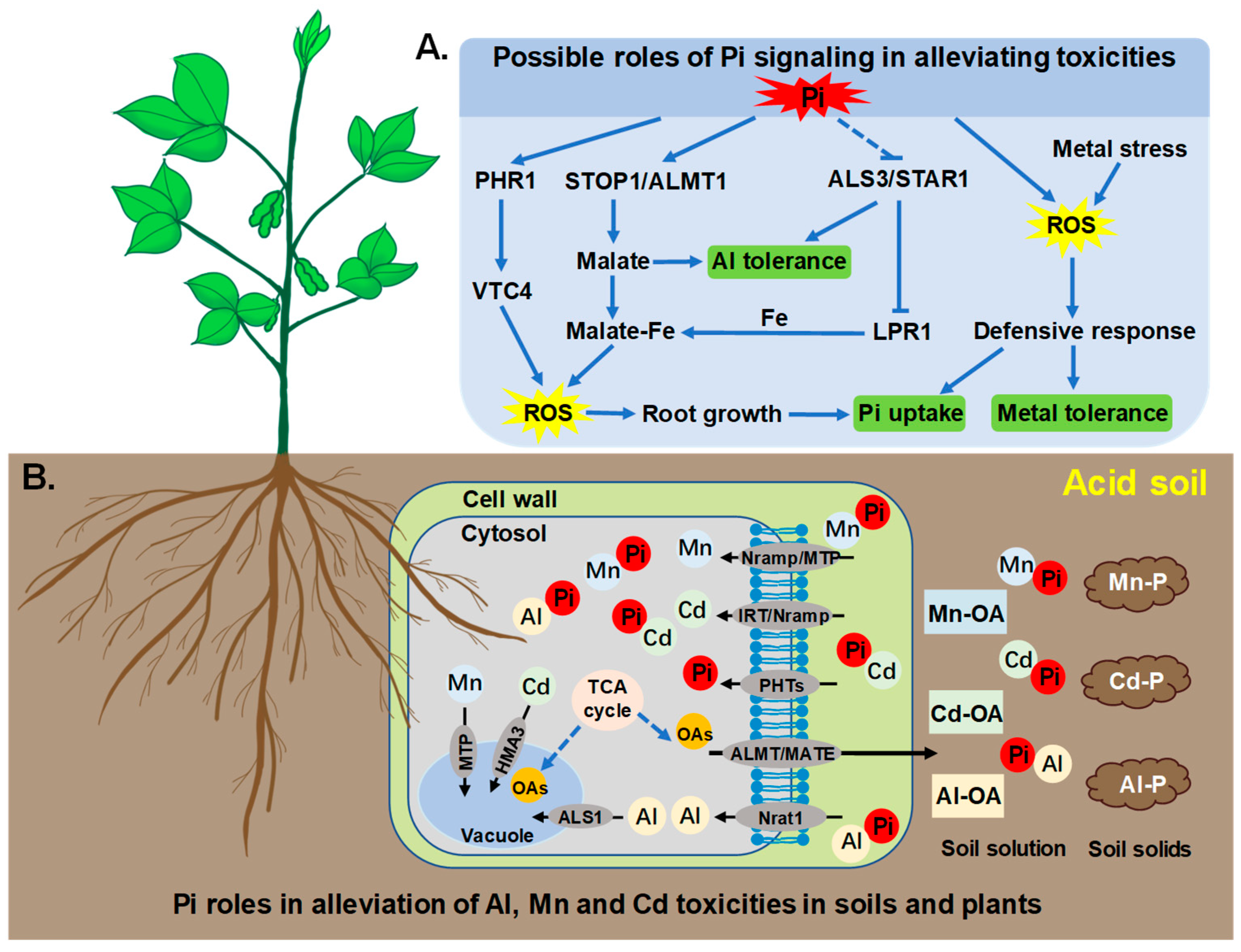
5. Phosphate Signaling and Its Roles in Alleviating Toxicity of Al, Mn, and Cd
5.1. Phosphate Signaling in Plants
5.2. The Role of Pi Signaling in Plant Resistance to Al, Mn, Cd
6. Applying Knowledge of P Interactions with Al, Mn, and Cd in Crop Improvement Efforts on Acid Soils
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils?—Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Diaz, M.; Alberdi, M.; Ivanov, A.; Krol, M.; Huner, N. Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 343–354. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Maistri, S.; Furini, A. How plants cope with cadmium: Staking all on metabolism and gene expression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Yang, G.; Rizwan, M.; Ali, S.; Zhou, Y.; Wang, Q.; Deng, L.; Wang, Y.; et al. Boron supply alleviates cadmium toxicity in rice (Oryza sativa L.) by enhancing cadmium adsorption on cell wall and triggering antioxidant defense system in roots. Chemosphere 2021, 266, 128938. [Google Scholar] [CrossRef]
- Chen, R.F.; Zhang, F.L.; Zhang, Q.M.; Sun, Q.B.; Dong, X.Y.; Shen, R.F. Aluminium-phosphorus interactions in plants growing on acid soils: Does phosphorus always alleviate aluminium toxicity? J. Sci. Food Agric. 2012, 92, 995–1000. [Google Scholar] [CrossRef]
- Du, J.; Yan, C.; Li, Z. Phosphorus and cadmium interactions in Kandelia obovate (S. L.) in relation to cadmium tolerance. Environ. Sci. Pollut. Res. 2014, 21, 355–365. [Google Scholar] [CrossRef]
- Berrios, G.A.; Luengo Escobar, A.; Alberdi, M.R.; Nunes-Nesi, A.; Reyes-Diaz, M.M. Manganese toxicity amelioration by phosphorus supply in contrasting Mn resistant genotypes of ryegrass. Plant Physiol. Biochem. 2019, 144, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.; Chen, J.; Yan, Y.; Lucas, W. Insights into plant phosphate sensing and signaling. Curr. Opin. Biotechnol. 2018, 49, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Kuo, H.; Chiou, T. Intracellular phosphate sensing and regulation of phosphate transport systems in plants. Plant Physiol. 2021, 187, 2043–2055. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Poirier, Y.; Jaskolowski, A.; Clúa, J. Phosphate acquisition and metabolism in plants. Curr. Biol. 2022, 32, R623–R629. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, J.; Pan, L.; Cao, H.; Li, C.; Lambers, H.; Wang, X. Soil property determines the ability of rhizobial inoculation to enhance nitrogen fixation and phosphorus acquisition in soybean. Appl. Soil Ecol. 2022, 171, 104346. [Google Scholar] [CrossRef]
- Wang, X.R.; Yan, X.L.; Liao, H. Genetic improvement for phosphorus efficiency in soybean: A radical approach. Ann. Bot. 2010, 106, 215–222. [Google Scholar] [CrossRef]
- Cong, W.; Suriyagoda, L.; Lambers, H. Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci. 2020, 25, 967–975. [Google Scholar] [CrossRef]
- Wang, F.; Deng, M.; Xu, J.; Zhu, X.; Mao, C. Molecular mechanisms of phosphate transport and signaling in higher plants. Semin. Cell Dev. Biol. 2018, 74, 114–122. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Kochian, L.; Piñeros, M.; Liu, J.; Magalhaes, J. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yan, W.; Sun, L.; Tian, J.; Liao, H. Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. J. Proteome 2016, 30, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.; Pena, L.; Barcia, R.; Azpilicueta, C.; Iannone, M.; Rosales, E.; Zawoznik, M.; Groppa, M.; Benavides, M. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Parveen, A.; Malik, Z.; Wang, X. Cadmium uptake and translocation: Selenium and silicon roles in Cd detoxification for the production of low Cd crops: A critical review. Chemosphere 2021, 273, 129690. [Google Scholar] [CrossRef]
