Stable Dietary Ora-Curcumin Formulation Protects from Experimental Colitis and Colorectal Cancer
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of OC-S Complexes
2.3. Preparation of Functional Dietary Products (DP) with OC-S Complexes (OC-S-DP)
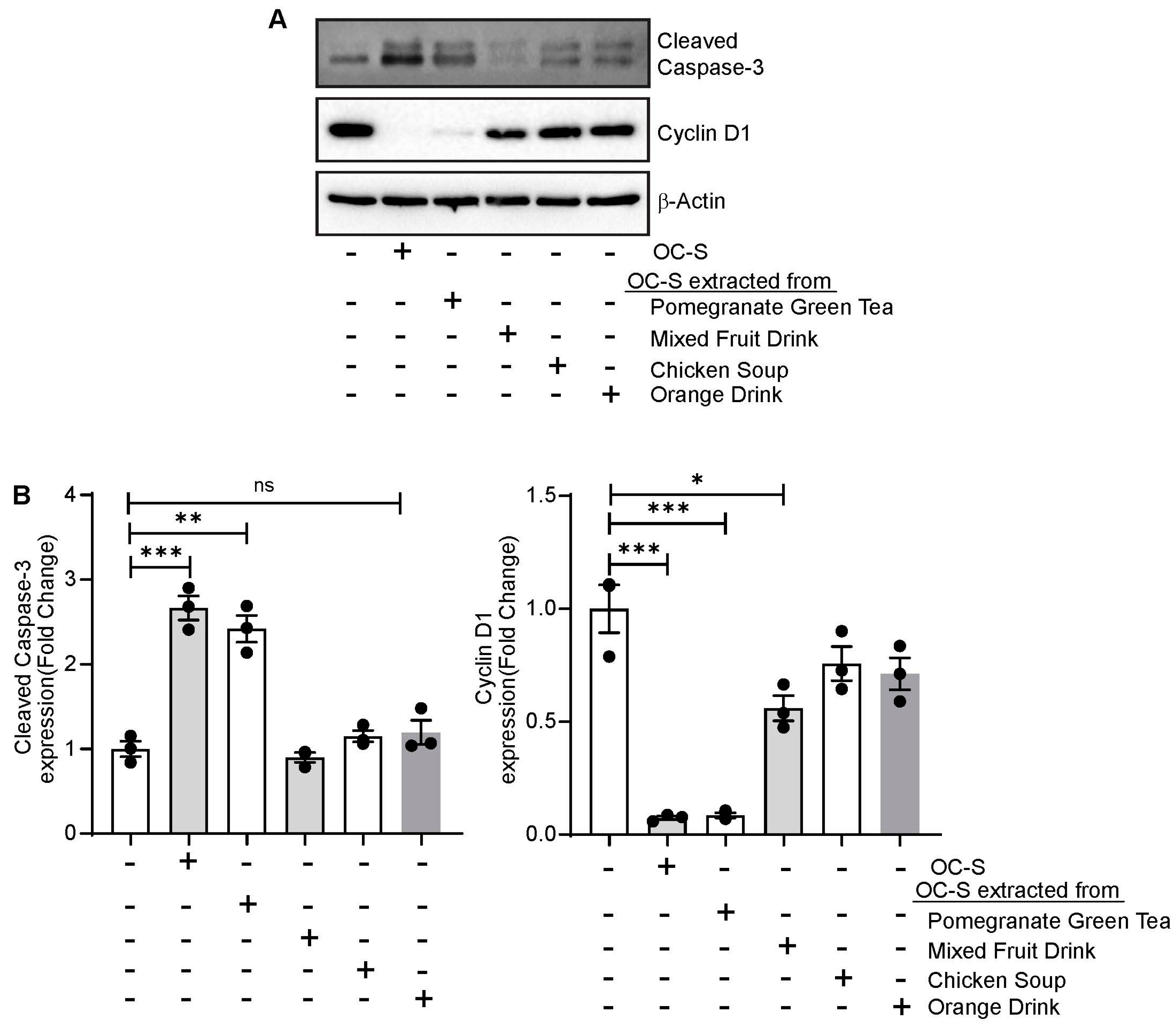
2.4. The Extraction of Curcumin from OC-S-DP
2.5. Quantification of Curcumin Content from the OC-S-DP
2.6. Curcumin Stability with OC-S-DP
2.7. Simulated Gastric Extraction of OC-S-DP for Functional Studies
2.8. Preparation of OC-S and Curcumin Solutions for In Vitro Use
2.9. CRC Cells and APCmin Tumoroids Culture
2.10. Western Blot Analysis
2.11. RNA Isolation and Real-Time qPCR Analysis
2.12. Animals and Colitis Induction
2.13. Evaluation of Inflammation-Specific Binding of OC-S Complexes
2.14. Statistical Analysis
3. Results
3.1. OC-S Is an “Inflammation Site-Targeted Local Drug Delivery System”
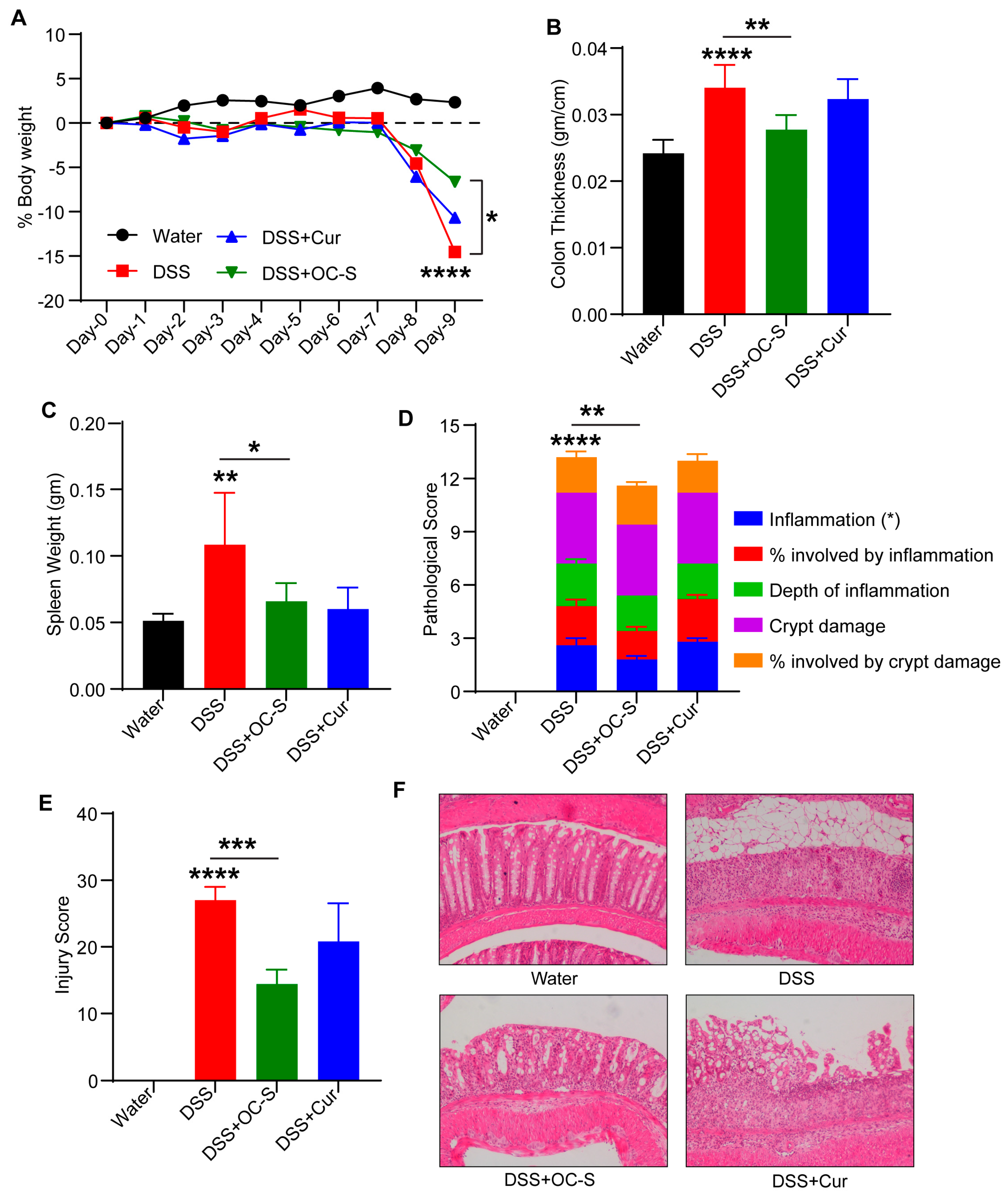
3.2. OC-S Attenuates Dextran-Sulfate-Sodium-Induced Mouse Colitis at a Very Low Dose
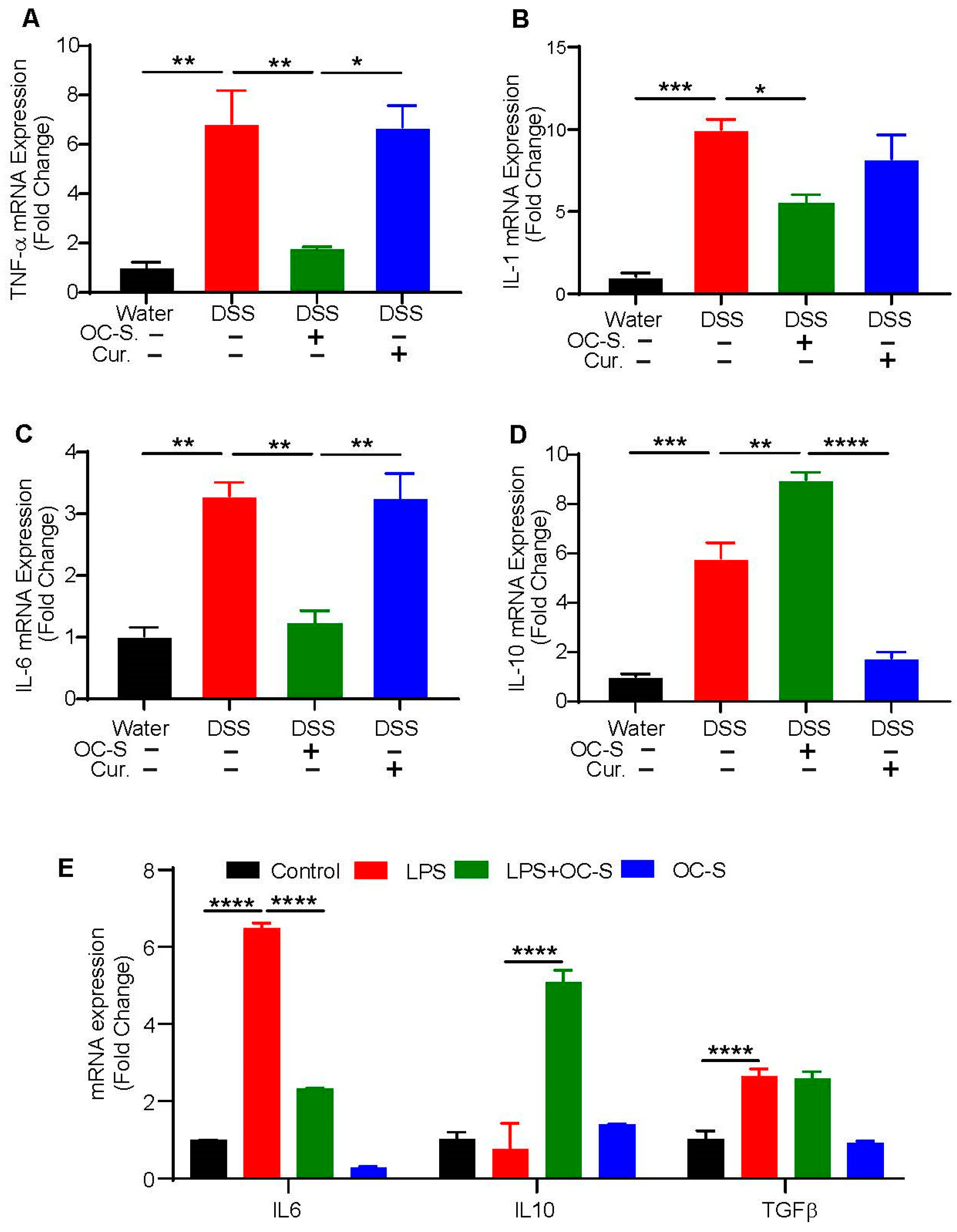
3.3. OC-S Treatment Re-Equilibrates Inflammatory Cytokines to Reduce Colonic Inflammation
3.4. OC-S Modulates LPS-Induced Inflammatory Responses in Intestinal Epithelial Cells
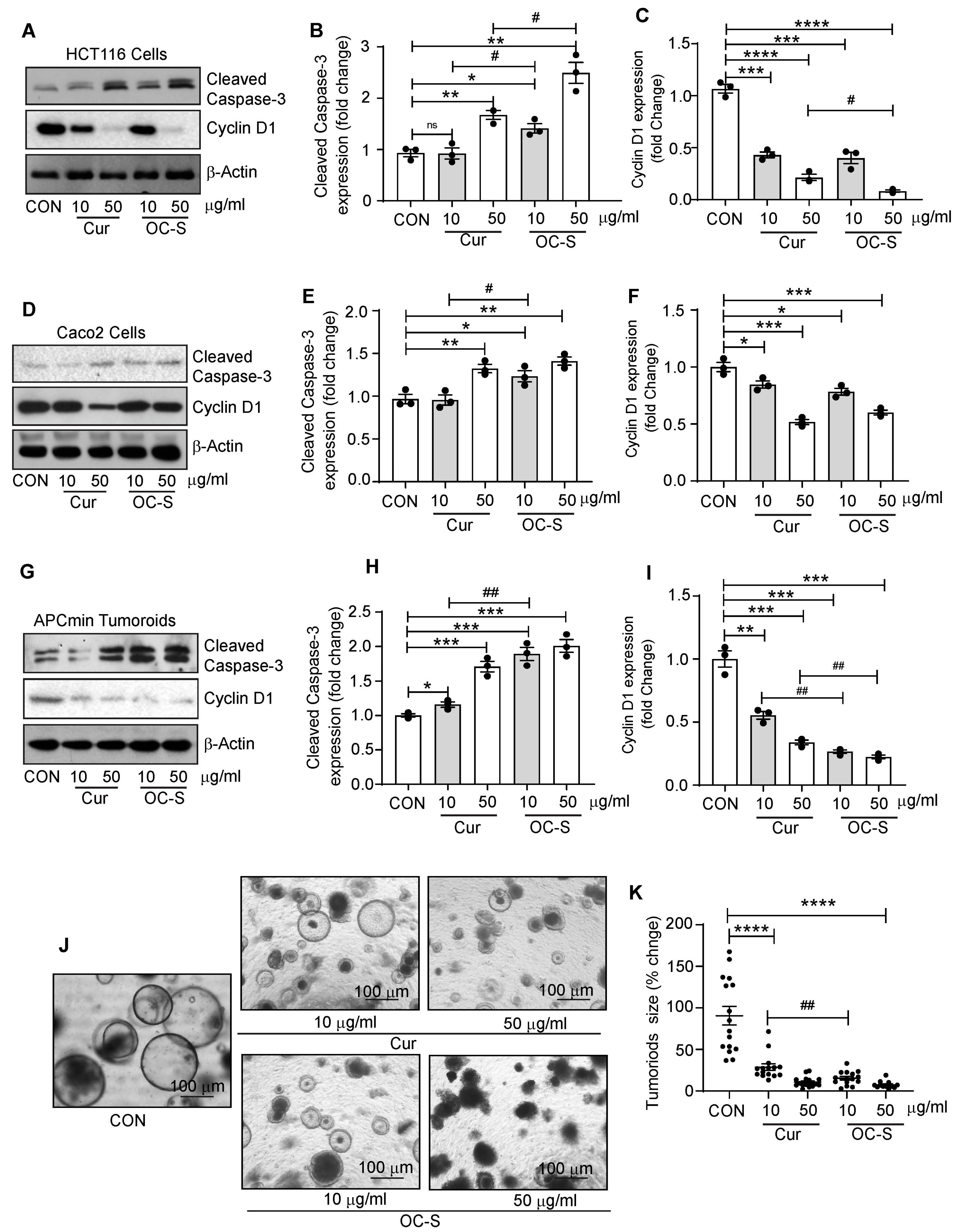
3.5. OC-S Improved Therapeutic Efficacy of Curcumin in CRC Cells and Colon Tumoroids
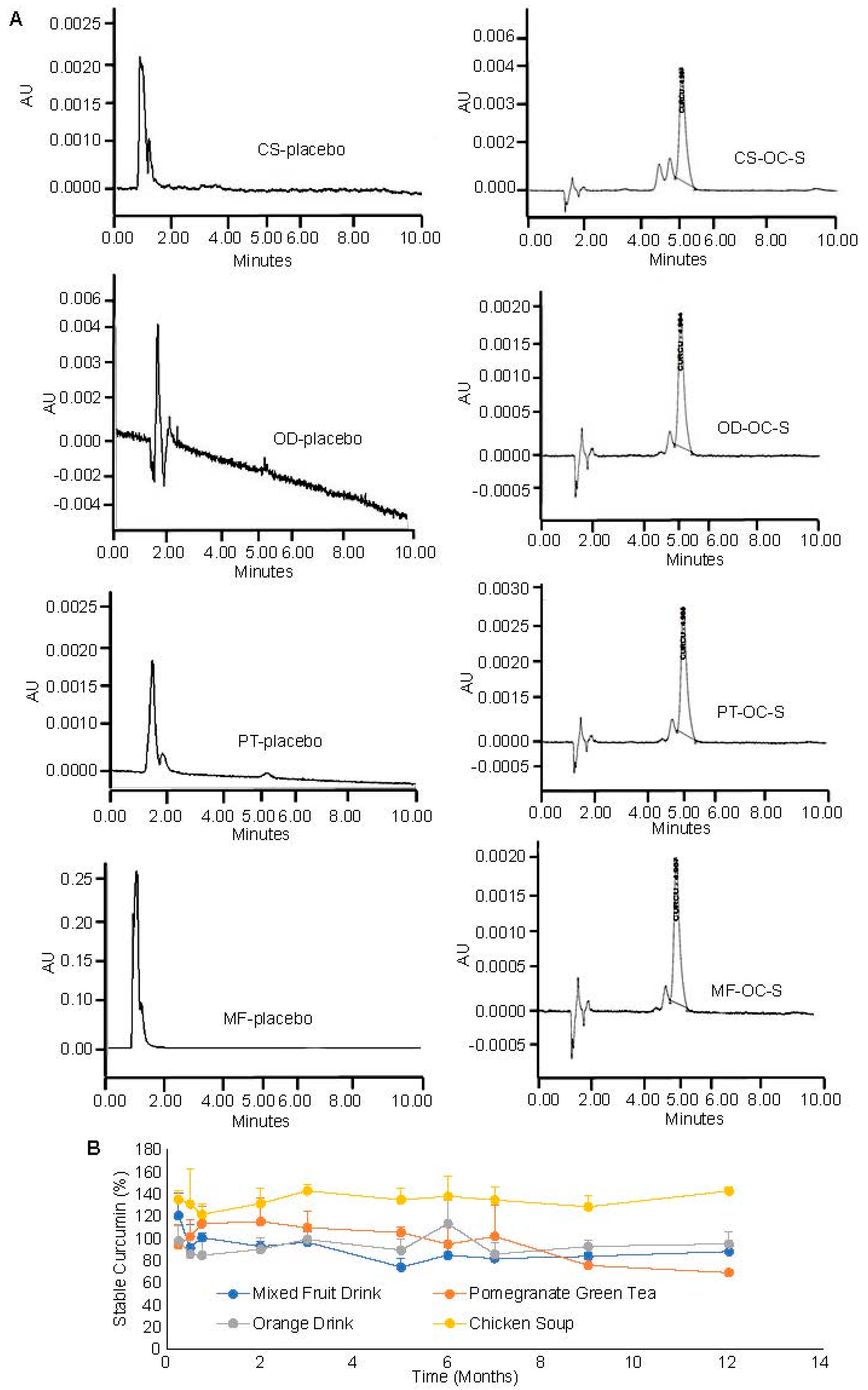
3.6. Compatibility and Stability of OC-S in Dietary Products as a Supplement
