Administration of Human-Derived Mesenchymal Stem Cells Activates Locally Stimulated Endogenous Neural Progenitors and Reduces Neurological Dysfunction in Mice after Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Induction of Ischemic Stroke
2.3. Cell Transplantation
2.4. Behavioral Tests
2.5. Preparation of Brain Samples Following Ischemic Stroke
2.6. Immunohistochemistry
2.7. Cell Culture
2.8. Microarray Analysis
2.9. Statistical Analysis
3. Results
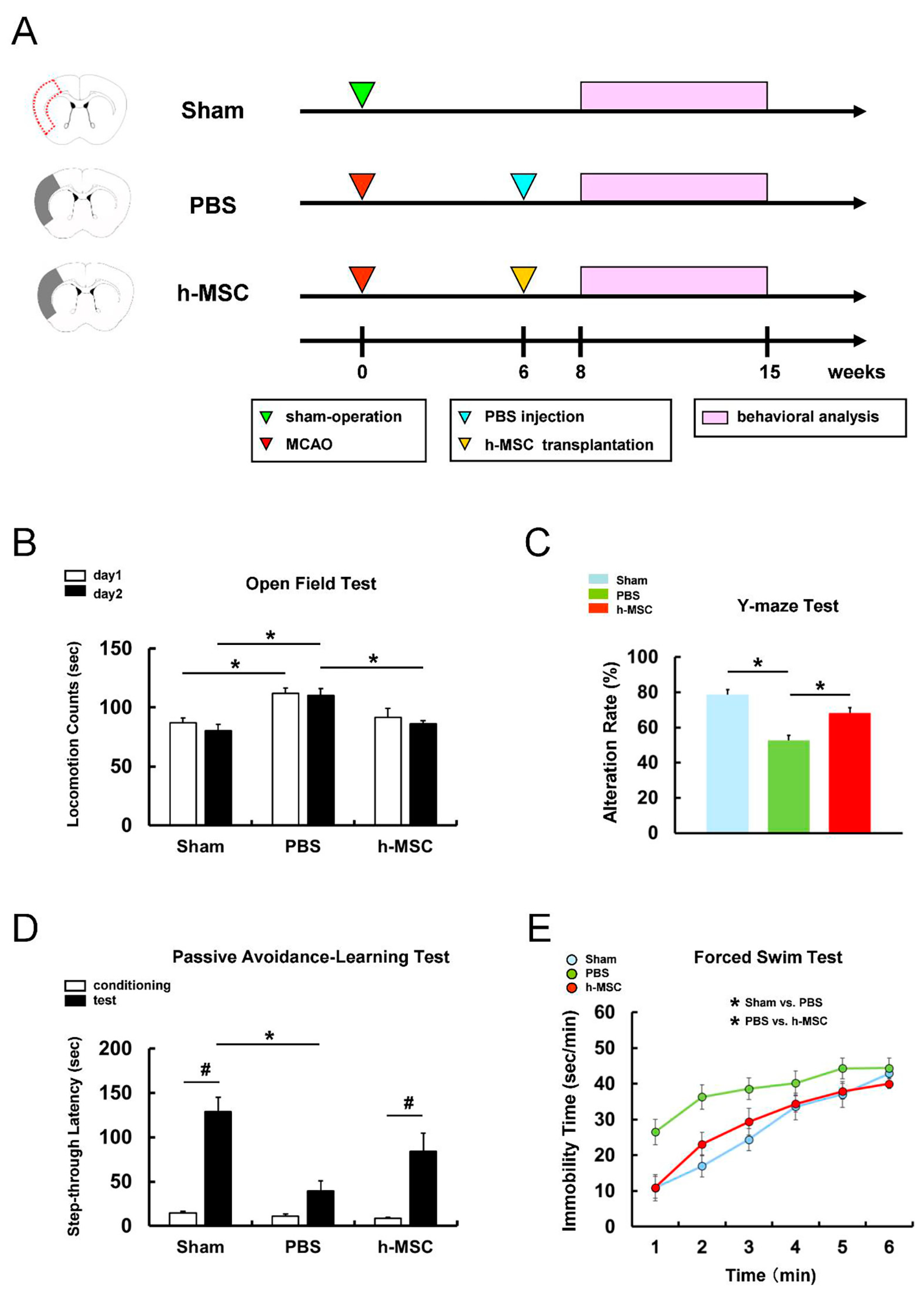
3.1. h-MSC Transplantation Improves Neurological Function after Ischemic Stroke
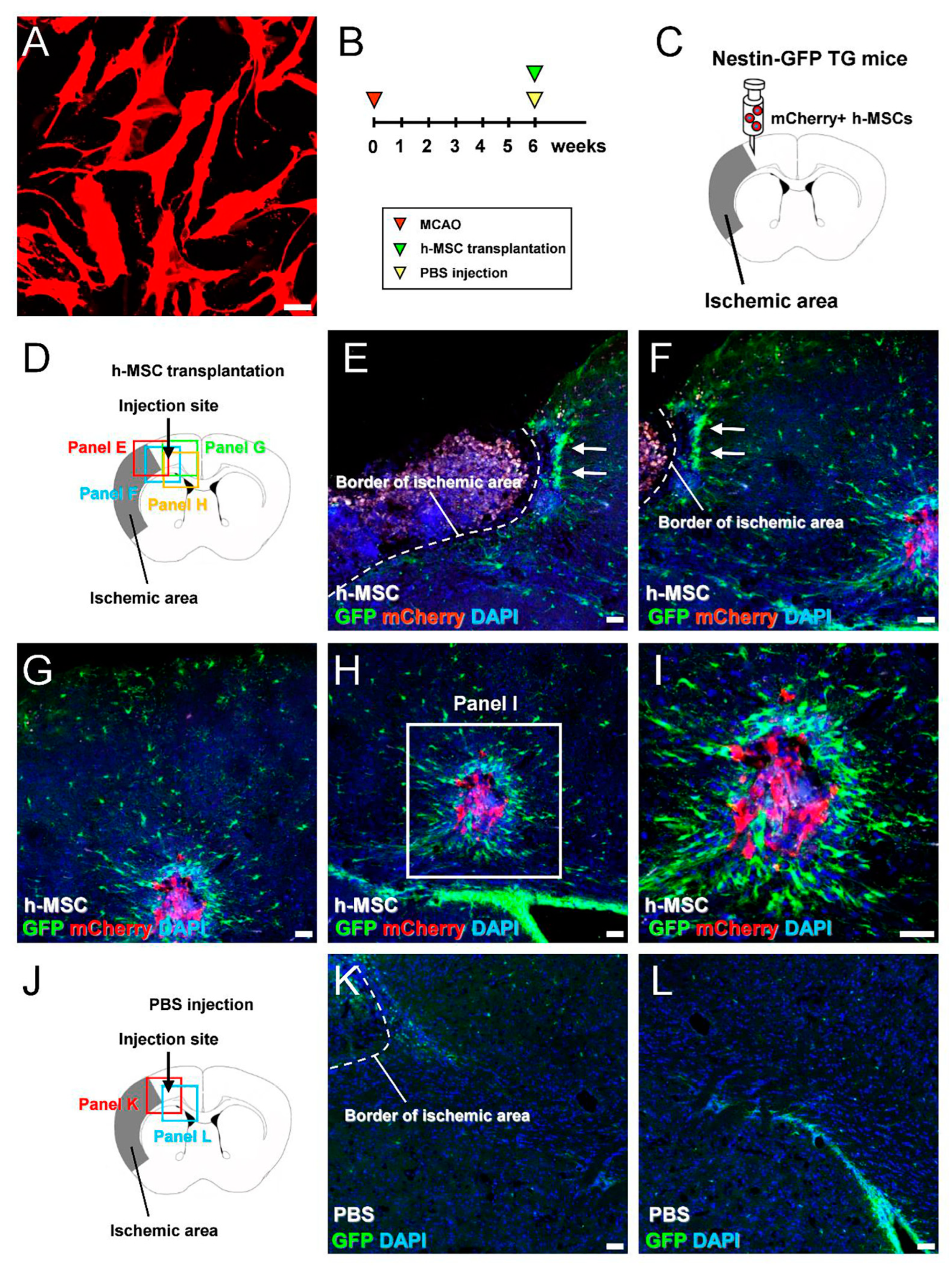
3.2. h-MSCs Transplanted into Mouse Brains Activate Endogenous iNSPCs in Mice after Ischemic Stroke
3.3. h-MSCs Promote the Proliferation of iNSPCs
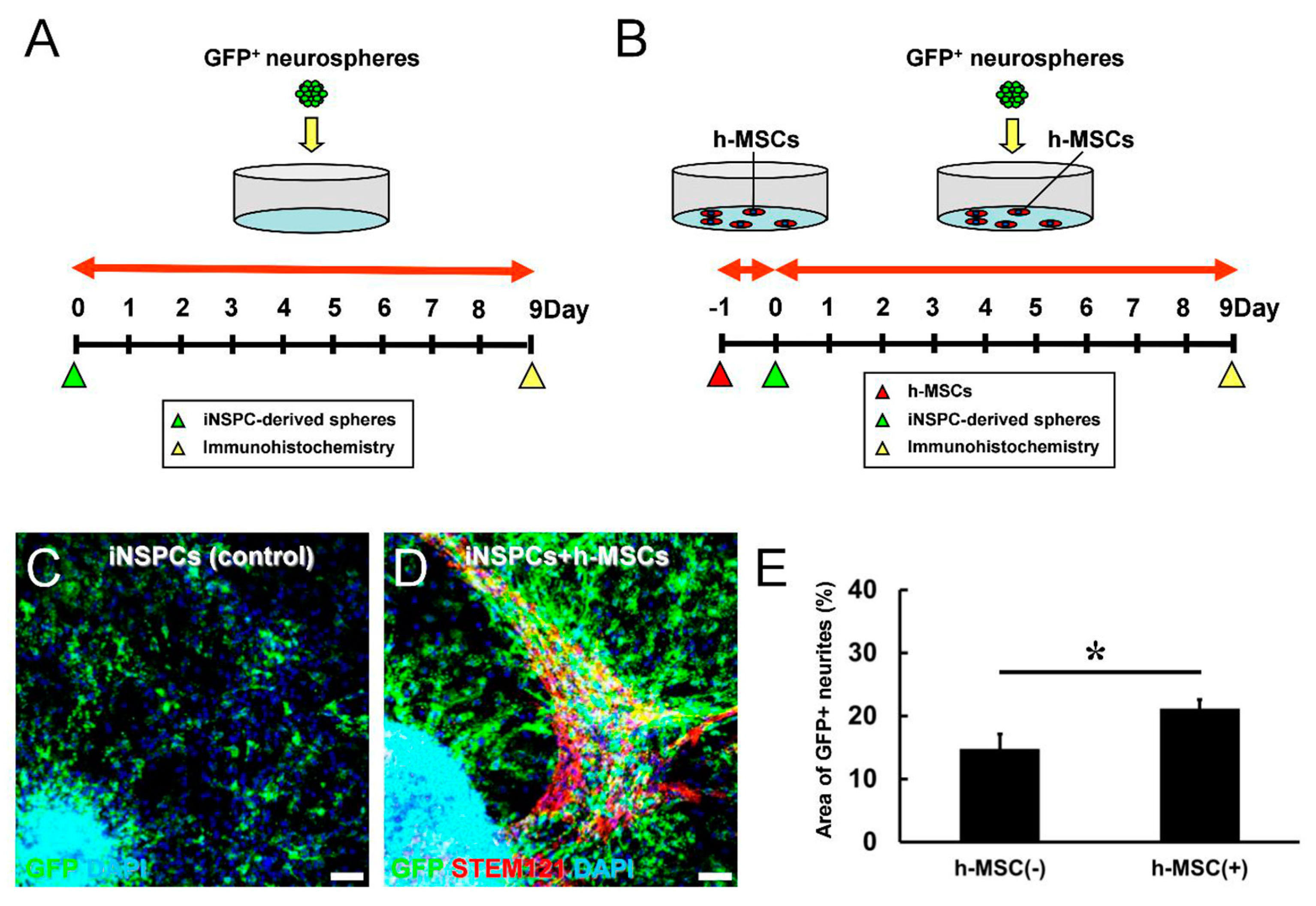
3.4. h-MSCs Promote the Differentiation of iNSPCs
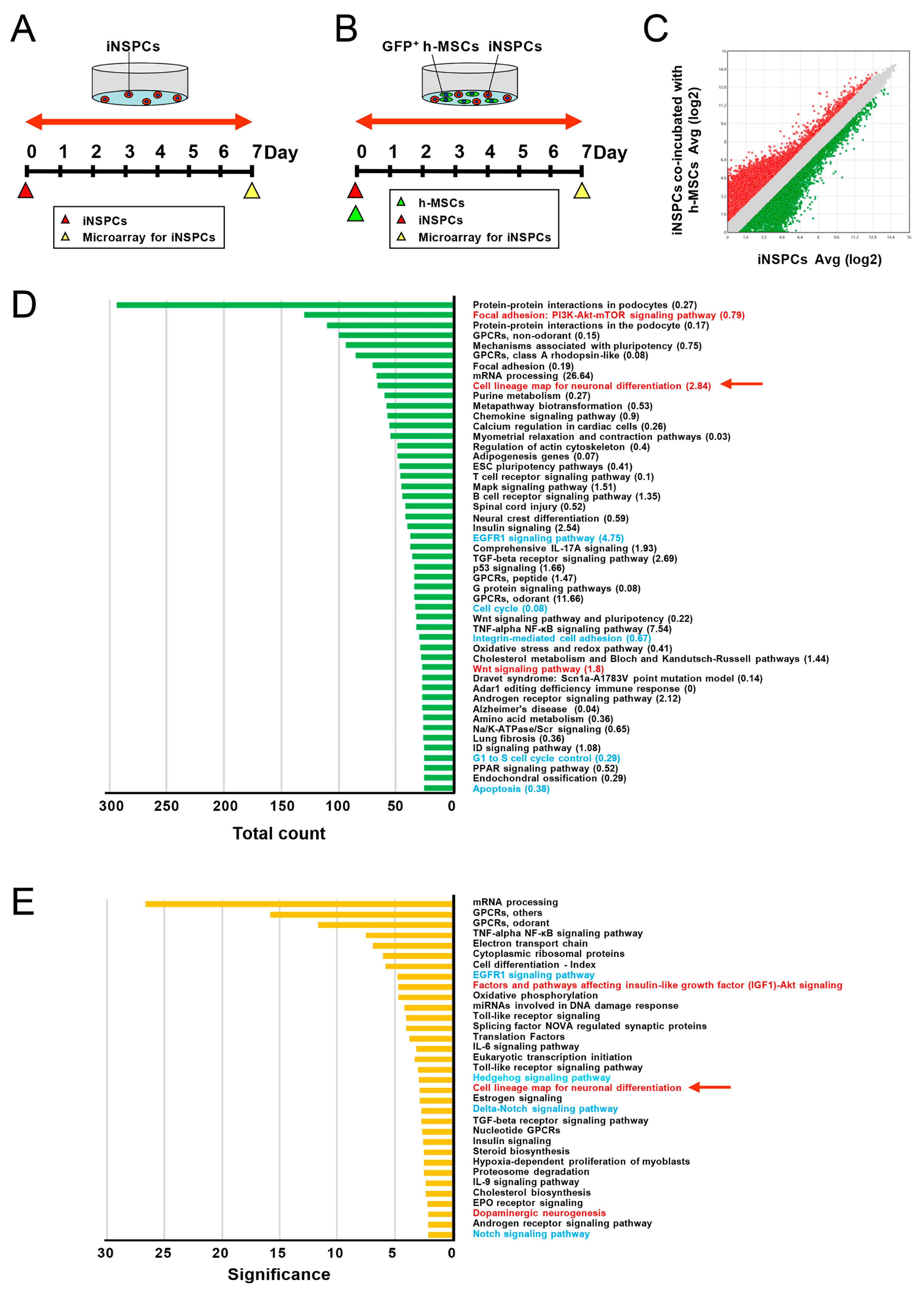
3.5. h-MSCs Regulate Genes Related to Self-Renewal and Maintenance in iNSPCs
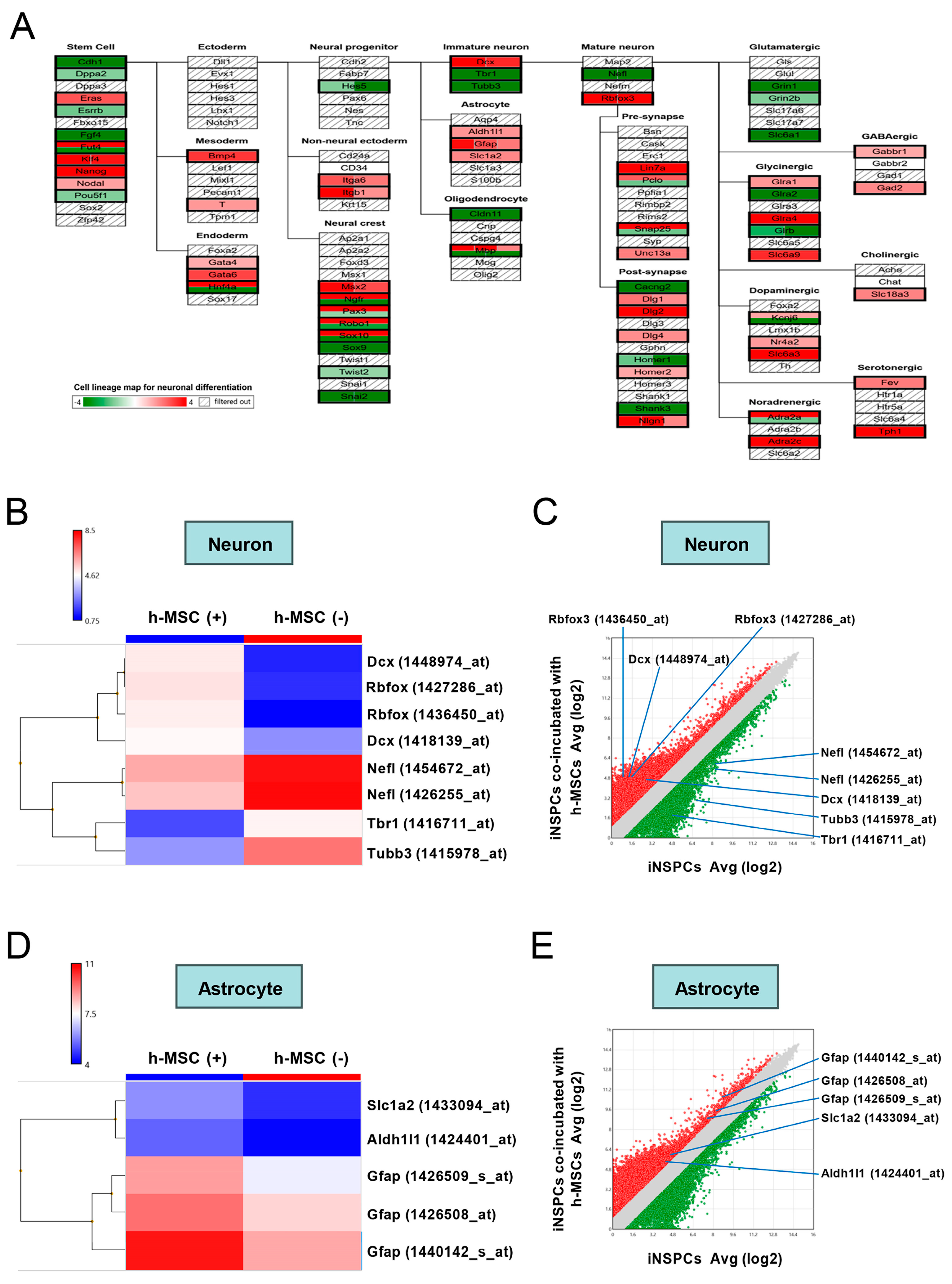
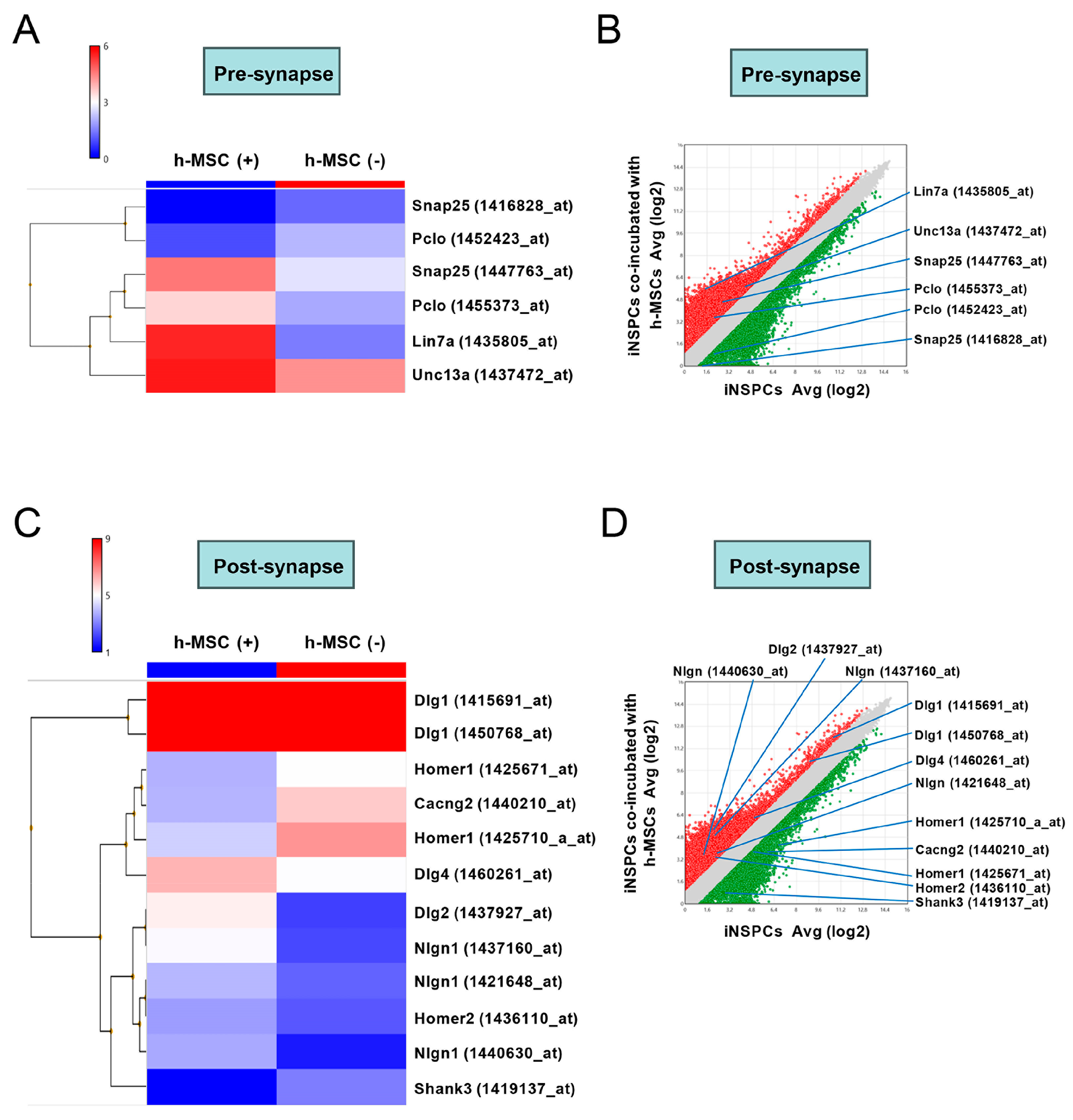
3.6. h-MSCs Regulate Genes Related to Neural Lineage Differentiation and Synapses in iNSPCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokin, M.; Kass-Hout, T.; Kass-Hout, O.; Dumont, T.M.; Kan, P.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: A systematic review of clinical outcomes. Stroke 2012, 43, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Zaidat, O.O.; Suarez, J.I.; Sunshine, J.L.; Tarr, R.W.; Alexander, M.J.; Smith, T.P.; Enterline, D.S.; Selman, W.R.; Landis, D.M. Thrombolytic therapy of acute ischemic stroke: Correlation of angiographic recanalization with clinical outcome. AJNR Am. J. Neuroradiol. 