Cellular Factors That Shape the Activity or Function of Nitric Oxide-Stimulated Soluble Guanylyl Cyclase
Abstract
:1. Introduction
1.1. Cytosolic Guanylyl Cyclase Mediates Diverse Physiological Functions of NO
1.2. SGC Is a Highly Sensitive NO Receptor
2. Modulation of SGC Activity by Cell- and Tissue-Derived Small Molecules
2.1. Role of Additional NO as Regulating Cellular Factor
2.2. Role of Free Cellular Thiols in SGC Function
2.3. Role of Ca2+ Ion in the Activity of SGC
2.4. Cell- and Tissue-Derived Allosteric Factors
3. Modulation of SGC Activity by Cellular Proteins
3.1. Proteins Affecting the Redox Status of SGC Thiols
3.2. Proteins Affecting the Redox Status of SGC Heme
3.3. Proteins Affecting the Assembly of SGC Heterodimer
3.4. Proteins Affecting Sub-Cellular Localization of SGC
3.5. Interacting Proteins Altering the Response to NO
3.6. Role of Protein Kinases in SGC Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martin, E.; Sharina, I.; Seminara, A.R.; Krumenacker, J.; Murad, F. Nitric Oxide cell signalling mediated by cGMP. In Nitric Oxide, Cell Signalling and Gene Expression; Cadenas, E., Lamas, S., Eds.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Foster, M.W.; McMahon, T.J.; Stamler, J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003, 9, 160–168. [Google Scholar] [CrossRef]
- Zabel, U.; Kleinschnitz, C.; Oh, P.; Nedvetsky, P.; Smolenski, A.; Müller, H.; Kronich, P.; Kugler, P.; Walter, U.; Schnitzer, J.E.; et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nature 2002, 4, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, M.; Wittau, N.; Koesling, D. Guanylyl cyclase/PSD-95 interaction: Targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J. Biol. Chem. 2001, 276, 44647–44652. [Google Scholar] [CrossRef]
- Gerzer, R.; Böhme, E.; Hofmann, F.; Schultz, G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981, 132, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Marletta, M.A. Soluble Guanylate Cyclase from Bovine Lung: Activation with Nitric Oxide and Carbon Monoxide and Spectral Characterization of the Ferrous and Ferric States. Biochemistry 1994, 33, 5636–5640. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.-P.; Becker, E.M.; Alonso-Alija, C.; Apeler, H.; Dembowsky, K.; Feurer, A.; Gerzer, R.; Minuth, T.; Perzborn, E.; Pleiß, U.; et al. NO-independent regulatory site on soluble guanylate cyclase. Nature 2001, 410, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 330, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, S.; Walter, E.; Pagel, O.; Walter, U.; Sickmann, A.; Gambaryan, S.; Smolenski, A.; Zahedi, R.P.; Jurk, K. Effects of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets. Nitric Oxide 2018, 76, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Noiri, E.; Hu, Y.; Bahou, W.F.; Keese, C.R.; Giaever, I.; Goligorsky, M.S. Permissive Role of Nitric Oxide in Endothelin-induced Migration of Endothelial Cells. J. Biol. Chem. 1997, 272, 1747–1752. [Google Scholar] [CrossRef] [Green Version]
- Noiri, E.; Lee, E.; Testa, J.; Quigley, J.; Colflesh, D.; Keese, C.R.; Giaever, I.; Goligorsky, M.S. Podokinesis in endothelial cell migration: Role of nitric oxide. Am. J. Physiol. Physiol. 1998, 274, C236–C244. [Google Scholar] [CrossRef] [PubMed]
- Seki, J.; Nishio, M.; Kato, Y.; Motoyama, Y.; Yoshida, K. FK409, a new nitric-oxide donor, suppresses smooth muscle proliferation in the rat model of balloon angioplasty. Atherosclerosis 1995, 117, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Mergia, E.; Dangel, O.; Lange, A.; Koesling, D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci. USA 2007, 104, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.T.; Zheng, F.; Martin, E.; Kots, A.Y.; Krumenacker, J.S.; Choi, B.-K.; McCutcheon, I.E.; Weisbrodt, N.; Bögler, O.; et al. Restoring Soluble Guanylyl Cyclase Expression and Function Blocks the Aggressive Course of Glioma. Mol. Pharmacol. 2011, 80, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Fabbro, D.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The concise guide to pharmacology 2021/22: Catalytic receptors. Br. J. Pharmacol. 2021, 178 (Suppl. 1), S264–S312. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, M.; Behrends, S.; Harteneck, C.; Koesling, D. Functional properties of a naturally occurring isoform of soluble guanylyl cyclase. Biochem. J. 1998, 335, 125–130. [Google Scholar] [CrossRef]
