Choroidal Mast Cells and Pathophysiology of Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Choroidal Mast Cells and AMD
2.1. Choroidal Mast Cells and Angioinflammatory Processes
2.2. Mast Cells in Retinal Inflammation and Oxidative Stress
2.3. Mast Cells in the Choroid
2.4. Choroidal Mast Cell Activation in AMD
3. The Sodium Iodate (NaIO3) Acute Model of AMD
3.1. NaIO3-Induced Oxidative Stress and Inflammation
3.2. NaIO3, RPE Cell Damage, and Its Implications in Dry AMD
3.3. Therapeutic Targets in NaIO3-Induced Outer Retinal Damage
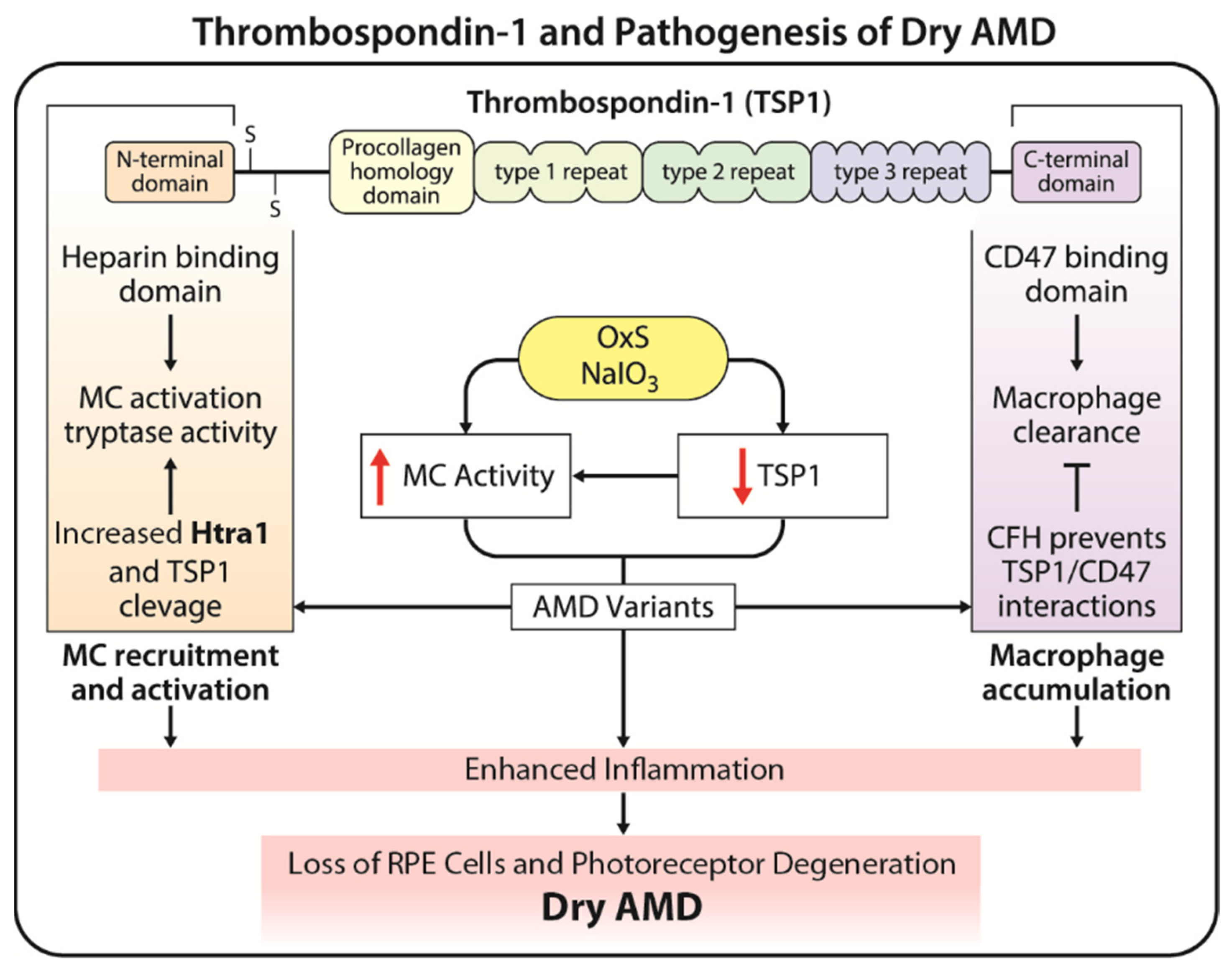
4. Thrombospondin-1 (TSP1) and Pathogenesis of AMD
4.1. Dysregulation of TSP1 in Pathogenesis of AMD
4.2. Therapeutic Potential of TSP1 in AMD
5. The Interplay between Oxidative Stress, Mast Cells, and TSP1
Potential Therapeutic Avenues Targeting This Interplay
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global burden of dry age-related macular degeneration: A targeted literature review. Clin. Ther. 2021, 43, 1792–1818. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.S.; Corneo, B.; Miller, J.D.; Kiehl, T.R.; Wang, Q.; Boles, N.C.; Blenkinsop, T.A.; Stern, J.H.; Temple, S. Nicotinamide ameliorates disease phenotypes in a human ipsc model of age-related macular degeneration. Cell Stem Cell 2017, 20, 635–647.e637. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Shahid, H.; Khan, J.C.; Cipriani, V.; Sepp, T.; Matharu, B.K.; Bunce, C.; Harding, S.P.; Clayton, D.G.; Moore, A.T.; Yates, J.R. Age-related macular degeneration: The importance of family history as a risk factor. Br. J. Ophthalmol. 2012, 96, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, A.; Del Priore, L.; Zarbin, M.A. Drusen in age-related macular degeneration: Pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 1999, 44, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The role of inflammation in age-related macular degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Haines, J.L.; Hauser, M.A.; Schmidt, S.; Scott, W.K.; Olson, L.M.; Gallins, P.; Spencer, K.L.; Kwan, S.Y.; Noureddine, M.; Gilbert, J.R.; et al. Complement factor h variant increases the risk of age-related macular degeneration. Science 2005, 308, 419–421. [Google Scholar] [CrossRef]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the htra1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef]
- Schustak, J.; Twarog, M.; Wu, X.; Wu, H.Y.; Huang, Q.; Bao, Y. Mechanism of nucleic acid sensing in retinal pigment epithelium (rpe): Rig-i mediates type i interferon response in human rpe. J. Immunol. Res. 2021, 2021, 9975628. [Google Scholar] [CrossRef]
- Dewan, A.; Liu, M.; Hartman, S.; Zhang, S.S.; Liu, D.T.; Zhao, C.; Tam, P.O.; Chan, W.M.; Lam, D.S.; Snyder, M.; et al. Htra1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314, 989–992. [Google Scholar] [CrossRef] [PubMed]
- McHarg, S.; Booth, L.; Perveen, R.; Riba Garcia, I.; Brace, N.; Bayatti, N.; Sergouniotis, P.I.; Phillips, A.M.; Day, A.J.; Black, G.C.M.; et al. Mast cell infiltration of the choroid and protease release are early events in age-related macular degeneration associated with genetic risk at both chromosomes 1q32 and 10q26. Proc. Natl. Acad. Sci. USA 2022, 119, e2118510119. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Jenny, L.A.; Li, Y.S.; Cui, X.; Kong, Y.; Li, Y.; Sparrow, J.R.; Tsang, S.H. Crispr editing demonstrates rs10490924 raised oxidative stress in ipsc-derived retinal cells from patients with arms2/htra1-related amd. Proc. Natl. Acad. Sci. USA 2023, 120, e2215005120. [Google Scholar] [CrossRef] [PubMed]