2005, 26, 880–884. [Google Scholar] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, J.; Huang, J.; Song, X.; Wu, Y.; Chen, X.; Tan, Y.; Yang, Q. The role of mesenchymal stem cell transplantation for ischemic stroke and recent research developments. Front. Neurol. 2022, 13, 1000777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Duan, W.M.; Reyes, M.; Keene, C.D.; Verfaillie, C.M.; Low, W.C. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 2002, 174, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, H.; Cai, X.; Sun, W.; He, B.; Yang, Z.; Xu, P. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Brain Res. Bull. 2019, 153, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Strzemecki, D.; Muraca, M.; Janowski, M.; Lukomska, B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J. Neuroinflamm. 2019, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Xie, J.; Zhong, J.; Liu, Y.; Du, L.; Zhou, B.; Xu, J.; Liu, P.; Yang, S.; Wang, J.; et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation 2009, 87, 350–359. [Google Scholar] [CrossRef]
- Tate, C.C.; Fonck, C.; McGrogan, M.; Case, C.C. Human mesenchymal stromal cells and their derivative, SB623 cells, rescue neural cells via trophic support following in vitro ischemia. Cell Transplant. 2010, 19, 973–984. [Google Scholar] [CrossRef]
- Son, J.P.; Kim, E.H.; Shin, E.K.; Kim, D.H.; Sung, J.H.; Oh, M.J.; Cha, J.M.; Chopp, M.; Bang, O.Y. Mesenchymal Stem Cell-Extracellular Vesicle Therapy for Stroke: Scalable Production and Imaging Biomarker Studies. Stem Cells Transl. Med. 2023, 12, 459–473. [Google Scholar] [CrossRef]
- Xin, H.; Liu, Z.; Buller, B.; Li, Y.; Golembieski, W.; Gan, X.; Wang, F.; Lu, M.; Ali, M.M.; Zhang, Z.G.; et al. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J. Cereb. Blood Flow Metab. 2021, 41, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Goings, G.E.; Soderstrom, K.E.; Szele, F.G.; Kozlowski, D.A. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005, 1053, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011, 1367, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Yoshida, M.; Horie, N.; Satoh, K.; Fukuda, Y.; Ishizaka, S.; Ogawa, K.; Morofuji, Y.; Hiu, T.; Izumo, T.; et al. Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials. Bioengineering 2022, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Kim, S.S.; Lee, S.Y.; Lee, H.S.; Kim, H.S.; Lee, Y.D.; Suh-Kim, H. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp. Mol. Med. 2008, 40, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Hirota, Y.; Ema, M.; Takahashi, S.; Miyoshi, I.; Okano, H.; Sawamoto, K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 2010, 28, 545–554. [Google Scholar] [CrossRef]
- Nishie, H.; Nakano-Doi, A.; Sawano, T.; Nakagomi, T. Establishment of a Reproducible Ischemic Stroke Model in Nestin-GFP Mice with High Survival Rates. Int. J. Mol. Sci. 2021, 22, 12997. [Google Scholar] [CrossRef] [PubMed]
- Minato, Y.; Nakano-Doi, A.; Maeda, S.; Nakagomi, T.; Yagi, H. A Bone Morphogenetic Protein Signaling Inhibitor, LDN193189, Converts Ischemia-Induced Multipotent Stem Cells into Neural Stem/Progenitor Cell-Like Cells. Stem Cells Dev. 2022, 31, 756–765. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakagomi, N.; Doe, N.; Nakano-Doi, A.; Sawano, T.; Takagi, T.; Matsuyama, T.; Yoshimura, S.; Nakagomi, T. Early Reperfusion Following Ischemic Stroke Provides Beneficial Effects, Even After Lethal Ischemia with Mature Neural Cell Death. Cells 2020, 9, 1374. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Saito, H.; Suzuki, M.; Mori, K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 2000, 11, 1991–1996. [Google Scholar] [CrossRef]