- Mergia, E.; Russwurm, M.; Zoidl, G.; Koesling, D. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell. Signal. 2003, 15, 189–195. [Google Scholar] [CrossRef]
- Mergia, E.; Koesling, D.; Friebe, A. Genetic mouse models of the NO receptor ‘soluble’ guanylyl cyclases. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2009; Volume 191, pp. 33–46. [Google Scholar]
- Derbyshire, E.R.; Marletta, M.A.; Hogg, N.; Fukuto, J.M.; Switzer, C.H.; Miranda, K.M.; Wink, D.A.; Griffith, O.W.; Stuehr, D.J.; Masters, B.S.S.; et al. Structure and Regulation of Soluble Guanylate Cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef]
- Wedel, B.; Humbert, P.; Harteneck, C.; Foerster, J.; Malkewitz, J.; Böhme, E.; Schultz, G.; Koesling, D. Mutation of His-105 in the beta 1 subunit yields a nitric oxide-insensitive form of soluble guanylyl cyclase. Proc. Natl. Acad. Sci. USA 1994, 91, 2592–2596. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.M.; Schramm, M.; Schröder, H.; Wunder, F.; Stasch, J.-P. Identification of Residues Crucially Involved in the Binding of the Heme Moiety of Soluble Guanylate Cyclase. J. Biol. Chem. 2004, 279, 3025–3032. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Liu, R.; Wu, J.-X.; Chen, L. Structural insights into the mechanism of human soluble guanylate cyclase. Nature 2019, 574, 206–210. [Google Scholar] [CrossRef]
- Zhao, Y.; Brandish, P.E.; Ballou, D.P.; Marletta, M.A. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1999, 96, 14753–14758. [Google Scholar] [CrossRef] [PubMed]
- Makino, R.; Matsuda, H.; Obayashi, E.; Shiro, Y.; Iizuka, T.; Hori, H. EPR Characterization of Axial Bond in Metal Center of Native and Cobalt-substituted Guanylate Cyclase. J. Biol. Chem. 1999, 274, 7714–7723. [Google Scholar] [CrossRef] [PubMed]
- Horst, B.; Marletta, M.A. Physiological activation and deactivation of soluble guanylate cyclase. Nitric Oxide 2018, 77, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sharina, I.; Lezgyieva, K.; Krutsenko, Y.; Martin, E. Higher susceptibility to heme oxidation and lower protein stability of the rare alpha1C517Ybeta1 sGC variant associated with moyamoya syndrome. Biochem. Pharmacol. 2021, 186, 114459. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Martin, E.; Murad, F. Human recombinant soluble guanylyl cyclase: Expression, purification, and regulation. Proc. Natl. Acad. Sci. USA 2000, 97, 10763–10768. [Google Scholar] [CrossRef]
- Martin, E.; Berka, V.; Tsai, A.; Murad, F. Soluble Guanylyl Cyclase: The Nitric Oxide Receptor. Methods Enzymol. 2005, 396, 478–492. [Google Scholar] [CrossRef]
- Martin, E.; Berka, V.; Bogatenkova, E.; Murad, F.; Tsai, A.L. Ligand selectivity of soluble guanylyl cyclase: Effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric oxide, and carbon monoxide. J. Biol. Chem. 2006, 281, 27836–27845. [Google Scholar] [CrossRef]
- Friebe, A.; Schultz, G.; Koesling, D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996, 15, 6863–6868. [Google Scholar] [CrossRef]
- Kharitonov, V.G.; Sharma, V.S.; Magde, D.; Koesling, D. Kinetics and Equilibria of Soluble Guanylate Cyclase Ligation by CO: Effect of YC-1. Biochemistry 1999, 38, 10699–10706. [Google Scholar] [CrossRef]
- Coburn, R.F. Endogenous Carbon Monoxide Metabolism. Annu. Rev. Med. 1973, 24, 241–250. [Google Scholar] [CrossRef]
- Coburn, R.F.; Blakemore, W.S.; Forster, R.E. Endogenous carbon monoxide production in man. J. Clin. Investig. 1963, 42, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.S.; Vreman, H.J.; McLaughlin, B.E.; Brien, J.F.; Nakatsu, K. Measurement of Endogenous Carbon Monoxide Formation in Biological Systems. Antioxidants Redox Signal. 2002, 4, 271–277. [Google Scholar] [CrossRef]
- Tsai, A.L.; Berka, V.; Sharina, I.; Martin, E. Dynamic Ligand Exchange in Soluble Guanylyl Cyclase (sGC): Implications for sGC regulation and desensitization. J. Biol. Chem. 2011, 286, 43182–43192. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sharina, I.; Martin, E. Soluble guanylyl cyclase: Molecular basis for ligand selectivity and action in vitro and in vivo. Front. Mol. Biosci. 2022, 9, 1007768. [Google Scholar] [CrossRef]