- Toops, K.A.; Tan, L.X.; Jiang, Z.; Radu, R.A.; Lakkaraju, A. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol. Biol. Cell 2015, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, S.S.; Sohn, E.H.; Chirco, K.R.; Drack, A.V.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Complement activation and choriocapillaris loss in early amd: Implications for pathophysiology and therapy. Prog. Retin. Eye Res. 2015, 45, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lejoyeux, R.; Benillouche, J.; Ong, J.; Errera, M.H.; Rossi, E.A.; Singh, S.R.; Dansingani, K.K.; da Silva, S.; Sinha, D.; Sahel, J.A.; et al. Choriocapillaris: Fundamentals and advancements. Prog. Retin. Eye Res. 2022, 87, 100997. [Google Scholar] [CrossRef] [PubMed]
- Mulfaul, K.; Russell, J.F.; Voigt, A.P.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. The essential role of the choriocapillaris in vision: Novel insights from imaging and molecular biology. Annu. Rev. Vis. Sci. 2022, 8, 33–52. [Google Scholar] [CrossRef]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between rpe and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef]
- Sohn, E.H.; Flamme-Wiese, M.J.; Whitmore, S.S.; Workalemahu, G.; Marneros, A.G.; Boese, E.A.; Kwon, Y.H.; Wang, K.; Abramoff, M.D.; Tucker, B.A.; et al. Choriocapillaris degeneration in geographic atrophy. Am. J. Pathol. 2019, 189, 1473–1480. [Google Scholar] [CrossRef]
- Calippe, B.; Augustin, S.; Beguier, F.; Charles-Messance, H.; Poupel, L.; Conart, J.B.; Hu, S.J.; Lavalette, S.; Fauvet, A.; Rayes, J.; et al. Complement factor h inhibits cd47-mediated resolution of inflammation. Immunity 2017, 46, 261–272. [Google Scholar] [CrossRef]
- Chen, C.Y.; Melo, E.; Jakob, P.; Friedlein, A.; Elsässer, B.; Goettig, P.; Kueppers, V.; Delobel, F.; Stucki, C.; Dunkley, T.; et al. N-terminomics identifies htra1 cleavage of thrombospondin-1 with generation of a proangiogenic fragment in the polarized retinal pigment epithelial cell model of age-related macular degeneration. Matrix Biol. 2018, 70, 84–101. [Google Scholar] [CrossRef]
- Lin, M.K.; Yang, J.; Hsu, C.W.; Gore, A.; Bassuk, A.G.; Brown, L.M.; Colligan, R.; Sengillo, J.D.; Mahajan, V.B.; Tsang, S.H. Htra1, an age-related macular degeneration protease, processes extracellular matrix proteins efemp1 and tsp1. Aging cell 2018, 17, e12710. [Google Scholar] [CrossRef] [PubMed]
- Sethna, S.; Scott, P.A.; Giese, A.P.J.; Duncan, T.; Jian, X.; Riazuddin, S.; Randazzo, P.A.; Redmond, T.M.; Bernstein, S.L.; Riazuddin, S.; et al. Cib2 regulates mtorc1 signaling and is essential for autophagy and visual function. Nat. Commun. 2021, 12, 3906. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Bhutto, I.; Edwards, M.M.; Gedam, M.; Baldeosingh, R.; Lutty, G.A. Mast cell-derived tryptase in geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Baldeosingh, R.; Bhutto, I.A.; Kambhampati, S.P.; Scott McLeod, D.; Edwards, M.M.; Rais, R.; Schubert, W.; Lutty, G.A. A role for mast cells in geographic atrophy. FASEB J. 2020, 34, 10117–10131. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; McLeod, D.S.; Jing, T.; Sunness, J.S.; Seddon, J.M.; Lutty, G.A. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Beghdadi, W.; Madjene, L.C.; Benhamou, M.; Charles, N.; Gautier, G.; Launay, P.; Blank, U. Mast cells as cellular sensors in inflammation and immunity. Front. Immunol. 2011, 2, 37. [Google Scholar] [CrossRef]
- Zaitoun, I.S.; Song, Y.S.; Zaitoun, H.B.; Sorenson, C.M.; Sheibani, N. Assessment of choroidal vasculature and innate immune cells in the eyes of albino and pigmented mice. Cells 2022, 11, 3329. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, nk cells. Cell Death Differ. 2019, 26, 703–714. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast cells and complement system: Ancient interactions between components of innate immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 2018, 174, 1293–1308.e1236. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Okamoto, N.; Kondo, M.; Arkwright, P.D.; Karasawa, K.; Ishizaka, S.; Yokota, S.; Matsuda, A.; Jung, K.; Oida, K.; et al. Mast cell hyperactivity underpins the development of oxygen-induced retinopathy. J. Clin. Investig. 2017, 127, 3987–4000. [Google Scholar] [CrossRef] [PubMed]
- Clare, A.J.; Liu, J.; Copland, D.A.; Theodoropoulou, S.; Dick, A.D. Unravelling the therapeutic potential of il-33 for atrophic amd. Eye 2022, 36, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100a8/a9 in inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Nagata, Y.; Suzuki, R. Fcεri: A master regulator of mast cell functions. Cells 2022, 11, 622. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Fedorov, A.V.; Ilyinskaya, O.P.; Zinovkin, R.A.; Chernyak, B.V. Role of reactive oxygen species in mast cell degranulation. Biochemistry 2016, 81, 1564–1577. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (amd): Relationships between the photoreceptor/retinal pigment epithelium/bruch’s membrane/choriocapillaris complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Rickman, C.B.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imagingdry amd mechanisms, targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, H.; Lu, S.; Li, X.; Wang, S.; Wang, W. Tryptase and exogenous trypsin: Mechanisms and ophthalmic applications. J. Inflamm. Res. 2023, 16, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.; Ness, S. The role of oxidative stress in the aging eye. Life 2023, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, F.; Ding, X.Q. Inhibition of thyroid hormone signaling protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration. Cell Death Dis. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Hsiao, Y.P.; Lin, Y.T.; Chen, C.; Lee, C.M.; Liao, W.C.; Tsou, S.C.; Lin, H.W.; Chang, Y.Y. Quercetin alleviates the accumulation of superoxide in sodium iodate-induced retinal autophagy by regulating mitochondrial reactive oxygen species homeostasis through enhanced deacetyl-sod2 via the nrf2-pgc-1α-sirt1 pathway. Antioxidants 2021, 10, 1125. [Google Scholar] [CrossRef]