- Horst, W.J.; Schmohl, N.; Kollmeier, M.; Baluska, F.; Sivaguru, M. Does aluminium affect root growth of maize through interaction with the cell wall–plasma membrane–cytoskeleton continuum? Plant Soil 1999, 215, 163–174. [Google Scholar] [CrossRef]
- Clarkson, D.T. Interactions between aluminum and phosphorus on root surfaces and cell wall material. Plant Soil 1967, 27, 347–356. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Peng, Y.; Zheng, C.; Li, G.; Liu, Y.; Shi, Y.; Zheng, S. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011, 155, 1885–1892. [Google Scholar] [CrossRef]
- Ma, J.F.; Shen, R.; Nagao, S.; Tanimoto, E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 2004, 45, 583–589. [Google Scholar] [CrossRef]
- Zhu, X.; Lei, G.; Wang, Z.; Shi, Y.; Braam, J.; Li, G.; Zheng, S. Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol. 2013, 162, 1947–1955. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Piñeros, M.A.; Kochian, L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014, 56, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Tsutsui, T.; Yokosho, K.; Yamaji, N.; Ma, J. Functional characterization of an aluminum (Al)-inducible transcription factor, ART2, revealed a different pathway for Al tolerance in rice. New Phytol. 2018, 220, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.; Liu, J.; Guimaraes, C.; Lana, U.; Alves, V.; Wang, Y.; Schaffert, R.; Hoekenga, O.; Pineros, M.; Shaff, J.; et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar] [CrossRef]
- Liu, J.; Magalhaes, J.; Shaff, J.; Kochian, L. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Maron, L.; Piñeros, M.; Guimarães, C.; Magalhaes, J.; Pleiman, J.; Mao, C.; Shaff, J.; Belicuas, S.; Kochian, L. Two functionally distinct members of the MATE multidrug and toxic compound extrusion family of transporters potentially underlie two major Al tolerance QTL in maize. Plant J. 2010, 61, 728–740. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Zhou, Y.; Piñeros, M.; Kochian, L.; Li, G.; Zheng, S. A de novo synthesis citrate transporter, Vigna umbellate multidrug and toxic compound extrusion, implicated in Al-activated citrate efflux in rice bean (Vigna umbellata) root apex. Plant Cell Environ. 2011, 34, 2138–2148. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011, 68, 1061–1069. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhang, Y.; Zhang, S.; Wu, Y.; Wu, P.; Zheng, S. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, Y.; Lei, G.; Fry, S.; Zhang, B.; Zhou, Y.; Braam, J.; Jiang, T.; Xu, X.; Mao, C.; et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Cancel, J.; Rounds, M.; Ochoa, V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 2007, 225, 1447–1458. [Google Scholar] [CrossRef]
- Sawaki, Y.; Iuchi, S.; Kobayashi, Y.; Kobayashi, Y.; Ikka, T.; Sakurai, N.; Fujita, M.; Shinozaki, K.; Shibata, D.; Kobayashi, M. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009, 150, 281–294. [Google Scholar] [CrossRef]
