AMPK Phosphorylation Impacts Apoptosis in Differentiating Myoblasts Isolated from Atrophied Rat Soleus Muscle
Abstract
1. Introduction
2. Materials and Methods
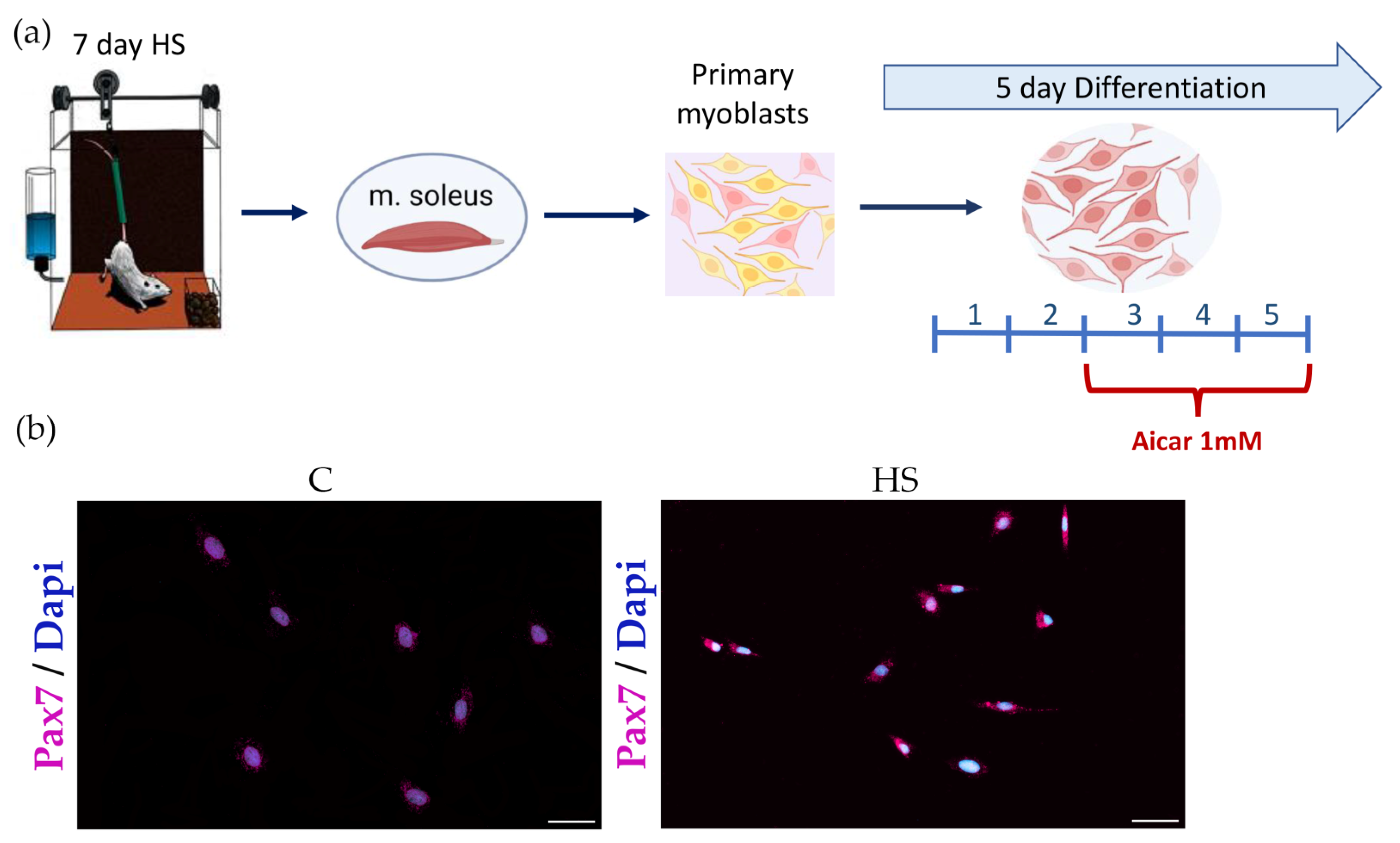
2.1. Experimental Design
2.2. In Situ Detection of Apoptotic Cells
2.3. Immunocytochemistry for Pax7 Detection
2.4. Gene Expression Analysis
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Body Weight and Soleus Muscle Weight
3.2. Phosphorylation Status of AMPK (Thr172), ACC (Ser79) and rpS6 (Ser 240/244) in Differentiating Myoblasts
3.3. Effect of AICAR Treatment on Morphological Characteristics and Expression of Differentiation Markers in Myoblasts Derived from the Atrophied Rat Soleus Muscle
3.4. The Number of Apoptotic Cells in Myoblast Cultures
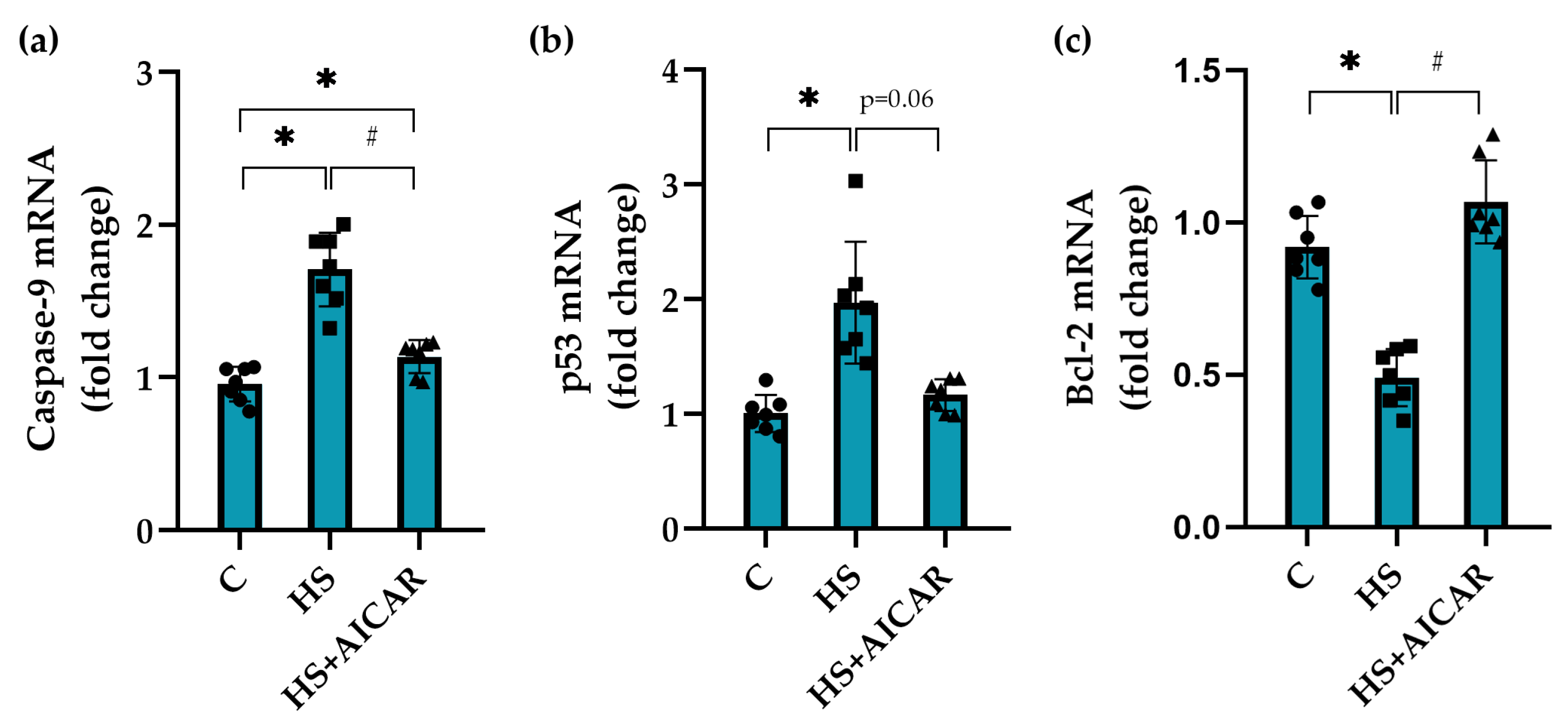
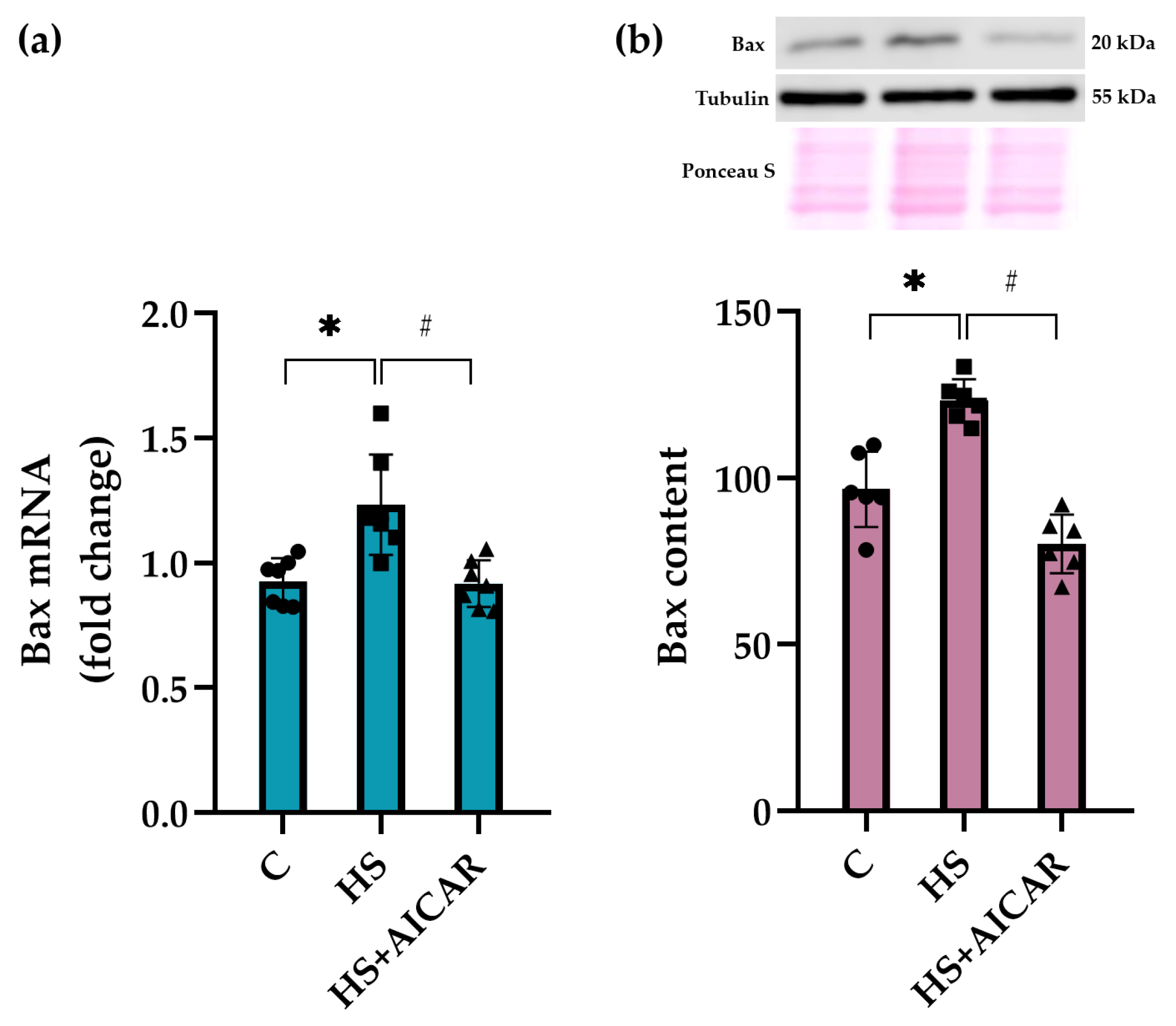
3.5. Expression of Apoptotic Markers in Myoblast Cultures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S.; Turtikova, O.V.; Nemirovskaya, T.L.; Grigoriev, A.I. Skeletal muscle activity and the fate of myonuclei. Acta Nat. 2010, 2, 59–66. [Google Scholar] [CrossRef]
- Nakanishi, R.; Hirayama, Y.; Tanaka, M.; Maeshige, N.; Kondo, H.; Ishihara, A.; Roy, R.R.; Fujino, H. Nucleoprotein supplementation enhances the recovery of rat soleus mass with reloading after hindlimb unloading-induced atrophy via myonuclei accretion and increased protein synthesis. Nutr. Res. 2016, 36, 1335–1344. [Google Scholar] [CrossRef]
- Wang, X.D.; Kawano, F.; Matsuoka, Y.; Fukunaga, K.; Terada, M.; Sudoh, M.; Ishihara, A.; Ohira, Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol. 2006, 290, C981–C989. [Google Scholar] [CrossRef]
- Mitchell, P.O.; Pavlath, G.K. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1753–C1762. [Google Scholar] [CrossRef]
- Stewart, C.E.; Newcomb, P.V.; Holly, J.M. Multifaceted roles of TNF-alpha in myoblast destruction: A multitude of signal transduction pathways. J. Cell. Physiol. 2004, 198, 237–247. [Google Scholar] [CrossRef]