- Trakkides, T.O.; Schäfer, N.; Reichenthaler, M.; Kühn, K.; Brandwijk, R.; Toonen, E.J.M.; Urban, F.; Wegener, J.; Enzmann, V.; Pauly, D. Oxidative stress increases endogenous complement-dependent inflammatory and angiogenic responses in retinal pigment epithelial cells independently of exogenous complement sources. Antioxidants 2019, 8, 548. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Q.; Tang, M.; Zhang, J.; Fan, W. Complement factor h expressed by retinal pigment epithelium cells can suppress neovascularization of human umbilical vein endothelial cells: An in vitro study. PLoS ONE 2015, 10, e0129945. [Google Scholar] [CrossRef] [PubMed]
- Brandstetter, C.; Holz, F.G.; Krohne, T.U. Complement component c5a primes retinal pigment epithelial cells for inflammasome activation by lipofuscin-mediated photooxidative damage. J. Biol. Chem. 2015, 290, 31189–31198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, H.; Pang, L.; Qiu, B.; Yuan, Y.; Guan, X.; Xiang, X. Qihuang granule protects the retinal pigment epithelium from oxidative stress via regulation of the alternative complement pathway. BMC Complement. Med. Ther. 2023, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Hsiao, G.; Chen, Y.S.; Lin, W.W.; Chan, C.M. P2x7 is involved in the mouse retinal degeneration via the coordinated actions in different retinal cell types. Antioxidants 2023, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2x7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Enzbrenner, A.; Zulliger, R.; Biber, J.; Pousa, A.M.Q.; Schäfer, N.; Stucki, C.; Giroud, N.; Berrera, M.; Kortvely, E.; Schmucki, R.; et al. Sodium iodate-induced degeneration results in local complement changes and inflammatory processes in murine retina. Int. J. Mol. Sci. 2021, 22, 9218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiu, Y.; Feng, Y.; Zhang, Y.; Li, Y.; Wang, B.; Wei, H.; Chen, X.; Shen, L.; Li, W.; et al. Calpain-2 facilitates autophagic/lysosomal defects and apoptosis in arpe-19 cells and rats induced by exosomes from rpe cells under naio(3) stimulation. Oxid. Med. Cell Longev. 2023, 2023, 3310621. [Google Scholar] [CrossRef]
- Kannan, R.; Hinton, D.R. Sodium iodate induced retinal degeneration: New insights from an old model. Neural Regen. Res. 2014, 9, 2044–2045. [Google Scholar]
- Wang, K.; Chen, Y.S.; Chien, H.W.; Chiou, H.L.; Yang, S.F.; Hsieh, Y.H. Melatonin inhibits naio(3)-induced arpe-19 cell apoptosis via suppression of hif-1α/bnip3-lc3b/mitophagy signaling. Cell Biosci. 2022, 12, 133. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Girardot, P.E.; Chrenek, M.A.; Sellers, J.T.; Li, Y.; Kim, Y.K.; Summers, V.R.; Ferdous, S.; Shelton, D.A.; et al. Electrophysiologic and morphologic strain differences in a low-dose naio3-induced retinal pigment epithelium damage model. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xie, J.; Hu, J.; Huang, X.; Ren, F.; Li, L. Protective effect of hydrogen on sodium iodate-induced age-related macular degeneration in mice. Front. Aging Neurosci. 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.; Kwon, M.Y.; Woo, J.M.; Chung, S.W. Oxidative stress-induced pentraxin 3 expression human retinal pigment epithelial cells is involved in the pathogenesis of age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 6028. [Google Scholar] [CrossRef] [PubMed]