- Tokizawa, M.; Enomoto, T.; Ito, H.; Wu, L.; Kobayashi, Y.; Mora-Macías, J.; Armenta-Medina, D.; Iuchi, S.; Kobayashi, M.; Nomoto, M.; et al. High affinity promoter binding of STOP1 is essential for the early aluminum-inducible expression of novel Al resistance genes GDH1 and GDH2 in Arabidopsis. J. Exp. Bot. 2021, 72, 2769–2789. [Google Scholar] [CrossRef] [PubMed]
- Arenhart, R.; Bai, Y.; de Oliveira, L.; Neto, L.; Schunemann, M.; Maraschin, F.; Mariath, J.; Silverio, A.; Sachetto-Martins, G.; Margis, R.; et al. New insights into aluminum tolerance in rice: The ASR5 protein binds the STAR1 promoter and other aluminum-responsive genes. Mol. Plant 2014, 7, 709–721. [Google Scholar] [CrossRef]
- Ma, J.F.; Chen, Z.C.; Shen, R.F. Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 2014, 381, 1–12. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.; Li, G.; Wu, Y.; Yang, J.; Ma, J.; et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef]
- Liao, H.; Wan, H.; Shaff, J.; Wang, X.; Yan, X.; Kochian, L.V. Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol. 2006, 141, 674–684. [Google Scholar] [CrossRef]
- Tan, K.; Keltjens, W.G. Interaction between aluminium and phosphorus in sorghum plants. I. Studies with the aluminium sensitive sorghum genotype TAM428. Plant Soil 1990, 124, 15–23. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef]
- Maejima, E.; Watanabe, T.; Osaki, M.; Wagatsuma, T. Phosphorus deficiency enhances aluminum tolerance of rice (Oryza sativa) by changing the physicochemical characteristics of root plasma membranes and cell walls. J. Plant Physiol. 2014, 171, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.L.; Kleinert, A.; Scortecci, K.C.; Benedito, V.A.; Valentine, A.J. Phosphorus-deficiency reduces aluminium toxicity by altering uptake and metabolism of root zone carbon dioxide. J. Plant Physiol. 2011, 168, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Parra-Almuna, L.; Diaz-Cortez, A.; Ferrol, N.; de la Luz Mora, M. Aluminium toxicity and phosphate deficiency activates antioxidant systems and up-regulates expression of phosphate transporters gene in ryegrass (Lolium perenne L.) plants. Plant Physiol. Biochem. 2018, 130, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Wang, G.; Wang, X.; Liao, H. Role of mycorrhizal symbiosis in aluminum and phosphorus interactions in relation to aluminum tolerance in soybean. Appl. Microbiol. Biotechnol. 2015, 99, 10225–10235. [Google Scholar] [CrossRef]
- Sun, Q.B.; Shen, R.F.; Zhao, X.Q.; Chen, R.F.; Dong, X.Y. Phosphorus enhances Al resistance in Al-resistant Lespedeza bicolor but not in Al-sensitive L. cuneata under relatively high Al stress. Ann. Bot. 2008, 102, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.M.; Tian, J.; Liao, H.; Bai, C.J.; Yan, X.L.; Liu, G.D. Aluminium tolerance and high phosphorus efficiency helps Stylosanthes better adapt to low-P acid soils. Ann. Bot. 2009, 103, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Taylor, P.; Hocking, P.J.; Simpson, R.J.; Ryan, P.R.; Richardson, A.E. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnol. J. 2009, 7, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, C.; Chen, Z.; Liu, P.; Tian, J.; Liu, G.; Liao, H. Superior aluminium (Al) tolerance of Stylosanthes is achieved mainly by malate synthesis through an Al-enhanced malic enzyme, SgME1. New Phytol. 2014, 202, 209–219. [Google Scholar] [CrossRef]
- Sparrow, L.A.; and Uren, N.C. Manganese oxidation and reduction in soils: Effects of temperature, water potential, pH and their interactions. Soil Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]