- Jejurikar, S.S.; Kuzon, W.M., Jr. Satellite cell depletion in degenerative skeletal muscle. Apoptosis 2003, 8, 573–578. [Google Scholar] [CrossRef]
- Matsuba, Y.; Goto, K.; Morioka, S.; Naito, T.; Akema, T.; Hashimoto, N.; Sugiura, T.; Ohira, Y.; Beppu, M.; Yoshioka, T. Gravitational unloading inhibits the regenerative potential of atrophied soleus muscle in mice. Acta Physiol. 2009, 196, 329–339. [Google Scholar] [CrossRef]
- Radugina, E.A.; Almeida, E.A.C.; Blaber, E.; Poplinskaya, V.A.; Markitantova, Y.V.; Grigoryan, E.N. Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and impaired regeneration in murine femoral Quadriceps. Life Sci. Space Res. 2018, 16, 18–25. [Google Scholar] [CrossRef]
- Ferreira, R.; Neuparth, M.J.; Ascensao, A.; Magalhaes, J.; Vitorino, R.; Duarte, J.A.; Amado, F. Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first week of hindlimb suspension. Eur. J. Appl. Physiol. 2006, 97, 340–346. [Google Scholar] [CrossRef]
- Guitart, M.; Lloreta, J.; Manas-Garcia, L.; Barreiro, E. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J. Cell. Physiol. 2018, 233, 4360–4372. [Google Scholar] [CrossRef]
- Hardie, D.G. New roles for the LKB1→AMPK pathway. Curr. Opin. Cell Biol. 2005, 17, 167–173. [Google Scholar] [CrossRef]
- Mounier, R.; Lantier, L.; Leclerc, J.; Sotiropoulos, A.; Pende, M.; Daegelen, D.; Sakamoto, K.; Foretz, M.; Viollet, B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. FASEB J. Off. Public Fed. Am. Soc. Exp. Biol. 2009, 23, 2264–2273. [Google Scholar] [CrossRef]
- Villanueva-Paz, M.; Cotan, D.; Garrido-Maraver, J.; Oropesa-Avila, M.; de la Mata, M.; Delgado-Pavon, A.; de Lavera, I.; Alcocer-Gomez, E.; Alvarez-Cordoba, M.; Sanchez-Alcazar, J.A. AMPK Regulation of Cell Growth, Apoptosis, Autophagy, and Bioenergetics. Exp. Suppl. 2016, 107, 45–71. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, M.; Zhang, S.; Foretz, M.; Viollet, B.; Du, M. Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK. Diabetes 2016, 65, 188–200. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, M.J.; Dodson, M.V.; Du, M. AMP-activated protein kinase stimulates Warburg-like glycolysis and activation of satellite cells during muscle regeneration. J. Biol. Chem. 2015, 290, 26445–26456. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, J.X.; Zhu, M.J.; Foretz, M.; Viollet, B.; Dodson, M.V.; Du, M. AMP-activated protein kinase alpha1 but not alpha2 catalytic subunit potentiates myogenin expression and myogenesis. Mol. Cell. Biol. 2013, 33, 4517–4525. [Google Scholar] [CrossRef]
- Williamson, D.L.; Butler, D.C.; Alway, S.E. AMPK inhibits myoblast differentiation through a PGC-1alpha-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E304–E314. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Niesler, C.U.; Myburgh, K.H.; Moore, F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp. Physiol. 2007, 92, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Komarova, M.Y.; Rozhkov, S.V.; Ivanova, O.A.; Turtikova, O.V.; Mirzoev, T.M.; Dmitrieva, R.I.; Shenkman, B.S.; Vilchinskaya, N.A. Cultured Myoblasts Derived from Rat Soleus Muscle Show Altered Regulation of Proliferation and Myogenesis during the Course of Mechanical Unloading. Int. J. Mol. Sci. 2022, 23, 9150. [Google Scholar] [CrossRef] [PubMed]
- Vilchinskaya, N.A.; Rozhkov, S.V.; Komarova, M.Y.; Dmitrieva, R.I.; Shenkman, B.S. Effect of simulated gravitational unloading on m. Soleus satellite cells. Aviakosmicheskaya I Ekol. Meditsina 2022, 56, 20–29. [Google Scholar] [CrossRef]
- Jejurikar, S.S.; Marcelo, C.L.; Kuzon, W.M., Jr. Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plast. Reconstr. Surg. 2002, 110, 160–168. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Sancilio, S.; Nobilio, S.; Ruggiero, A.G.; Filippo, E.S.D.; Stati, G.; Fulle, S.; Bellomo, R.G.; Saggini, R.; Pietro, R.D. Effects of Focused Vibrations on Human Satellite Cells. Int. J. Mol. Sci. 2022, 23, 6026. [Google Scholar] [CrossRef]
- Vilchinskaya, N.A.; Mochalova, E.P.; Nemirovskaya, T.L.; Mirzoev, T.M.; Turtikova, O.V.; Shenkman, B.S. Rapid decline in MyHC I(beta) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation. J. Physiol. 2017, 595, 7123–7134. [Google Scholar] [CrossRef]