- Russwurm, M.; Koesling, D. Isoforms of NO-sensitive guanylyl cyclase. Mol. Cell. Biochem. 2002, 230, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.L.; Martin, E.; Berka, V.; Olson, J.S. How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c’, Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase. Antioxid. Redox Signal. 2012, 17, 1246–1263. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef]
- Sato, M.; Nakajima, T.; Goto, M.; Umezawa, Y. Cell-Based Indicator to Visualize Picomolar Dynamics of Nitric Oxide Release from Living Cells. Anal. Chem. 2006, 78, 8175–8182. [Google Scholar] [CrossRef]
- Chen, K.; Popel, A. Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free. Radic. Biol. Med. 2006, 41, 668–680. [Google Scholar] [CrossRef]
- Tsai, A.L.; Berka, V.; Martin, E.; Olson, J.S. A "Sliding Scale Rule" for Selectivity among NO, CO, and O2 by Heme Protein Sensors. Biochemistry 2012, 51, 172–186. [Google Scholar] [CrossRef]
- Kharitonov, V.G.; Sharma, V.S.; Magde, D.; Koesling, D. Kinetics of Nitric Oxide Dissociation from Five- and Six-Coordinate Nitrosyl Hemes and Heme Proteins, Including Soluble Guanylate Cyclase. Biochemistry 1997, 36, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Brandish, P.E.; Buechler, W.; Marletta, M.A. Regeneration of the Ferrous Heme of Soluble Guanylate Cyclase from the Nitric Oxide Complex: Acceleration by Thiols and Oxyhemoglobin. Biochemistry 1998, 37, 16898–16907. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, V.G.; Russwurm, M.; Magde, D.; Sharma, V.S.; Koesling, D. Dissociation of Nitric Oxide from Soluble Guanylate Cyclase. Biochem. Biophys. Res. Commun. 1997, 239, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, M.; Mergia, E.; Mullershausen, F.; Koesling, D. Inhibition of Deactivation of NO-sensitive Guanylyl Cyclase Accounts for the Sensitizing Effect of YC-1. J. Biol. Chem. 2002, 277, 24883–24888. [Google Scholar] [CrossRef]
- Margulis, A.; Sitaramayya, A. Rate of Deactivation of Nitric Oxide-Stimulated Soluble Guanylate Cyclase: Influence of Nitric Oxide Scavengers and Calcium. Biochemistry 2000, 39, 1034–1039. [Google Scholar] [CrossRef]
- Bellamy, T.C.; Garthwaite, J. Sub-second Kinetics of the Nitric Oxide Receptor, Soluble Guanylyl Cyclase, in Intact Cerebellar Cells. J. Biol. Chem. 2001, 276, 4287–4292. [Google Scholar] [CrossRef]
- Batchelor, A.M.; Bartus, K.; Reynell, C.; Constantinou, S.; Halvey, E.J.; Held, K.F.; Dostmann, W.R.; Vernon, J.; Garthwaite, J. Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 22060–22065. [Google Scholar] [CrossRef]
- Mergia, E.; Friebe, A.; Dangel, O.; Russwurm, M.; Koesling, D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J. Clin. Investig. 2006, 116, 1731–1737. [Google Scholar] [CrossRef]
- Bellamy, T.C.; Wood, J.; Goodwin, D.A.; Garthwaite, J. Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc. Natl. Acad. Sci. USA 2000, 97, 2928–2933. [Google Scholar] [CrossRef]
- Sharma, V.S.; Magde, D. Activation of Soluble Guanylate Cyclase by Carbon Monoxide and Nitric Oxide: A Mechanistic Model. Methods 1999, 19, 494–505. [Google Scholar] [CrossRef]
- Schmidt, K.; Schrammel, A.; Koesling, R.; Mayer, B. Molecular Mechanisms Involved in the Synergistic Activation of Soluble Guanylyl Cyclase by YC-1 and Nitric Oxide in Endothelial Cells. Mol. Pharmacol. 2001, 59, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Balashova, N.; Chang, F.-J.; Lamothe, M.; Sun, Q.; Beuve, A. Characterization of a Novel Type of Endogenous Activator of Soluble Guanylyl Cyclase. J. Biol. Chem. 2005, 280, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.-P. Soluble Guanylate Cyclase Stimulators and Activators. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2019; Volume 264, pp. 355–391. [Google Scholar]
- Ghofrani, H.A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event: Insights From the VICTORIA Trial. JAMA Cardiol. 2020, 6, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Follmann, M.; Becker-Pelster, E.; Hahn, M.G.; Meier, C.; Freitas, C.; Roessig, L.; Stasch, J. Soluble GC stimulators and activators: Past, present and future. Br. J. Pharmacol. 2021, 178, 1–22. [Google Scholar] [CrossRef]
- Hahn, M.G.; Lampe, T.; El Sheikh, S.; Griebenow, N.; Woltering, E.; Schlemmer, K.-H.; Dietz, L.; Gerisch, M.; Wunder, F.; Becker-Pelster, E.-M.; et al. Discovery of the Soluble Guanylate Cyclase Activator Runcaciguat (BAY 1101042). J. Med. Chem. 2021, 64, 5323–5344. [Google Scholar] [CrossRef]
- Russwurm, M.; Koesling, D. NO activation of guanylyl cyclase. Embo. J. 2004, 23, 4443–4450. [Google Scholar] [CrossRef] [PubMed]