- Alejandro, S.; Holler, S.; Meier, B.; and Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Shao, J.; Yamaji, N.; Shen, R.F.; Ma, J. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; and Ma, J. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Sasaki, A.; Yamaji, N.; Miyaji, T.; Fujii, Y.; Takemoto, Y.; Moriyama, S.; Che, J.; Moriyama, Y.; Iwasaki, K.; et al. A polarly localized transporter for efficient manganese uptake in rice. Nat. Plants 2015, 1, 15170. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal–nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sasaki, A.; Xia, J.X.; Yokosho, K.; and Ma, J. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef]
- Delhaize, E.; Kataoka, T.; Hebb, D.; White, R.; Ryan, P. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 2003, 15, 1131–1142. [Google Scholar] [CrossRef]
- Chen, Z.; Fujii, Y.; Yamaji, N.; Masuda, S.; Takemoto, Y.; Kamiya, T.; Yusuyin, Y.; Iwasaki, K.; Kato, S.; Maeshima, M.; et al. Mn tolerance in rice is mediated by MTP8.1, a member of the cation diusion facilitator family. J. Exp. Bot. 2013, 64, 4375–4387. [Google Scholar] [CrossRef]
- Eroglu, S.; Meier, B.; Wirén, N.; Peiter, E. The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiol. 2016, 170, 1030–1045. [Google Scholar] [CrossRef]
- Pittman, J.; Shigaki, T.; Marshall, J.; Morris, J.; Cheng, N.; Hirschi, K. Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol. Biol. 2004, 56, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Akahori, T.; Maeshima, M. Expression profile of the genes for rice cation/HC exchanger family and functional analysis in yeast. Plant Cell Physiol. 2005, 46, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanism. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Peiter, E.; Montanini, B.; Gobert, A.; Pedas, P.; Husted, S.; Maathuis, F.; Blaudez, D.; Chalot, M.; Sanders, D. A secretory pathway-localized cation diusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 8532–8537. [Google Scholar] [CrossRef]
- Tsunemitsu, Y.; Genga, M.; Okada, T.; Yamaji, N.; Ma, J.F.; Miyazaki, A.; Kato, S.; Iwasaki, K.; Ueno, D. A member of cation diusion facilitator family, MTP11, is required for manganese tolerance and high fertility in rice. Planta 2018, 248, 231–241. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, F.; Hong, B.; Young, J.C.; Sussman, M.R.; Harper, J.F.; Sze, H. An endoplasmic reticulum-bound Ca(2+)/Mn(2+) pump, ECA1, supports plant growth and confers tolerance to Mn(2+) stress. Plant Physiol. 2002, 130, 128–137. [Google Scholar] [CrossRef]
- Mills, R.F.; Doherty, M.L.; Lopez-Marques, R.L.; Weimar, T.; Dupree, P.; Palmgren, M.G.; Pittman, J.K.; Williams, L.E. ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol. 2008, 146, 116–128. [Google Scholar] [CrossRef]
- de la Luz Mora, M.; Rosas, A.; Ribera, A.; Rengel, Z. Differential tolerance to Mn toxicity in perennial ryegrass genotypes: Involvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil 2009, 320, 79–89. [Google Scholar] [CrossRef]
- Blamey, F.; Hernandez-Soriano, M.; Cheng, M.; Tang, C.; Paterson, D.; Lombi, E.; Wang, W.; Scheckel, K.; Kopittke, P. Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiol. 2015, 169, 2006–2020. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Steffen, K.; Lynch, J. Light and excess manganese implications for oxidative stress in common bean. Plant Physiol. 1998, 118, 493–504. [Google Scholar] [CrossRef]