- Mirzoev, T.; Tyganov, S.; Vilchinskaya, N.; Lomonosova, Y.; Shenkman, B. Key Markers of mTORC1-Dependent and mTORC1-Independent Signaling Pathways Regulating Protein Synthesis in Rat Soleus Muscle During Early Stages of Hindlimb Unloading. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 39, 1011–1020. [Google Scholar] [CrossRef]
- Smith, H.K.; Maxwell, L.; Martyn, J.A.; Bass, J.J. Nuclear DNA fragmentation and morphological alterations in adult rabbit skeletal muscle after short-term immobilization. Cell Tissue Res. 2000, 302, 235–241. [Google Scholar] [CrossRef]
- Sandri, M.; Cantini, M.; Massimino, M.L.; Geromel, V.; Arslan, P. Myoblasts and myotubes in primary cultures deprived of growth factors undergo apoptosis. Basic Appl. Myol. 1996, 6, 257–260. [Google Scholar]
- Wang, J.; Walsh, K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 1996, 273, 359–361. [Google Scholar] [CrossRef]
- Tews, D.S.; Goebel, H.H. DNA fragmentation and BCL-2 expression in infantile spinal muscular atrophy. Neuromuscul. Disord. 1996, 6, 265–273. [Google Scholar] [CrossRef]
- Tews, D.S.; Goebel, H.H. DNA-fragmentation and expression of apoptosis-related proteins in muscular dystrophies. Neuropathol. Appl. Neurobiol. 1997, 23, 331–338. [Google Scholar] [CrossRef]
- Tidball, J.G.; Albrecht, D.E.; Lokensgard, B.E.; Spencer, M.J. Apoptosis precedes necrosis of dystrophin-deficient muscle. J. Cell Sci. 1995, 108, 2197–2204. [Google Scholar] [CrossRef]
- Andrianjafiniony, T.; Dupre-Aucouturier, S.; Letexier, D.; Couchoux, H.; Desplanches, D. Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. Am. J. Physiol. Cell Physiol. 2010, 299, C307–C315. [Google Scholar] [CrossRef]
- Loro, E.; Rinaldi, F.; Malena, A.; Masiero, E.; Novelli, G.; Angelini, C.; Romeo, V.; Sandri, M.; Botta, A.; Vergani, L. Normal myogenesis and increased apoptosis in myotonic dystrophy type-1 muscle cells. Cell Death Differ. 2010, 17, 1315–1324. [Google Scholar] [CrossRef]
- Liang, J.; Shao, S.H.; Xu, Z.X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef]