3.7. OC-S Food Extract Shows Therapeutic Efficacy in Colon Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fumery, M.; Dulai, P.S.; Gupta, S.; Prokop, L.J.; Ramamoorthy, S.; Sandborn, W.J.; Singh, S. Incidence, Risk Factors, and Outcomes of Colorectal Cancer in Patients With Ulcerative Colitis With Low-Grade Dysplasia: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 665–674 e665. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P.; Sorensen, T.I. Increased risk of intestinal cancer in Crohn’s disease: A meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 2005, 100, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, N.; Polydorides, A.D. Colorectal dysplasia in chronic inflammatory bowel disease: Pathology, clinical implications, and pathogenesis. Arch. Pathol. Lab. Med. 2010, 134, 876–895. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Danial, D.; Youssef, E.D.; Maryam, B.M.; Mohammad, A.; Moein, B.M.; Liliane, D. Risk Factors of Young-Onset Colorectal Cancer: Analysis of a Large Population-Based Registry. Can. J. Gastroenterol. Hepatol. 2022, 2022, 3582443. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fernandez-Ortega, P.; Pud, D.; Ozden, G.; Platin, N.; Hummerston, S.; Scott, J.A.; Panteli, V.; Gudmundsdottir, G.; Selvekerova, S.; et al. Complementary and alternative medicine use in colorectal cancer patients in seven European countries. Complement. Ther. Med. 2005, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Saberi-Karimian, M.; Valizadeh, O.; Behnam, B.; Saadat, A.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids on Systemic Inflammation and Quality of Life in Patients with Colorectal Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. Adv. Exp. Med. Biol. 2021, 1328, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449 e1441. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414 e396. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Chan, E.; Zhou, S.F. ADME properties of herbal medicines in humans: Evidence, challenges and strategies. Curr. Pharm. Des. 2011, 17, 357–407. [Google Scholar] [CrossRef]
- Bisht, S.; Maitra, A. Systemic Delivery of Curcumin: 21st Century Solutions for an Ancient Conundrum. Curr. Drug Discov. Technol. 2009, 6, 192–199. [Google Scholar] [CrossRef]
- Mahran, R.I.; Hagras, M.M.; Sun, D.; Brenner, D.E. Bringing Curcumin to the Clinic in Cancer Prevention: A Review of Strategies to Enhance Bioavailability and Efficacy. AAPS J. 2017, 19, 54–81. [Google Scholar] [CrossRef]
- Chandan, S.; Mohan, B.P.; Chandan, O.C.; Ahmad, R.; Challa, A.; Tummala, H.; Singh, S.; Dhawan, P.; Ponnada, S.; Singh, A.B.; et al. Curcumin use in ulcerative colitis: Is it ready for prime time? A systematic review and meta-analysis of clinical trials. Ann. Gastroenterol. 2020, 33, 53–58. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef]
- Kumar, S.; Kesharwani, S.S.; Mathur, H.; Tyagi, M.; Bhat, G.J.; Tummala, H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur. J. Pharm. Sci. 2016, 82, 86–96. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Chen, F.; Cheng, K.W. Polysaccharide-Zein Composite Nanoparticles for Enhancing Cellular Uptake and Oral Bioavailability of Curcumin: Characterization, Anti-colorectal Cancer Effect, and Pharmacokinetics. Front. Nutr. 2022, 9, 846282. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; Gonzalez-Navarro, C.J.; Gil, A.G.; Asin-Prieto, E.; Saiz, M.J.; Llabres, J.M. Zein Nanoparticles Improve the Oral Bioavailability of Curcumin in Wistar Rats. Pharmaceutics 2021, 13, 361. [Google Scholar] [CrossRef]