- Fecht-Christoffers, M.; Führs, H.; Braun, H.; Horst, W. The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiol. 2006, 140, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Ribera-Fonseca, A.; Inostroza-Blancheteau, C.; Cartes, P.; Rengel, Z.; Mora, M. Early induction of Fe-SOD gene expression is involved in tolerance to Mn toxicity in perennial ryegrass. Plant Physiol. Biochen. 2013, 73, 77–82. [Google Scholar] [CrossRef]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Sulfur mediated alleviation of Mn toxicity in polish wheat relates to regulating Mn allocation and improving antioxidant system. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Neilsen, D.; Neilsen, G.; Sinclair, A.; Linehan, D. Soil phosphorus status, pH and the manganese nutrition of wheat. Plant Soil 1992, 145, 45–50. [Google Scholar] [CrossRef]
- Pedas, P.; Husted, S.; Skytte, K.; Schjoerring, J. Elevated phosphorus impedes manganese acquisition by barley plants. Front Plant Sci. 2011, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Magalhães, G.; Cardoso, E. Manganese toxicity in mycorrhizal and phosphorus-fertilized soybean plants. J. Plant Nutr. Soil Sci. 2004, 27, 141–156. [Google Scholar] [CrossRef]
- Sarkar, D.; Pandey, S.; Sud, K.; Chanemougasoundharam, A. In vitro characterization of manganese toxicity in relation to phosphorus nutrition in potato (Solanum tuberosum L.). J. Plant Sci. 2004, 167, 977–986. [Google Scholar] [CrossRef]
- Rosas, A.; Rengel, Z.; Ribera, A.; Mora, M. Phosphorus nutrition alleviates manganese toxicity in Lolium perenne and Trifolium repens. J. Plant Nutr. Soil Sci. 2011, 174, 210–219. [Google Scholar] [CrossRef]
- Farcasanu, I.; Hirata, D.; Tsuchiya, E.; Nishiyama, F.; Miyakawa, T. Protein phosphatase 2B of Saccharomyces cerevisiae is required for tolerance to manganese, in blocking the entry of ions into the cells. Eur. J. Biochem. 1995, 232, 712–717. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Hasanuzzaman, M.; Hussain, S.; Li, G.; Liu, J. Phosphorus confers tolerance against manganese toxicity in Prunus persica by reducing oxidative stress and improving chloroplast ultrastructure. Chemosphere 2022, 291, 132999. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Hayes, P.; Laliberté, E.; Oliveira, R.; Turner, B. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- George, T.; French, A.; Brown, L.; Karley, A.; White, P.; Ramsay, L.; Daniell, T. Genotypic variation in the ability of landraces and commercial cereal varieties to avoid manganese deficiency in soils with limited manganese availability: Is there a role for root-exuded phytases? Physiol. Plant 2014, 151, 243–256. [Google Scholar] [CrossRef]
- Dučić, T.; Polle, A. Manganese toxicity in two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply. Funct. Plant Biol. 2007, 34, 31–40. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Grotz, N.; Dedaldechamp, F.; Gaymard, F.; Guerinot, M.; Briata, J.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Cailliatte, R.; Schikora, A.; Briat, J.; Mari, S.; Curie, C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 2010, 22, 904–917. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.; Zhao, F. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 function synergistically in zinc/cadmium uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Cobbett, C. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009, 181, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.; Peaston, K.; Runions, J.; Williams, L. HvHMA2, a P1b-ATPase from barley, is highly conserved among cereals and functions in Zn and Cd transport. PLoS ONE 2012, 7, e42640. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Luo, J.; Huang, J.; Zeng, D.; Peng, J.; Zhang, G.; Ma, H.; Guan, Y.; Yi, H.; Fu, Y.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Ma, J. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.; Ando, T.; Yano, M.; Ma, J. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Yang, G.; Fu, S.; Huang, J.; Li, L.; Long, Y.; Wei, Q.; Wang, Z.; Chen, Z.; Xia, J. The tonoplast-localized transporter OsABCC9 is involved in cadmium tolerance and accumulation in rice. Plant Sci. 2021, 307, 110894. [Google Scholar] [CrossRef]
- Thomine, S.; Lelievre, F.; Debarbieux, E.; Schroeder, J.; Barbier-Brygoo, H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003, 34, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Choppala, G.; Saifullah; Bolan, N.; Bibi, S.; Iqbal, M.; Rengel, Z.; Kunhikrishnan, A.; Ashwath, N.; Ok, Y. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit. Rev. Plant Sci. 2014, 33, 374–391. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, Z.; Song, J.; Huang, X.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Ma, J. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, L.; Tang, Z.; Huang, X.; Ma, J.; Zhao, F. Producing cadmium-free Indica rice by overexpressing OsHMA3. Environ. Int. 2019, 126, 619–626. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, L.; Hu, J.; Zhang, X.; Lu, K.; Dong, H.; Wang, D.; Zhao, F.; Huang, C.; et al. OsNramp5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y.; et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tian, H.; Li, L.; Guan, C.; Zhang, Z. Higher Cd-accumulating oilseed rape has stronger Cd tolerance due to stronger Cd fixation in pectin and hemicellulose and higher Cd chelation. Environ. Pollut. 2021, 285, 117218. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Dai, X.; Xu, W.; Ma, M. Over expressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 2008, 72, 1020–1026. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Bigalke, M.; Imseng, M.; Keller, A.; Rehkämper, M.; Wilcke, W.; Frossard, E. Using isotopes to trace freshly applied cadmium through mineral phosphorus fertilization in soil-fertilizer-plant systems. Sci. Total Environ. 2019, 648, 779–786. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.; Jung, K.; Lee, S.; Hong, C. Cadmium phytoavailability from 1976 through 2016: Changes in soil amended with phosphate fertilizer and compost. Sci. Total Environ. 2021, 762, 143132. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, B.; Bolan, N.; Wijesekara, H.; Kunhikrishnan, A.; Thangarajan, R.; Qi, F.; Matheyarasu, R.; Rocco, C.; Mbene, K.; Naidu, R. Phosphorus–cadmium interactions in paddy soils. Geoderma 2016, 270, 43–59. [Google Scholar] [CrossRef]