- Kefas, B.A.; Heimberg, H.; Vaulont, S.; Meisse, D.; Hue, L.; Pipeleers, D.; Van de Casteele, M. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 2003, 46, 250–254. [Google Scholar] [CrossRef]
- Kilbride, S.M.; Farrelly, A.M.; Bonner, C.; Ward, M.W.; Nyhan, K.C.; Concannon, C.G.; Wollheim, C.B.; Byrne, M.M.; Prehn, J.H. AMP-activated protein kinase mediates apoptosis in response to bioenergetic stress through activation of the pro-apoptotic Bcl-2 homology domain-3-only protein BMF. J. Biol. Chem. 2010, 285, 36199–36206. [Google Scholar] [CrossRef]
- Morishita, M.; Kawamoto, T.; Hara, H.; Onishi, Y.; Ueha, T.; Minoda, M.; Katayama, E.; Takemori, T.; Fukase, N.; Kurosaka, M.; et al. AICAR induces mitochondrial apoptosis in human osteosarcoma cells through an AMPK-dependent pathway. Int. J. Oncol. 2017, 50, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kewalramani, G.; Puthanveetil, P.; Wang, F.; Kim, M.S.; Deppe, S.; Abrahani, A.; Luciani, D.S.; Johnson, J.D.; Rodrigues, B. AMP-activated protein kinase confers protection against TNF-alpha-induced cardiac cell death. Cardiovasc. Res. 2009, 84, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Sun, Y.; Zhang, M.H.; Jin, Z. Insulin-like growth factor-1 inhibits apoptosis of rat gastric smooth muscle cells under high glucose condition via adenosine monophosphate-activated protein kinase (AMPK) pathway. Folia Histochem. Cytobiol. 2022, 60, 74–88. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Billin, A.N.; Campbell, M.E.; Russell, A.J.; Huffman, K.M.; Kraus, W.E. The AMPK/p27(Kip1) Axis Regulates Autophagy/Apoptosis Decisions in Aged Skeletal Muscle Stem Cells. Stem Cell Rep. 2018, 11, 425–439. [Google Scholar] [CrossRef]
- Bolster, D.R.; Crozier, S.J.; Kimball, S.R.; Jefferson, L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002, 277, 23977–23980. [Google Scholar] [CrossRef]
- Williamson, D.L.; Bolster, D.R.; Kimball, S.R.; Jefferson, L.S. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E80–E89. [Google Scholar] [CrossRef]