- Panuthai, P.; Phumsuay, R.; Muangnoi, C.; Maitreesophone, P.; Kongkatitham, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Isolation and identification of dihydrophenanthrene derivatives from dendrobium virgineum with protective effects against hydrogen-peroxide-induced oxidative stress of human retinal pigment epithelium arpe-19 cells. Antioxidants 2023, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Cai, Y.; Li, Y.; Yu, X.; Lu, L. Protection of retina by mini-αa in naio3-induced retinal pigment epithelium degeneration mice. Int. J. Mol. Sci. 2015, 16, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Santhoshkumar, P.; Krishna Sharma, K. Alpha-crystallin-derived peptides as therapeutic chaperones. Biochim. Biophys. Acta 2016, 1860, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Phadte, A.S.; Sluzala, Z.B.; Fort, P.E. Therapeutic potential of α-crystallins in retinal neurodegenerative diseases. Antioxidants 2021, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, H.; Li, S.; Deng, X.; Zhang, J. Mini-αa upregulates the mir-155-5p target gene cdk2 and plays an antiapoptotic role in retinal pigment epithelial cells during oxidative stress. J. Ophthalmol. 2023, 2023, 6713094. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.M.; Drago, F.; Salomone, S.; Ragusa, M.; Barbagallo, C.; Di Pietro, C.; Purrello, M.; Reibaldi, M.; Avitabile, T.; et al. Retinal and circulating mirnas in age-related macular degeneration: An in vivo animal and human study. Front. Pharmacol. 2017, 8, 168. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Wang, S. Rpe necroptosis in response to oxidative stress and in amd. Ageing Res. Rev. 2015, 24, 286–298. [Google Scholar] [CrossRef]
- Tao, Y.; Murakami, Y.; Vavvas, D.G.; Sonoda, K.H. Necroptosis and neuroinflammation in retinal degeneration. Front. Neurosci. 2022, 16, 911430. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Matsumoto, H.; Roh, M.; Giani, A.; Kataoka, K.; Morizane, Y.; Kayama, M.; Thanos, A.; Nakatake, S.; Notomi, S.; et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and damp-mediated inflammation in dsrna-induced retinal degeneration. Cell Death Differ. 2014, 21, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, X.M.; Zhao, W.J.; Ban, X.X.; Li, Y.; Huang, Y.X.; Wan, H.; He, Y.; Liao, L.S.; Shang, L.; et al. Targeting necroptosis: A novel therapeutic option for retinal degenerative diseases. Int. J. Biol. Sci. 2023, 19, 658–674. [Google Scholar] [CrossRef]
- Cao, L.; Mu, W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications. Pharmacol. Res. 2021, 163, 105297. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Totsuka, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihara, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, X.; Sun, Y. Iron accumulation and lipid peroxidation in the aging retina: Implication of ferroptosis in age-related macular degeneration. Aging Dis. 2021, 12, 529–551. [Google Scholar] [CrossRef]
- Gupta, U.; Ghosh, S.; Wallace, C.T.; Shang, P.; Xin, Y.; Nair, A.P.; Yazdankhah, M.; Strizhakova, A.; Ross, M.A.; Liu, H.; et al. Increased lcn2 (lipocalin 2) in the rpe decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry amd. Autophagy 2023, 19, 92–111. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium arpe-19 cells. Cell Death Dis 2021, 12, 230. [Google Scholar] [CrossRef]