- Hong, C.; Chung, D.; Lee, D.; Kim, P. Comparison of phosphate materials for immobilizing cadmium in soil. Arch. Environ. Contam. Toxicol. 2010, 58, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Owens, V.; Park, S.; Kim, J.; Hong, C. Adsorption and precipitation of cadmium affected by chemical form and addition rate of phosphate in soils having different levels of cadmium. Chemosphere 2018, 206, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Increasing phosphate inhibits cadmium uptake in plants and promotes synthesis of amino acids in grains of rice. Environ. Pollut. 2020, 257, 113496. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Lu, H.; Liu, W.; Jia, H.; Hong, H.; Liu, J.; Yan, C. Phosphorus mediation of cadmium stress in two mangrove seedlings Avicennia marina and Kandelia obovata differing in cadmium accumulation. Ecotoxicol. Environ. Saf. 2017, 139, 272–279. [Google Scholar] [CrossRef]
- Xue, M.; Zhou, Y.; Yang, Z.; Lin, B.; Yuan, J.; Wu, S. Comparisons in subcellular and biochemical behaviors of cadmium between low-Cd and high-Cd accumulation cultivars of pakchoi (Brassica chinensis L.). Front. Environ. Sci. Eng. 2014, 8, 226–238. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, Q. Growth responses and cadmium accumulation of Mirabilis jalapa L. under interaction between cadmium and phosphorus. J. Hazard. Mater. 2009, 167, 38–43. [Google Scholar] [CrossRef]
- Shi, G.L.; Zhu, S.; Bai, S.; Xia, Y.; Lou, L.; Cai, Q. The transportation accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars their relationships with each other. J. Hazard. Mater. 2015, 299, 94–102. [Google Scholar] [CrossRef]
- Wang, S.; Huang, D.; Zhu, Q.; Zhu, H.; Liu, S.; Luo, Z.; Cao, X.; Wang, J.; Rao, Z.; Shen, X. Speciation and phytoavailability of cadmium in soil treated with cadmium-contaminated rice straw. Environ. Sci. Pollut. Res. 2015, 22, 2679–2686. [Google Scholar] [CrossRef]
- Ahonen-Jonnarth, U.; Finlay, R. Effects of elevated nickel and cadmium concentrations on growth and nutrient uptake of mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Plant Soil 2001, 236, 129–138. [Google Scholar] [CrossRef]
- Cui, G.; Ai, S.; Chen, K.; Wang, X. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef]
- Wang, X.; Ruan, W.; Yi, K. Internal phosphate starvation signaling and external phosphate availability have no obvious effect on the accumulation of cadmium in rice. J. Integr. Agric. 2019, 18, 2153–2161. [Google Scholar] [CrossRef]
- Corso, M.; Schvartzman, M.; Guzzo, F.; Souard, F.; Malkowski, E.; Hanikenne, M.; Verbruggen, N. Contrasting cadmium resistance strategies in two metallicolous populations of Arabidopsis halleri. New Phytol. 2018, 218, 283–297. [Google Scholar] [CrossRef]
- Chien, P.; Chiang, C.; Leong, S.; Chiou, T. Sensing and signaling of phosphate starvation: From local to long distance. Plant Cell Physiol. 2018, 59, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Balzergue, C.; Dartevelle, T.; Godon, C.; Laugier, E.; Meisrimler, C.; Teulon, J.; Creff, A.; Bissler, M.; Brouchoud, C.; Hagège, A.; et al. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat. Commun. 2017, 8, 15300. [Google Scholar] [CrossRef] [PubMed]
- Mora-Macías, J.; Ojeda-Rivera, J.; Gutiérrez-Alanís, D.; Yong-Villalobos, L.; Oropeza-Aburto, A.; Raya-González, J.; Jiménez-Domínguez, G.; Chávez-Calvillo, G.; Rellán-Álvarez, R.; Herrera-Estrella, L.; et al. Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc. Natl. Acad. Sci. USA 2017, 114, E3563–E3572. [Google Scholar] [CrossRef]
- Cederholm, H.; Benfey, P. Distinct sensitivities to phosphate deprivation suggest that RGF peptides play disparate roles in Arabidopsis thaliana root development. New Phytol. 2015, 207, 683–691. [Google Scholar] [CrossRef]