- Jiang, Z.; Gan, J.; Wang, L.; Lv, C. Binding of curcumin to barley protein Z improves its solubility, stability and bioavailability. Food Chem. 2023, 399, 133952. [Google Scholar] [CrossRef]
- Arvapalli, D.M.; Sheardy, A.T.; Allado, K.; Chevva, H.; Yin, Z.; Wei, J. Design of Curcumin Loaded Carbon Nanodots Delivery System: Enhanced Bioavailability, Release Kinetics, and Anticancer Activity. ACS Appl. Bio Mater. 2020, 3, 8776–8785. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Ahmad, R.; Bakkari, M.A.; Rajput, M.K.S.; Dachineni, R.; Valiveti, C.K.; Kapur, S.; Jayarama Bhat, G.; Singh, A.B.; Tummala, H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J. Control Release 2018, 290, 165–179. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Ahmad, A.; Zughaibi, T.A.; Alhosin, M.; Tabrez, S. Curcumin, its derivatives, and their nanoformulations: Revolutionizing cancer treatment. Cell Biochem. Funct. 2024, 42, e3911. [Google Scholar] [CrossRef]
- Kumar, B.; Ahmad, R.; Sharma, S.; Gowrikumar, S.; Primeaux, M.; Rana, S.; Natarajan, A.; Oupicky, D.; Hopkins, C.R.; Dhawan, P.; et al. PIK3C3 Inhibition Promotes Sensitivity to Colon Cancer Therapy by Inhibiting Cancer Stem Cells. Cancers 2021, 13, 2168. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, B.; Tamang, R.L.; Talmon, G.A.; Dhawan, P.; Singh, A.B. P62/SQSTM1 binds with claudin-2 to target for selective autophagy in stressed intestinal epithelium. Commun. Biol. 2023, 6, 740. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, B.; Thapa, I.; Talmon, G.A.; Salomon, J.; Ramer-Tait, A.E.; Bastola, D.K.; Dhawan, P.; Singh, A.B. Loss of claudin-3 expression increases colitis risk by promoting Gut Dysbiosis. Gut Microbes 2023, 15, 2282789. [Google Scholar] [CrossRef]
- Zhang, S.F.; Ermann, J.; Succi, M.D.; Zhou, A.; Hamilton, M.J.; Cao, B.N.; Korzenik, J.R.; Glickman, J.N.; Vemula, P.K.; Glimcher, L.H.; et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 2015, 7, 300ra128. [Google Scholar] [CrossRef]
- Tirosh, B.; Khatib, N.; Barenholz, Y.; Nissan, A.; Rubinstein, A. Transferrin as a Luminal Target for Negatively Charged Liposomes in the Inflamed Colonic Mucosa. Mol. Pharmaceut 2009, 6, 1083–1091. [Google Scholar] [CrossRef]
- Harel, E.; Rubinstein, A.; Nissan, A.; Khazanov, E.; Milbauer, M.N.; Barenholz, Y.; Tirosh, B. Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa. PLoS ONE 2011, 6, e24202. [Google Scholar] [CrossRef]
- Moldoveanu, A.C.; Diculescu, M.; Braticevici, C.F. Cytokines in inflammatory bowel disease. Rom. J. Intern. Med. 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Neuman, M.G. Immune dysfunction in inflammatory bowel disease. Transl. Res. 2007, 149, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Pratap Mouli, V.; Garg, S.K.; Rai, T.; Choudhury, B.N.; Verma, P.; Deb, R.; Tiwari, V.; Rohatgi, S.; Dhingra, R.; et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis—A randomized, placebo-controlled, pilot study. J. Crohns Colitis 2014, 8, 208–214. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Prasad, S.; Tyagi, A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015, 6, 3412–3419. [Google Scholar] [CrossRef]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Groundwater, P.W.; Narlawar, R.; Liao, V.W.; Bhattacharya, A.; Srivastava, S.; Kunal, K.; Doddareddy, M.; Oza, P.M.; Mamidi, R.; Marrs, E.C.; et al. A Carbocyclic Curcumin Inhibits Proliferation of Gram-Positive Bacteria by Targeting FtsZ. Biochemistry 2017, 56, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Ji, J.; Bucci, L.R.; Preuss, H.G. A Comparative Pharmacokinetic Assessment of a Novel Highly Bioavailable Curcumin Formulation with 95% Curcumin: A Randomized, Double-Blind, Crossover Study. J. Am. Coll. Nutr. 2018, 37, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Volak, L.P.; Hanley, M.J.; Masse, G.; Hazarika, S.; Harmatz, J.S.; Badmaev, V.; Majeed, M.; Greenblatt, D.J.; Court, M.H. Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br. J. Clin. Pharmacol. 2013, 75, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Peterson, K.H.; Olaison, G.; Franzén, L.E.; Weström, B.; Magnusson, K.E.; Sjödahl, R. Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology 1999, 117, 65–72. [Google Scholar] [CrossRef]
- Turpin, W.; Lee, S.-H.; Raygoza Garay, J.A.; Madsen, K.L.; Meddings, J.B.; Bedrani, L.; Power, N.; Espin-Garcia, O.; Xu, W.; Smith, M.I.; et al. Increased Intestinal Permeability Is Associated With Later Development of Crohn’s Disease. Gastroenterology 2020, 159, 2092–2100.e2095. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, L.; Gammella, E.; De Ponti, C.; Recalcati, S.; Cairo, G. Role of HIF-1 and NF-kappaB transcription factors in the modulation of transferrin receptor by inflammatory and anti-inflammatory signals. J. Biol. Chem. 2008, 283, 2067420686. [Google Scholar] [CrossRef]