- Nakagomi, T.; Nakano-Doi, A.; Kubo, S.; Sawano, T.; Kuramoto, Y.; Yamahara, K.; Matsuyama, T.; Takagi, T.; Doe, N.; Yoshimura, S. Transplantation of Human Brain-Derived Ischemia-Induced Multipotent Stem Cells Ameliorates Neurological Dysfunction in Mice After Stroke. Stem Cells Transl. Med. 2023, 12, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Nakagomi, T.; Nakano-Doi, A.; Kubo, S.; Minato, Y.; Sawano, T.; Sakagami, M.; Tsuzuki, K. Microglia Negatively Regulate the Proliferation and Neuronal Differentiation of Neural Stem/Progenitor Cells Isolated from Poststroke Mouse Brains. Cells 2023, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Tatebayashi, K.; Tanaka, Y.; Nakano-Doi, A.; Sakuma, R.; Kamachi, S.; Shirakawa, M.; Uchida, K.; Kageyama, H.; Takagi, T.; Yoshimura, S.; et al. Identification of Multipotent Stem Cells in Human Brain Tissue Following Stroke. Stem Cells Dev. 2017, 26, 787–797. [Google Scholar] [CrossRef]
- Beppu, M.; Nakagomi, T.; Takagi, T.; Nakano-Doi, A.; Sakuma, R.; Kuramoto, Y.; Tatebayashi, K.; Matsuyama, T.; Yoshimura, S. Isolation and Characterization of Cerebellum-Derived Stem Cells in Poststroke Human Brain. Stem Cells Dev. 2019, 28, 528–542. [Google Scholar] [CrossRef]
- Mazzio, E.; Badisa, R.; Mack, N.; Cassim, S.; Zdralevic, M.; Pouyssegur, J.; Soliman, K.F.A. Whole-transcriptome Analysis of Fully Viable Energy Efficient Glycolytic-null Cancer Cells Established by Double Genetic Knockout of Lactate Dehydrogenase A/B or Glucose-6-Phosphate Isomerase. Cancer Genom. Proteom. 2020, 17, 469–497. [Google Scholar] [CrossRef]
- Chou, M.Y.; Appan, D.; Chang, K.W.; Chou, C.H.; Lin, C.Y.; Gau, S.S.; Huang, H.S. Mouse hybrid genome mediates diverse brain phenotypes with the specificity of reciprocal crosses. FASEB J. 2022, 36, e22232. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Huttner, W.B.; Calegari, F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 2009, 5, 320–331. [Google Scholar] [CrossRef]