- Zhou, J.; Jiao, F.; Wu, Z.; Li, Y.; Wang, X.; He, X.; Zhong, W.; Wu, P. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol. 2008, 146, 1673–1686. [Google Scholar] [CrossRef]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.; Rubio, V.; Perez-Perez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef]
- Ruan, W.; Guo, M.; Cai, L.; Hu, H.; Li, C.; Liu, Y.; Wu, Z.; Mao, C.; Yi, K.; Wu, P.; et al. Genetic manipulation of a high-affinity PHR1 target cis-element to improve phosphorous uptake in Oryza sativa L. Plant Mol. Biol. 2015, 87, 429–440. [Google Scholar] [CrossRef]
- Gonzalez, E.; Solano, R.; Rubio, V.; Leyva, A.; Paz-Ares, J. PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 2015, 17, 3500–3512. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.; de Lorenzo, L.; Irigoyen, M.; Masiero, S.; Bustos, R.; Rodríguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of phosphate starvation response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ruan, W.; Shi, J.; Zhang, L.; Xiang, D.; Yang, C.; Li, C.; Wu, Z.; Liu, Y.; Yu, Y.; et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 14953–14958. [Google Scholar] [CrossRef] [PubMed]
- Belal, R.; Tang, R.; Li, Y.; Mabrouk, Y.; Badr, E.; Luan, S. An ABC transporter complex encoded by ALUMINUM SENSITIVE 3 and NAP3 is required for phosphate deficiency responses in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 463, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Pineros, M.; Li, X.; Yang, H.; Liu, Y.; Murphy, A.; Kochian, L.; Liu, D. An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol. Plant 2017, 10, 244–259. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Yang, L.; Zhu, B.; Luo, F. Phosphorus regulates the level of signaling molecules in rice to reduce cadmium toxicity. Curr. Issues Mol. Biol. 2022, 44, 4070–4086. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Abel, S. Phosphate scouting by root tips. Curr. Opin. Plant Biol. 2017, 39, 168–177. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.; Pazmiño, D.; Testillano, P.; Risueño, M.; del Río, L.; Sandalio, L. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef]
- Sivaguru, M.; Liu, J.; Kochian, L. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013, 76, 297–307. [Google Scholar] [CrossRef]
- Sun, L.; Tian, J.; Zhang, H.; Liao, H. Phytohormone regulation of root growth triggered by P deficiency or Al toxicity. J. Exp. Bot. 2016, 67, 3655–3664. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, Y.; Liang, C. Evaluation of phosphate fertilizers for the immobilization of Cd in contaminated soils. PLoS ONE 2015, 10, e0124022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Kou, J.; Shi, L.; Zhang, C.; Xu, F. Dual effects of transgenic Brassica napus overexpressing CS gene on tolerances to aluminum toxicity and phosphorus deficiency. Plant Soil 2013, 362, 231–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ai, S.; Liao, H. Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil. Cells 2023, 12, 441. https://doi.org/10.3390/cells12030441
Wang X, Ai S, Liao H. Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil. Cells. 2023; 12(3):441. https://doi.org/10.3390/cells12030441
Chicago/Turabian StyleWang, Xiurong, Shaoying Ai, and Hong Liao. 2023. "Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil" Cells 12, no. 3: 441. https://doi.org/10.3390/cells12030441
APA StyleWang, X., Ai, S., & Liao, H. (2023). Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil. Cells, 12(3), 441. https://doi.org/10.3390/cells12030441