- Cary, S.P.L.; Winger, J.A.; Marletta, M.A. Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP. Proc. Natl. Acad. Sci. USA 2005, 102, 13064–13069. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Berka, V.; Sharina, I.; Tsai, A.-L. Mechanism of Binding of NO to Soluble Guanylyl Cyclase: Implication for the Second NO Binding to the Heme Proximal Site. Biochemistry 2012, 51, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.M.; Stevenson, C.E.; Andrew, C.R.; Eady, R.R. Unprecedented proximal binding of nitric oxide to heme: Implications for guanylate cyclase. EMBO J. 2000, 19, 5661–5671. [Google Scholar] [CrossRef]
- Herzik, M.A.; Jonnalagadda, R.; Kuriyan, J.; Marletta, M.A. Structural insights into the role of iron–histidine bond cleavage in nitric oxide-induced activation of H-NOX gas sensor proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E4156–E4164. [Google Scholar] [CrossRef]
- Wu, G.; Martin, E.; Berka, V.; Liu, W.; Garcin, E.D.; Tsai, A.-L. A new paradigm for gaseous ligand selectivity of hemoproteins highlighted by soluble guanylate cyclase. J. Inorg. Biochem. 2020, 214, 111267. [Google Scholar] [CrossRef]
- Fernhoff, N.B.; Derbyshire, E.R.; Marletta, M.A. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 2009, 106, 21602–21607. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.D.S. Transphosphorylation and G protein activation. Biochem. Pharmacol. 1990, 39, 1399–1404. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Harada, T.; Kagamiyama, H. IMP dehydrogenase inhibitors reduce intracellular tetrahydrobiopterin levels through reduction of intracellular GTP levels. Indications of the regulation of GTP cyclohydrolase I activity by restriction of GTP availability in the cells. J. Biol. Chem. 1992, 267, 20734–20739. [Google Scholar] [CrossRef]
- Schmidt, P.; Schramm, M.; Schröder, H.; Stasch, J.-P. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur. J. Pharmacol. 2003, 468, 167–174. [Google Scholar] [CrossRef]
- Stomberski, C.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Edwards, J.C.; Gruetter, D.Y.; Barry, B.K.; Gruetter, C.A. Possible involvement of S -nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980, 110, 275–278. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Gruetter, C.S. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite possible involvement of S-nitrosothiols. Biochim. Biophys. Acta (BBA) Gen. Subj. 1980, 631, 221–231. [Google Scholar] [CrossRef]
- Mellion, B.T.; Ignarro, L.J.; Myers, C.B.; Ohlstein, E.H.; Ballot, B.A.; Hyman, A.L.; Kadowitz, P.J. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol. Pharmacol. 1983, 23, 653–664. [Google Scholar]
- Sayed, N.; Baskaran, P.; Ma, X.; van den Akker, F.; Beuve, A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc. Natl. Acad. Sci. USA 2007, 104, 12312–12317. [Google Scholar] [CrossRef]
- Sayed, N.; Kim, D.D.; Fioramonti, X.; Iwahashi, T.; Durán, W.N.; Beuve, A. Nitroglycerin-Induced S-nitrosylation and Desensitization of Soluble Guanylyl Cyclase Contribute to Nitrate Tolerance. Circ. Res. 2008, 103, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Zhang, Y.-Y.; Handy, D.E.; Beuve, A.; Tang, S.-S.; Loscalzo, J.; Leopold, J.A. Aldosterone Increases Oxidant Stress to Impair Guanylyl Cyclase Activity by Cysteinyl Thiol Oxidation in Vascular Smooth Muscle Cells. J. Biol. Chem. 2009, 284, 7665–7672. [Google Scholar] [CrossRef] [PubMed]
- Crassous, P.-A.; Couloubaly, S.; Huang, C.; Zhou, Z.; Baskaran, P.; Kim, D.D.; Papapetropoulos, A.; Fioramonti, X.; Durán, W.N.; Beuve, A. Soluble guanylyl cyclase is a target of angiotensin II-induced nitrosative stress in a hypertensive rat model. Am. J. Physiol. Circ. Physiol. 2012, 303, H597–H604. [Google Scholar] [CrossRef]
- Mayer, B.; Kleschyov, A.L.; Stessel, H.; Russwurm, M.; Münzel, T.; Koesling, D.; Schmidt, K. Inactivation of Soluble Guanylate Cyclase by Stoichiometric S-Nitrosation. Mol. Pharmacol. 2008, 75, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, M.; Suvorava, T.; Freudenberger, T.; Dao, V.T.-V.; Fischer, J.W.; Weber, M.; Kojda, G. Regulation of vascular guanylyl cyclase by endothelial nitric oxide-dependent posttranslational modification. Basic Res. Cardiol. 2011, 106, 539–549. [Google Scholar] [CrossRef]