- Nakashima, K.; Ishida, A. AMP-activated Protein Kinase Activation Suppresses Protein Synthesis and mTORC1 Signaling in Chick Myotube Cultures. J. Poult. Sci. 2022, 59, 81–85. [Google Scholar] [CrossRef]
- Belova, S.P.; Vilchinskaya, N.A.; Mochalova, E.P.; Mirzoev, T.M.; Nemirovskaya, T.L.; Shenkman, B.S. Elevated p70S6K phosphorylation in rat soleus muscle during the early stage of unloading: Causes and consequences. Arch. Biochem. Biophys. 2019, 674, 108105. [Google Scholar] [CrossRef]
- Liu, J.; Long, S.; Wang, H.; Liu, N.; Zhang, C.; Zhang, L.; Zhang, Y. Blocking AMPK/ULK1-dependent autophagy promoted apoptosis and suppressed colon cancer growth. Cancer Cell Int. 2019, 19, 336. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Concannon, C.G.; Tuffy, L.P.; Weisova, P.; Bonner, H.P.; Davila, D.; Bonner, C.; Devocelle, M.C.; Strasser, A.; Ward, M.W.; Prehn, J.H. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J. Cell Biol. 2010, 189, 83–94. [Google Scholar] [CrossRef]
- Faivre, S.; Kroemer, G.; Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006, 5, 671–688. [Google Scholar] [CrossRef]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef]
- Gil-Gomez, G.; Berns, A.; Brady, H.J. A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J. 1998, 17, 7209–7218. [Google Scholar] [CrossRef]
- Hiromura, K.; Pippin, J.W.; Fero, M.L.; Roberts, J.M.; Shankland, S.J. Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27(Kip1). J. Clin. Investig. 1999, 103, 597–604. [Google Scholar] [CrossRef]



| Gene Name | Sequence (5’->3’) | GenBank |
|---|---|---|
| Caspase 9 | 5’-gaagaacgacctgactgctaag-3’ 5’-atgagagaggatgaccacca-3’ | NM_031632.2 |
| Caspase 3 | 5’-gagcttggaacgcgaagaaa-3’ 5’-taaccgggtgcggtagagta-3’ | NM_012922.2 |
| p53 | 5’-cccctgaagactggataactgt-3’ 5’-gacctcaggtggctcatacg-3’ | NM_030989.3 |
| Bax | 5’-ggcctttttgctacagggtttc-3’ 5’-gggggtcccgaagtaggaaag-3’ | NM_017059.2 |
| Bcl-2 | 5’-tcatgtgtgtggagagcgtc-3’ 5’-agttccacaaaggcatcccag-3’ | NM_016993.2 |
| Ywhaz | 5’-cccactccggacacagaata-3’ 5’-tgtcatcgtatcgctctgcc-3’ | NM_013011.4 |