- Li, F.; Wei, H.; Li, H.; Li, X.; Hu, C.; Zhang, J.; Deng, Y.; Liao, X. miR-26a prevents neural stem cells from apoptosis via beta-catenin signaling pathway in cardiac arrest-induced brain damage. Biosci. Rep. 2019, 39, BSR20181635. [Google Scholar]
- Aguirre, A.; Rubio, M.E.; Gallo, V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 2010, 467, 323–327. [Google Scholar] [CrossRef]
- Porlan, E.; Marti-Prado, B.; Morante-Redolat, J.M.; Consiglio, A.; Delgado, A.C.; Kypta, R.; Lopez-Otin, C.; Kirstein, M.; Farinas, I. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat. Cell Biol. 2014, 16, 629–638. [Google Scholar] [CrossRef]
- Morante-Redolat, J.M.; Porlan, E. Neural Stem Cell Regulation by Adhesion Molecules within the Subependymal Niche. Front. Cell Dev. Biol. 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Haragopal, H.; Yu, D.; Zeng, X.; Kim, S.W.; Han, I.B.; Ropper, A.E.; Anderson, J.E.; Teng, Y.D. Stemness enhancement of human neural stem cells following bone marrow MSC coculture. Cell Transplant. 2015, 24, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Joyner, A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 2005, 437, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Varela-Nallar, L.; Inestrosa, N.C. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front. Cell. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Cheng, X.; Guo, Y.; Sun, X.; Chen, G.; Li, H.; Li, P.; Lu, X.; Tian, M.; et al. IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway. PLoS ONE 2014, 9, e113801. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, N.; Zhu, Y.; Jin, R.; Wu, F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials 2021, 265, 120448. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, N.; Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Saino, O.; Takata, M.; Yoshikawa, H.; Stern, D.M.; Matsuyama, T.; Taguchi, A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells 2009, 27, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ko, E.; Park, Y. Adipose Tissue-Derived Mesenchymal Stem Cell Inhibits Osteoclast Differentiation through Tumor Necrosis Factor Stimulated Gene-6. Tissue Eng. Regen. Med. 2024, 21, 587–594. [Google Scholar] [CrossRef]
- Oppliger, B.; Joerger-Messerli, M.S.; Simillion, C.; Mueller, M.; Surbek, D.V.; Schoeberlein, A. Mesenchymal stromal cells from umbilical cord Wharton’s jelly trigger oligodendroglial differentiation in neural progenitor cells through cell-to-cell contact. Cytotherapy 2017, 19, 829–838. [Google Scholar] [CrossRef]
- Qiu, X.C.; Jin, H.; Zhang, R.Y.; Ding, Y.; Zeng, X.; Lai, B.Q.; Ling, E.A.; Wu, J.L.; Zeng, Y.S. Donor mesenchymal stem cell-derived neural-like cells transdifferentiate into myelin-forming cells and promote axon regeneration in rat spinal cord transection. Stem Cell Res. Ther. 2015, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Cho, H.H.; Cho, Y.B.; Park, J.S.; Jeong, H.S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Duarte, A.S.; Longhini, A.L.; Pradella, F.; Farias, A.S.; Luzo, A.C.; Oliveira, A.L.; Olalla Saad, S.T. Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci. Rep. 2015, 5, 16167. [Google Scholar] [CrossRef]
- Chung, T.N.; Kim, J.H.; Choi, B.Y.; Jeong, J.Y.; Chung, S.P.; Kwon, S.W.; Suh, S.W. Effect of Adipose-Derived Mesenchymal Stem Cell Administration and Mild Hypothermia Induction on Delayed Neuronal Death After Transient Global Cerebral Ischemia. Crit. Care Med. 2017, 45, e508–e515. [Google Scholar] [CrossRef] [PubMed]