- Fernhoff, N.B.; Derbyshire, E.R.; Underbakke, E.S.; Marletta, M.A. Heme-assisted S-Nitrosation Desensitizes Ferric Soluble Guanylate Cyclase to Nitric Oxide. J. Biol. Chem. 2012, 287, 43053–43062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beuve, A.; Wu, C.; Cui, C.; Liu, T.; Jain, M.R.; Huang, C.; Yan, L.; Kholodovych, V.; Li, H. Identification of novel S-nitrosation sites in soluble guanylyl cyclase, the nitric oxide receptor. J. Proteom. 2016, 138, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.M.; Gladyshev, V.N. Cysteine Function Governs Its Conservation and Degeneration and Restricts Its Utilization on Protein Surfaces. J. Mol. Biol. 2010, 404, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Craven, P.A.; DeRubertis, F. Effects of thiol inhibitors on hepatic guanylate cylase activity. Biochim. Biophys. Acta 1978, 524, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, H.J.; Lewicki, J.A.; Murad, F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J. Biol. Chem. 1981, 256, 2958–2962. [Google Scholar] [CrossRef]
- Braughler, J. Soluble guanylate cyclase activation by nitric oxide and its reversal: Involvement of sulfhydryl group oxidation and reduction. Biochem. Pharmacol. 1983, 32, 811–818. [Google Scholar] [CrossRef]
- Alapa, M.; Cui, C.; Shu, P.; Li, H.; Kholodovych, V.; Beuve, A. Selective cysteines oxidation in soluble guanylyl cyclase catalytic domain is involved in NO activation. Free. Radic. Biol. Med. 2021, 162, 450–460. [Google Scholar] [CrossRef]
- Beuve, A.; Alruwaili, N.; Kandhi, S.; Sun, D.; Wolin, M.S. Thiol-Based Redox Modulation of Soluble Guanylyl Cyclase, the Nitric Oxide Receptor. Antioxid. Redox Signal. 2017, 26, 137–149. [Google Scholar] [CrossRef]
- Braughler, J.M.; Mittal, C.K.; Murad, F. Effects of thiols, sugars, and proteins on nitric oxide activation of guanylate cyclase. J. Biol. Chem. 1979, 254, 12450–12454. [Google Scholar] [CrossRef]
- Fritz, B.G.; Hu, X.; Brailey, J.L.; Berry, R.E.; Walker, F.A.; Montfort, W.R. Oxidation and Loss of Heme in Soluble Guanylyl Cyclase from Manduca sexta. Biochemistry 2011, 50, 5813–5815. [Google Scholar] [CrossRef]
- Stasch, J.-P.; Schmidt, P.M.; Nedvetsky, P.I.; Nedvetskaya, T.Y.; Kumar, A.H.S.; Meurer, S.; Deile, M.; Taye, A.; Knorr, A.; Lapp, H.; et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Investig. 2006, 116, 2552–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, L.; Schmidt, P.; Keim, Y.; Schaefer, S.; Schmidt, H.; Stasch, J. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br. J. Pharmacol. 2009, 157, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Sharina, I.G.; Jelen, F.; Bogatenkova, E.P.; Thomas, A.; Martin, E.; Murad, F. Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J. Biol. Chem. 2008, 283, 15104–15113. [Google Scholar] [CrossRef]
- Shah, R.C.; Sanker, S.; Wood, K.C.; Durgin, B.G.; Straub, A.C. Redox regulation of soluble guanylyl cyclase. Nitric Oxide 2018, 76, 97–104. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Nguyen, A.T.; Miller, M.P.; Hahn, S.A.; Sparacino-Watkins, C.; Jobbagy, S.; Carew, N.; Cantu-Medellin, N.; Wood, K.C.; Baty, C.J.; et al. Cytochrome b5 Reductase 3 Modulates Soluble Guanylate Cyclase Redox State and cGMP Signaling. Circ. Res. 2017, 121, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary diseas. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef]
- Durgin, B.G.; Hahn, S.A.; Schmidt, H.M.; Miller, M.P.; Hafeez, N.; Mathar, I.; Freitag, D.; Sandner, P.; Straub, A.C. Loss of smooth muscle CYB5R3 amplifies angiotensin II–induced hypertension by increasing sGC heme oxidation. J. Clin. Investig. 2019, 4, e129183. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.; Guo, D.C.; Regalado, E.; Mellor-Crummey, L.; Bamshad, M.; Nickerson, D.A.; Dauser, R.; Hanchard, N.; Marom, R.; Martin, E.; et al. Disrupted nitric oxide signaling due to GUCY1A3 mutations increases risk for moyamoya disease, achalasia and hypertension. Clin. Genet. 2016, 90, 351–360. [Google Scholar] [CrossRef]