- Kyosseva, S.V. Targeting mapk signaling in age-related macular degeneration. Ophthalmol. Eye Dis. 2016, 8, 23–30. [Google Scholar] [CrossRef]
- Villarejo-Zori, B.; Jiménez-Loygorri, J.I.; Zapata-Muñoz, J.; Bell, K.; Boya, P. New insights into the role of autophagy in retinal and eye diseases. Mol. Aspects Med. 2021, 82, 101038. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Song, Y.S.; Sorenson, C.M.; Sheibani, N. Thrombospondin-1 in vascular development, vascular function, and vascular disease. Semin. Cell Dev. Biol. 2023, 155, 32–44. [Google Scholar] [CrossRef]
- Lopez-Dee, Z.; Pidcock, K.; Gutierrez, L.S. Thrombospondin-1: Multiple paths to inflammation. Mediat. Inflamm. 2011, 2011, 296069. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Suto, M.J. Thrombospondin-1 regulation of latent tgf-β activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018, 68–69, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Fibroblast-extracellular matrix interactions in tissue fibrosis. Curr. Pathobiol. Rep. 2016, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Sweetwyne, M.T.; Murphy-Ullrich, J.E. Thrombospondin1 in tissue repair and fibrosis: Tgf-β-dependent and independent mechanisms. Matrix Biol. 2012, 31, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, X.; Yuan, J.; Han, S. Contribution of thrombospondin-1 and -2 to lipopolysaccharide-induced acute respiratory distress syndrome. Mediat. Inflamm. 2021, 2021, 8876484. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000, 6, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, B.; Volpert, O.V.; Reiher, F.; Chang, L.; Munoz, A.; Karin, M.; Bouck, N. C-jun n-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene 2001, 20, 3443–3448. [Google Scholar] [CrossRef]
- Uno, K.; Bhutto, I.A.; McLeod, D.S.; Merges, C.; Lutty, G.A. Impaired expression of thrombospondin-1 in eyes with age related macular degeneration. Br. J. Ophthalmol. 2006, 90, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; Uno, K.; Merges, C.; Zhang, L.; McLeod, D.S.; Lutty, G.A. Reduction of endogenous angiogenesis inhibitors in bruch’s membrane of the submacular region in eyes with age-related macular degeneration. Arch. Ophthalmol. 2008, 126, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sorenson, C.M.; Sheibani, N. Lack of thrombospondin 1 and exacerbation of choroidal neovascularization. Arch. Ophthalmol. 2012, 130, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Zaitoun, I.; Farnoodian, M.; Fisk, D.L.; Wang, S.; Sorenson, C.M.; Sheibani, N. Expression of thrombospondin-1 modulates the angioinflammatory phenotype of choroidal endothelial cells. PLoS ONE 2014, 9, e116423. [Google Scholar] [CrossRef] [PubMed]
- Farnoodian, M.; Wang, S.; Dietz, J.; Nickells, R.W.; Sorenson, C.M.; Sheibani, N. Negative regulators of angiogenesis: Important targets for treatment of exudative amd. Clin. Sci. 2017, 131, 1763–1780. [Google Scholar] [CrossRef] [PubMed]