| Gapdh | 5′-cggtgtgaacggatttggc-3′ 5′-ttgaggtcaatgaaggggtcg-3′ | NM_017008.4 |
| Groups | Body Weight, g | Soleus Wet Weight, mg | Soleus Weight-to-Body Weight Ratio, mg/g |
|---|---|---|---|
| C | 235 ± 4 | 78 ± 3 | 0.33 ± 0.01 |
| HS | 213 ± 5 * | 49 ± 3 * | 0.23 ± 0.01 * |
| Groups | Fusion Index, % | Area, µm² | Diameter, µm | Width, µm | Length, µm |
|---|---|---|---|---|---|
| C | 100 ± 4.3 | 8908 ± 814 | 70 ± 3 | 25 ± 1 | 510 ± 30 |
| HS | 143 ± 13.5 * | 5667 ± 569 * | 54 ± 3 * | 17 ± 1 * | 306 ± 38 * |
| HS+ AICAR | 102 ± 10.2 # | 7686 ± 759 # | 69 ± 3 # | 24 ± 2 # | 365 ± 31 * |
| Groups | Myogenin mRNA, 2−ΔΔCt | MyoD mRNA, 2−ΔΔCt | Myomaker mRNA, 2−ΔΔCt | Myomixer mRNA, 2−ΔΔCt |
|---|---|---|---|---|
| C | 1 ± 0.06 | 1 ± 0.04 | 1 ± 0.02 | 1 ± 0.05 |
| HS | 2.91 ± 0.22 * | 2.49 ± 0.28 * | 1.4 ± 0.15 * | 2.02 ± 0.11 * |
| HS + AICAR | 1.78 ± 0.15 *# | 1.63 ± 0.27 # | 1.14 ± 0.06 # | 1.67 ± 0.19 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilchinskaya, N.A.; Rozhkov, S.V.; Turtikova, O.V.; Mirzoev, T.M.; Shenkman, B.S. AMPK Phosphorylation Impacts Apoptosis in Differentiating Myoblasts Isolated from Atrophied Rat Soleus Muscle. Cells 2023, 12, 920. https://doi.org/10.3390/cells12060920
Vilchinskaya NA, Rozhkov SV, Turtikova OV, Mirzoev TM, Shenkman BS. AMPK Phosphorylation Impacts Apoptosis in Differentiating Myoblasts Isolated from Atrophied Rat Soleus Muscle. Cells. 2023; 12(6):920. https://doi.org/10.3390/cells12060920
Chicago/Turabian StyleVilchinskaya, Natalia A., Sergey V. Rozhkov, Olga V. Turtikova, Timur M. Mirzoev, and Boris S. Shenkman. 2023. "AMPK Phosphorylation Impacts Apoptosis in Differentiating Myoblasts Isolated from Atrophied Rat Soleus Muscle" Cells 12, no. 6: 920. https://doi.org/10.3390/cells12060920
APA StyleVilchinskaya, N. A., Rozhkov, S. V., Turtikova, O. V., Mirzoev, T. M., & Shenkman, B. S. (2023). AMPK Phosphorylation Impacts Apoptosis in Differentiating Myoblasts Isolated from Atrophied Rat Soleus Muscle. Cells, 12(6), 920. https://doi.org/10.3390/cells12060920