- Martin, E.; Lee, Y.-C.; Murad, F. YC-1 activation of human soluble guanylyl cyclase has both heme-dependent and heme-independent components. Proc. Natl. Acad. Sci. USA 2001, 98, 12938–12942. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Zhou, Z.; Martin, E.; Sharina, I.; Esposito, I.; Szabo, C.; Bucci, M.; Cirino, G.; Papapetropoulos, A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 2016, 111, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Serfass, L.; Carr, H.S.; Aschenbrenner, L.M.; Burstyn, J.N. Calcium Ion Downregulates Soluble Guanylyl Cyclase Activity: Evidence for a Two-metal Ion Catalytic Mechanism. Arch. Biochem. Biophys. 2001, 387, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, S.J.; Jovanovic, A.; Jovanovic, S.; Wagner, F.; Terzic, A.; Waldman, S. Regulation of Nitric Oxide-Responsive Recombinant Soluble Guanylyl Cyclase by Calcium. Biochemistry 1999, 38, 6441–6448. [Google Scholar] [CrossRef]
- Ramanathan, S.; Mazzalupo, S.; Boitano, S.; Montfort, W.R. Thrombospondin-1 and Angiotensin II Inhibit Soluble Guanylyl Cyclase through an Increase in Intracellular Calcium Concentration. Biochemistry 2011, 50, 7787–7799. [Google Scholar] [CrossRef]
- Kazerounian, S.; Pitari, G.M.; Ruiz-Stewart, I.; Schulz, S.; Waldman, S.A. Nitric Oxide Activation of Soluble Guanylyl Cyclase Reveals High and Low Affinity Sites That Mediate Allosteric Inhibition by Calcium. Biochemistry 2002, 41, 3396–3404. [Google Scholar] [CrossRef]
- Andric, S.; Kostic, T.; Tomić, M.; Koshimizu, T.-A.; Stojilkovic, S.S. Dependence of Soluble Guanylyl Cyclase Activity on Calcium Signaling in Pituitary Cells. J. Biol. Chem. 2001, 276, 844–849. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Pandol, S.J. Dual regulation of cGMP formation by calcium in pancreatic acinar cells. Am. J. Physiol. Content 1995, 268, G900–G907. [Google Scholar] [CrossRef]
- Friebe, A.; Koesling, D. Mechanism of YC-1-Induced Activation of Soluble Guanylyl Cyclase. Mol. Pharmacol. 1998, 53, 123–127. [Google Scholar] [CrossRef]
- Horst, B.G.; Yokom, A.L.; Rosenberg, D.J.; Morris, K.L.; Hammel, M.; Hurley, J.H.; A Marletta, M. Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy. Elife 2019, 8, e50634. [Google Scholar] [CrossRef]
- Sharina, I.; Sobolevsky, M.; Doursout, M.-F.; Gryko, D.; Martin, E. Cobinamides Are Novel Coactivators of Nitric Oxide Receptor That Target Soluble Guanylyl Cyclase Catalytic Domain. Experiment 2011, 340, 723–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guetterman, H.M.; Huey, S.L.; Knight, R.; Fox, A.M.; Mehta, S.; Finkelstein, J.L. Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review. Adv. Nutr. Int. Rev. J. 2021, 13, 530–558. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Baskaran, P.; Ma, X.; Dunten, P.W.; Schaefer, M.; Stasch, J.-P.; Beuve, A.; Akker, F.V.D. Structure of Cinaciguat (BAY 58–2667) Bound to Nostoc H-NOX Domain Reveals Insights into Heme-mimetic Activation of the Soluble Guanylyl Cyclase. J. Biol. Chem. 2010, 285, 22651–22657. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Wood, K.S.; Wolin, M.S. Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc. Natl. Acad. Sci. USA 1982, 79, 2870–2873. [Google Scholar] [CrossRef]
- Martin, E.; Sharina, I.; Kots, A.; Murad, F. A constitutively activated mutant of human soluble guanylyl cyclase (sGC): Implication for the mechanism of sGC activation. Proc. Natl. Acad. Sci. USA 2003, 100, 9208–9213. [Google Scholar] [CrossRef] [PubMed]
- Koglin, M.; Behrends, S. Biliverdin IX is an endogenous inhibitor of soluble guanylyl cyclase. Biochem. Pharmacol. 2002, 64, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Crush, K. Carnosine and related substances in animal tissues. Comp. Biochem. Physiol. 1970, 34, 3–30. [Google Scholar] [CrossRef]
- Solana-Manrique, C.; Sanz, F.J.; Martínez-Carrión, G.; Paricio, N. Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases. Antioxidants 2022, 11, 848. [Google Scholar] [CrossRef]
- Severina, I. Soluble guanylate cyclase of platelets: Function and regulation in normal and pathological states. Adv. Enzym. Regul. 1992, 32, 35–56. [Google Scholar] [CrossRef]
- Severina, I.S.; Busygina, O.G. Effect of carnosine on the activation of human platelet soluble guanylate cyclase by sodium nitroprusside and protoporphyrin IX. Biochem. Int. 1990, 22, 455–465. [Google Scholar] [PubMed]
- Severina, I.S.; Bussygina, O.G.; Pyatakova, N.V. Carnosine as a regulator of soluble guanylate cyclase. Biochemistry 2000, 65, 783–788. [Google Scholar] [PubMed]
- Wilkinson, B.; Gilbert, H.F. Protein disulfide isomerase. Biochim. Biophys. Acta 2004, 1699, 35–44. [Google Scholar] [CrossRef]