- Grimbert, P.; Bouguermouh, S.; Baba, N.; Nakajima, T.; Allakhverdi, Z.; Braun, D.; Saito, H.; Rubio, M.; Delespesse, G.; Sarfati, M. Thrombospondin/cd47 interaction: A pathway to generate regulatory t cells from human cd4+ cd25- t cells in response to inflammation. J. Immunol. 2006, 177, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 cd47 signalling: From mechanisms to medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef] [PubMed]
- Kaminuma, O.; Kitamura, N.; Gotoh, M.; Shindo, M.; Watanabe, N.; Saeki, M.; Nishimura, T.; Mori, A.; Nemoto, S.; Tatsumi, H.; et al. Thrombospondin 1-mediated suppression of mast cell degranulation is involved in the efficacy of sublingual immunotherapy. Allergo. Int. Off. J. Jpn. Soc. Allergol. 2019, 68s, S9–S10. [Google Scholar] [CrossRef]
- Sorenson, C.M.; Wang, S.; Darjatmoko, S.R.; Gurel, Z.; Liu, B.; Sheibani, N. Targeted thrombospondin-1 expression in ocular vascular development and neovascularization. Fron. Cell Dev. Biol. 2021, 9, 671989. [Google Scholar] [CrossRef]
- Crawford, S.E.; Stellmach, V.; Murphy-Ullrich, J.E.; Ribeiro, S.M.; Lawler, J.; Hynes, R.O.; Boivin, G.P.; Bouck, N. Thrombospondin-1 is a major activator of tgf-beta1 in vivo. Cell 1998, 93, 1159–1170. [Google Scholar] [CrossRef]
- Soriano-Romani, L.; Contreras-Ruiz, L.; Lopez-Garcia, A.; Diebold, Y.; Masli, S.; Contreras Ruiz, L.; Mir, F.A.; Turpie, B.; Masli, S. Topical application of tgf-beta-activating peptide, krfk, prevents inflammatory manifestations in the tsp-1-deficient mouse model of chronic ocular inflammation thrombospondin-derived peptide attenuates sjogren’s syndrome-associated ocular surface inflammation in mice. Int. J. Mol. Sci. 2018, 20, 86–95. [Google Scholar]
- Daftarian, N.; Rohani, S.; Kanavi, M.R.; Suri, F.; Mirrahimi, M.; Hafezi-Moghadam, A.; Soheili, Z.S.; Ahmadieh, H. Effects of intravitreal connective tissue growth factor neutralizing antibody on choroidal neovascular membrane-associated subretinal fibrosis. Exp. Eye Res. 2019, 184, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, N. Connective tissue growth factor: A key factor among mediators of tissue fibrosis. J. Ophthalmic. Vis. Res. 2022, 17, 449–452. [Google Scholar] [CrossRef]
- Picard, E.; Houssier, M.; Bujold, K.; Sapieha, P.; Lubell, W.; Dorfman, A.; Racine, J.; Hardy, P.; Febbraio, M.; Lachapelle, P.; et al. Cd36 plays an important role in the clearance of oxldl and associated age-dependent sub-retinal deposits. Aging 2010, 2, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Ramakrishnan, D.P.; Silverstein, R.L. Thrombospondin-1 modulates vegf signaling via cd36 by recruiting shp-1 to vegfr2 complex in microvascular endothelial cells. Blood 2013, 122, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Touhami, S.; Béguier, F.; Yang, T.; Augustin, S.; Roubeix, C.; Blond, F.; Conart, J.B.; Sahel, J.A.; Bodaghi, B.; Delarasse, C.; et al. Hypoxia inhibits subretinal inflammation resolution thrombospondin-1 dependently. Int. J. Mol. Sci. 2022, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 in maladaptive aging responses: A concept whose time has come. Am. J. Physiol. Cell Physiol. 2020, 319, C45–C63. [Google Scholar] [CrossRef]
- Ren, C.; Yu, J. Potential gene identification and pathway crosstalk analysis of age-related macular degeneration. Front. Genet. 2022, 13, 992328. [Google Scholar] [CrossRef]