- Cooney, D.S.; Wimmers, E.G.; Ibrahim, Z.; Grahammer, J.; Christensen, J.M.; Brat, G.A.; Wu, L.W.; Sarhane, K.A.; Lopez, J.; Wallner, C.; et al. Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model. Sci. Rep. 2016, 6, 31306. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, A.; Yasuhara, T.; Kameda, M.; Morimoto, J.; Takeuchi, H.; Wang, F.; Sasaki, T.; Sasada, S.; Shinko, A.; Wakamori, T.; et al. Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms. PLoS ONE 2015, 10, e0127302. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, A.; Rajagopalan, P.; Albarqi, Z.; Devaraj, A.; Mohamed, M.H.; Al-Hakami, A.; Chandramoorthy, H.C. Mesenchymal Stem Cells (MSCs) Coculture Protects [Ca2+]i Orchestrated Oxidant Mediated Damage in Differentiated Neurons In Vitro. Cells 2018, 7, 250. [Google Scholar] [CrossRef]
- Peng, Z.; Gao, W.; Yue, B.; Jiang, J.; Gu, Y.; Dai, J.; Chen, L.; Shi, Q. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J. Tissue Eng. Regen. Med. 2018, 12, e1725–e1736. [Google Scholar] [CrossRef]
- Robinson, A.P.; Foraker, J.E.; Ylostalo, J.; Prockop, D.J. Human stem/progenitor cells from bone marrow enhance glial differentiation of rat neural stem cells: A role for transforming growth factor beta and Notch signaling. Stem Cells Dev. 2011, 20, 289–300. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, S.W.; Han, J.E.; Choi, J.W.; Song, M.R. The complex morphology of reactive astrocytes controlled by fibroblast growth factor signaling. Glia 2014, 62, 1328–1344. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Polo-Parada, L.; Landmesser, L.T. Selective targeting of different neural cell adhesion molecule isoforms during motoneuron myotube synapse formation in culture and the switch from an immature to mature form of synaptic vesicle cycling. J. Neurosci. 2007, 27, 14481–14493. [Google Scholar] [CrossRef] [PubMed]