- Heckler, E.J.; Crassous, P.-A.; Baskaran, P.; Beuve, A. Protein disulfide-isomerase interacts with soluble guanylyl cyclase via a redox-based mechanism and modulates its activity. Biochem. J. 2013, 452, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Alapa, M.; Shu, P.; Nagarajan, N.; Wu, C.; Sadoshima, J.; Kholodovych, V.; Li, H.; Beuve, A. Guanylyl cyclase sensitivity to nitric oxide is protected by a thiol oxidation-driven interaction with thioredoxin-1. J. Biol. Chem. 2017, 292, 14362–14370. [Google Scholar] [CrossRef]
- Gupte, S.A.; Rupawalla, T.; Phillibert, D., Jr.; Wolin, M.S. NADPH and heme redox modulate pulmonary artery relaxation and guanylate cyclase activation by NO. Am. J. Physiol. Cell. Mol. Physiol. 1999, 277, L1124–L1132. [Google Scholar] [CrossRef]
- Ghosh, A.; Stasch, J.-P.; Papapetropoulos, A.; Stuehr, D.J. Nitric Oxide and Heat Shock Protein 90 Activate Soluble Guanylate Cyclase by Driving Rapid Change in Its Subunit Interactions and Heme Content. J. Biol. Chem. 2014, 289, 15259–15271. [Google Scholar] [CrossRef]
- Ghosh, A.; Stuehr, D.J. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 12998–13003. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, E.A.; Singh, A.B.; Chakravarti, R.; Martinez-Guzman, O.; Saini, A.; Haque, M.M.; Garee, G.; Dans, P.D.; Hannibal, L.; Reddi, A.R.; et al. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 2018, 293, 14557–14568. [Google Scholar] [CrossRef]
- Dai, Y.; Sweeny, E.A.; Schlanger, S.; Ghosh, A.; Stuehr, D.J. GAPDH delivers heme to soluble guanylyl cyclase. J. Biol. Chem. 2020, 295, 8145–8154. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Schlanger, S.; Haque, M.M.; Misra, S.; Stuehr, D.J. Heat shock protein 90 regulates soluble guanylyl cyclase maturation by a dual mechanism. J. Biol. Chem. 2019, 294, 12880–12891. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Misra, S.; Dai, Y.; Ghosh, A. Maturation, inactivation, and recovery mechanisms of soluble guanylyl cyclase. J. Biol. Chem. 2021, 296, 100336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Schelvis, J.P.; Babcock, G.T.; Marletta, M.A. Identification of histidine 105 in the beta1 subunit of soluble guanylate cyclase as the heme proximal ligand. Biochemistry 1998, 37, 4502–4509. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Faul, E.M.; Ghosh, A.; Stuehr, D.J. NO rapidly mobilizes cellular heme to trigger assembly of its own receptor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115774119. [Google Scholar] [CrossRef] [PubMed]
- Venema, R.C.; Venema, V.J.; Ju, H.; Harris, M.B.; Snead, C.; Jilling, T.; Dimitropoulou, C.; Maragoudakis, M.E.; Catravas, J.D. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am. J. Physiol. Circ. Physiol. 2003, 285, H669–H678. [Google Scholar] [CrossRef]
- Tsai, E.J.; Liu, Y.; Koitabashi, N.; Bedja, D.; Danner, T.; Jasmin, J.-F.; Lisanti, M.; Friebe, A.; Takimoto, E.; Kass, D.A. Pressure-Overload–Induced Subcellular Relocalization/Oxidation of Soluble Guanylyl Cyclase in the Heart Modulates Enzyme Stimulation. Circ. Res. 2012, 110, 295–303. [Google Scholar] [CrossRef]
- Meurer, S.; Pioch, S.; Wagner, K.; Müller-Esterl, W.; Gross, S. AGAP1, a Novel Binding Partner of Nitric Oxide-sensitive Guanylyl Cyclase. J. Biol. Chem. 2004, 279, 49346–49354. [Google Scholar] [CrossRef]
- Crassous, P.; Shu, P.; Huang, C.; Gordan, R.; Brouckaert, P.; Lampe, P.D.; Xie, L.; Beuve, A. Newly Identified NO-Sensor Guanylyl Cyclase/Connexin 43 Association Is Involved in Cardiac Electrical Function. J. Am. Hear. Assoc. 2017, 6, e006397. [Google Scholar] [CrossRef]
- Hanafy, K.A.; Martin, E.; Murad, F. CCTη, a Novel Soluble Guanylyl Cyclase-interacting Protein. J. Biol. Chem. 2004, 279, 46946–46953. [Google Scholar] [CrossRef]
- Smith, T.M.; Willardson, B.M. Mechanistic insights into protein folding by the eukaryotic chaperonin complex CCT. Biochem. Soc. Trans. 2022, 50, 1403–1414. [Google Scholar] [CrossRef]
- Chauhan, S.; Jelen, F.; Sharina, I.; Martin, E. The G-protein regulator LGN modulates the activity of the NO receptor soluble guanylate cyclase. Biochem. J. 2012, 446, 445–453. [Google Scholar] [CrossRef]