- Arai, R.; Usui-Ouchi, A.; Ito, Y.; Mashimo, K.; Murakami, A.; Ebihara, N. Effects of secreted mast cell mediators on retinal pigment epithelial cells: Focus on mast cell tryptase. Mediators Inflamm. 2017, 2017, 3124753. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi-functional master cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Uppal, M.; Khan, W.; Samad, I.; Obasanmi, G.; To, E.; Cui, J.Z.; Yeung, S.; Matsubara, J.A. Mast cells contribute to choroidal neovascularization in ex vivo model of amd. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2301. [Google Scholar]
- Wang, Y.; Wang, V.M.; Chan, C.C. The role of anti-inflammatory agents in age-related macular degeneration (amd) treatment. Eye 2011, 25, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Eamegdool, S.S.; Sitiwin, E.I.; Cioanca, A.V.; Madigan, M.C. Extracellular matrix and oxidative stress regulate human retinal pigment epithelium growth. Free Radic. Biol. Med. 2020, 146, 357–371. [Google Scholar] [CrossRef] [PubMed]
- García-Onrubia, L.; Valentín-Bravo, F.J.; Coco-Martin, R.M.; González-Sarmiento, R.; Pastor, J.C.; Usategui-Martín, R.; Pastor-Idoate, S. Matrix metalloproteinases in age-related macular degeneration (amd). Int. J. Mol. Sci. 2020, 21, 5934. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Jayesh Sodha, S.; Junnuthula, V.; Kolimi, P.; Dyawanapelly, S. Novel and investigational therapies for wet and dry age-related macular degeneration. Drug Discov Today 2022, 27, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent advances in age-related macular degeneration therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Kashkoli, H.H.; Weyland, D.E.; Pirkalkhoran, S.; Grabowska, W.R. Advanced therapy medicinal products for age-related macular degeneration; scaffold fabrication and delivery methods. Pharmaceuticals 2023, 16, 620. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.; Chen, F.; Goldberg, J.L.; Sabel, B.A. Nanomedicine and drug delivery to the retina: Current status and implications for gene therapy. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 1477–1507. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on rpe degeneration in non-neovascular amd. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Copland, D.A.; Liu, J.; Scott, L.; Wu, J.; Dick, A.D. Modulation of mast cells regulates retinal microenvironment and vascular integrity. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5555. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malih, S.; Song, Y.-S.; Sorenson, C.M.; Sheibani, N. Choroidal Mast Cells and Pathophysiology of Age-Related Macular Degeneration. Cells 2024, 13, 50. https://doi.org/10.3390/cells13010050
Malih S, Song Y-S, Sorenson CM, Sheibani N. Choroidal Mast Cells and Pathophysiology of Age-Related Macular Degeneration. Cells. 2024; 13(1):50. https://doi.org/10.3390/cells13010050
Chicago/Turabian StyleMalih, Sara, Yong-Seok Song, Christine M. Sorenson, and Nader Sheibani. 2024. "Choroidal Mast Cells and Pathophysiology of Age-Related Macular Degeneration" Cells 13, no. 1: 50. https://doi.org/10.3390/cells13010050
APA StyleMalih, S., Song, Y.-S., Sorenson, C. M., & Sheibani, N. (2024). Choroidal Mast Cells and Pathophysiology of Age-Related Macular Degeneration. Cells, 13(1), 50. https://doi.org/10.3390/cells13010050