- Polo-Parada, L.; Bose, C.M.; Plattner, F.; Landmesser, L.T. Distinct roles of different neural cell adhesion molecule (NCAM) isoforms in synaptic maturation revealed by analysis of NCAM 180 kDa isoform-deficient mice. J. Neurosci. 2004, 24, 1852–1864. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, S.; Nakano-Doi, A.; Sawano, T.; Kubo, S.; Doe, N.; Nakagomi, T. Administration of Human-Derived Mesenchymal Stem Cells Activates Locally Stimulated Endogenous Neural Progenitors and Reduces Neurological Dysfunction in Mice after Ischemic Stroke. Cells 2024, 13, 939. https://doi.org/10.3390/cells13110939
Fujiwara S, Nakano-Doi A, Sawano T, Kubo S, Doe N, Nakagomi T. Administration of Human-Derived Mesenchymal Stem Cells Activates Locally Stimulated Endogenous Neural Progenitors and Reduces Neurological Dysfunction in Mice after Ischemic Stroke. Cells. 2024; 13(11):939. https://doi.org/10.3390/cells13110939
Chicago/Turabian StyleFujiwara, Shuichi, Akiko Nakano-Doi, Toshinori Sawano, Shuji Kubo, Nobutaka Doe, and Takayuki Nakagomi. 2024. "Administration of Human-Derived Mesenchymal Stem Cells Activates Locally Stimulated Endogenous Neural Progenitors and Reduces Neurological Dysfunction in Mice after Ischemic Stroke" Cells 13, no. 11: 939. https://doi.org/10.3390/cells13110939
APA StyleFujiwara, S., Nakano-Doi, A., Sawano, T., Kubo, S., Doe, N., & Nakagomi, T. (2024). Administration of Human-Derived Mesenchymal Stem Cells Activates Locally Stimulated Endogenous Neural Progenitors and Reduces Neurological Dysfunction in Mice after Ischemic Stroke. Cells, 13(11), 939. https://doi.org/10.3390/cells13110939