- Murthy, K.S. Activation of phosphodiesterase 5 and inhibition of guanylate cyclase by cGMP-dependent protein kinase in smooth muscle. Biochem. J. 2001, 360 Pt 1, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.S. Modulation of soluble guanylate cyclase activity by phosphorylation. Neurochem. Int. 2004, 45, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sayed, N.; Pyriochou, A.; Roussos, C.; Fulton, D.; Beuve, A.; Papapetropoulos, A. Protein Kinase G Phosphorylates Soluble Guanylyl Cyclase on Serine 64 and Inhibits Its Activity. Arter. Thromb. Vasc. Biol. 2008, 28, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.; Rodríguez-Pascual, F.; Miras-Portugal, M.T.; Torres, M. Nitric oxide-sensitive guanylyl cyclase activity inhibition through cyclic GMP-dependent dephosphorylation. J. Neurochem. 2000, 75, 2029–2039. [Google Scholar] [CrossRef]
- Zwiller, J.; Revel, M.-O.; Basset, P. Evidence for phosphorylation of rat brain guanylate cyclase by cyclic AMP-dependent protein kinase. Biochem. Biophys. Res. Commun. 1981, 101, 1381–1387. [Google Scholar] [CrossRef]
- Kostic, T.S.; Andric, S.A.; Stojilkovic, S.S. Receptor-Controlled Phosphorylation of α1Soluble Guanylyl Cyclase Enhances Nitric Oxide-Dependent Cyclic Guanosine 5′-Monophosphate Production in Pituitary Cells. Mol. Endocrinol. 2004, 18, 458–470. [Google Scholar] [CrossRef]
- Zwiller, J.; Revel, M.O.; Malviya, A.N. Protein kinase C catalyzes phosphorylation of guanylate cyclase in vitro. J. Biol. Chem. 1985, 260, 1350–1353. [Google Scholar] [CrossRef]
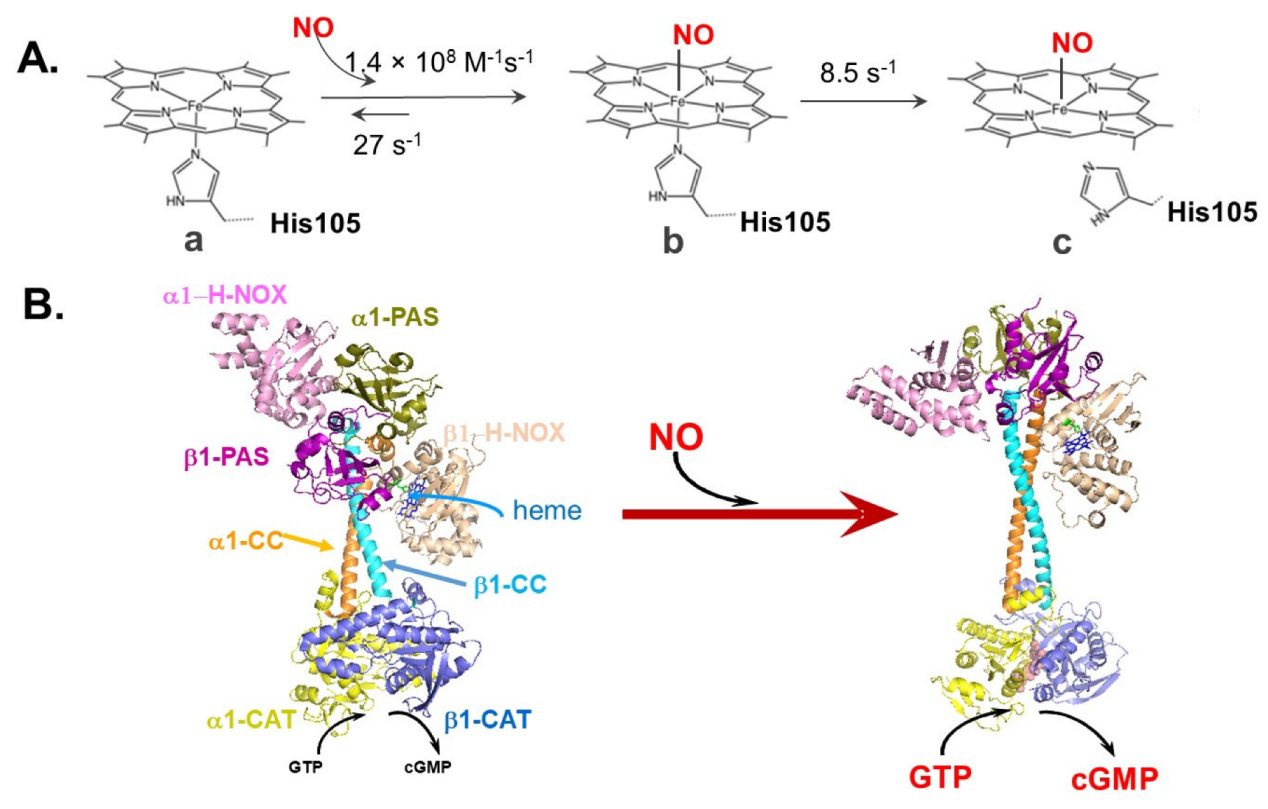
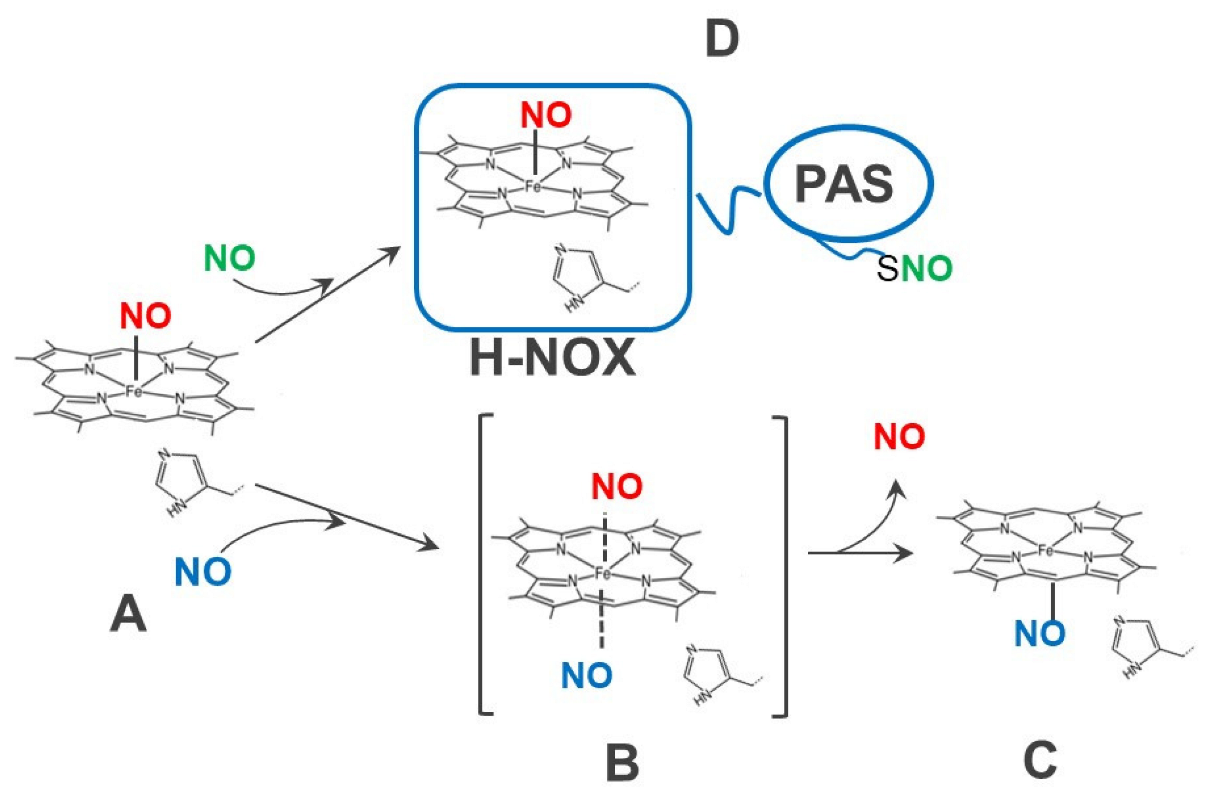
| Cellular Factor | Effect | References |
|---|---|---|
| Role of additional NO as an allosteric factor | ||
| Binding of additional NO to the proximal site of heme; Formation nitrosothiol or thionitroxide by additional NO Nitrosylation of sGC cysteines | Stabilizes of NO:SGC adduct; enhances SGC activity; Enhances SGC activity Desensitizes of SGC towards NO | [66] [70] [78,79,81,83,84,85] |
| Cell- and tissue-derived small molecules | ||
| Free cellular thiols | Reduction of oxidized SGC heme; Protects and reverses desensitization by nitrosothiols; Protects from inhibitory SGC thiol oxidation; | [27,102] [82] [87,88,89] |
| Hydrogen sulfide | Reduces oxidized SGC heme; | [104] |
| Ca2+ ion | Inhibits SGC via binding to two Ca2+-binding sites; Promotes translocation of SGC to membrane fraction. | [106,107,108] [3] |
| Protoporphyrin IX | Activates heme-deficient SGC | [117,118] |
| Biliverdin IX | Inhibits SGC activity | [119] |
| Carnosine | Inhibits SGC activation by NO | [122,123] |
| Cobinamide | Stimulates SGC activity | [114] |
| Cellular proteins | ||
| Protein targeting SGC thiols -protein disulfide isomerase (PDI) -thioredoxin-1 | Inhibits SGC activity Reverses S-nitrosylation of SGC | [126] [127] |
| Protein affecting SGC heme -CytB5R3 -Caveolin 3 | Maintains SGC heme in ferrous state Possibly protects SGC heme in cardiomyocytes | [138] |
| Proteins affecting SGC maturation -Hsp90 -GAPDH | Promotes maturation of the β1 subunit; prevents premature binding of α1 Delivers heme to the β1 subunit | [129,130] [131,132,133] |
| Protein affecting cellular localization -PSD95 -Hsp90 -Connexin 43 -AGAP1 | Localizes GC-2 to synaptosomes Directs SGC to caveolae in cardiomyocytes Binds SGC at the intercalating discs, affects cardiac electrical function Promotes SGC phosphorylation | [4] [137] [140] [139] |
| Proteins affecting SGC activity -CCTη -Hsp70 -LGN | Inhibits SGC activity upon binding Enhances SGC activity; promotes membrane localization Inhibits SGC in concert with unknown cellular factors | [141] [56] [143] |
| Protein kinases affecting SGC -PKG -PKA -PKC | Inhibition of SGC activity Stimulation of SGC activity Stimulation of SGC activity | [144,145,146,147] [148,149] [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharina, I.; Martin, E. Cellular Factors That Shape the Activity or Function of Nitric Oxide-Stimulated Soluble Guanylyl Cyclase. Cells 2023, 12, 471. https://doi.org/10.3390/cells12030471
Sharina I, Martin E. Cellular Factors That Shape the Activity or Function of Nitric Oxide-Stimulated Soluble Guanylyl Cyclase. Cells. 2023; 12(3):471. https://doi.org/10.3390/cells12030471
Chicago/Turabian StyleSharina, Iraida, and Emil Martin. 2023. "Cellular Factors That Shape the Activity or Function of Nitric Oxide-Stimulated Soluble Guanylyl Cyclase" Cells 12, no. 3: 471. https://doi.org/10.3390/cells12030471
APA StyleSharina, I., & Martin, E. (2023). Cellular Factors That Shape the Activity or Function of Nitric Oxide-Stimulated Soluble Guanylyl Cyclase. Cells, 12(3), 471. https://doi.org/10.3390/cells12030471