- Canny, G.; Levy, O.; Furuta, G.T.; Narravula-Alipati, S.; Sisson, R.B.; Serhan, C.N.; Colgan, S.P. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc. Natl. Acad. Sci. USA 2002, 99, 3902–3907. [Google Scholar] [CrossRef]
- Monajemi, H.; Meenan, J.; Lamping, R.; Obradov, D.O.; Radema, S.; Trown, P.; Tytgat, G.; Van Deventer, S. Inflammatory bowel disease is associated with increased mucosal levels of bactericidal/permeability-increasing protein. Gastroenterology 1996, 110, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Ramasundara, M.; Leach, S.T.; Lemberg, D.A.; Day, A.S. Defensins and inflammation: The role of defensins in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009, 24, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-y.; Zhong, X.; Kim, S.-J.; Kim, D.-H.; Kim, H.S.; Lee, J.-S.; Yum, H.-W.; Lee, J.; Na, H.-K.; Surh, Y.-J. Comparative effects of curcumin and tetrahydrocurcumin on dextran sulfate sodium-induced colitis and inflammatory signaling in mice. J. Cancer Prev. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Prev. Biomark. 2002, 11, 535–540. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Satoskar, R.; Shah, S.; Shenoy, S. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar]
- Ao, R.; Wang, Y.; Zhnag, D.R.; Du, Y.Q. Role of TLR4 rs4986790A>G and rs4986791C>T Polymorphisms in the Risk of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2015, 2015, 141070. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Shi, R.H.; Zhang, H.J.; Li, K.B.; Zhao, Y.; Zhang, R.Y. The Toll-Like Receptor 4 D299G and T399I Polymorphisms Are Associated with Crohn’s Disease and Ulcerative Colitis: A Meta-Analysis. Digestion 2010, 81, 69–77. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Huang, X.P.; Zhang, W.; Han, Z.L.; Liu, S.D. Association between TLR2 and TLR4 Gene Polymorphisms and the Susceptibility to Inflammatory Bowel Disease: A Meta-Analysis. PLoS ONE 2015, 10, e0126803. [Google Scholar] [CrossRef]
- Fukata, M.; Shang, L.; Santaolalla, R.; Sotolongo, J.; Pastorini, C.; España, C.; Ungaro, R.; Harpaz, N.; Cooper, H.S.; Elson, G. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm. Bowel Dis. 2011, 17, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Fukata, M.; Hernandez, Y.; Conduah, D.; Cohen, J.; Chen, A.; Breglio, K.; Goo, T.; Hsu, D.; Xu, R.; Abreu, M.T. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm. Bowel Dis. 2009, 15, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Frosali, S.; Pagliari, D.; Gambassi, G.; Landolfi, R.; Pandolfi, F.; Cianci, R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J. Immunol. Res. 2015, 2015, 489821. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Lahiff, C.; Moss, A.C. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, E66. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Samba-Mondonga, M.; Constante, M.; Fragoso, G.; Calvé, A.; Santos, M.M. Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLoS ONE 2019, 14, e0208677. [Google Scholar] [CrossRef]
- Burkina, V.; Zamaratskaia, G.; Rasmussen, M.K. Curcumin and quercetin modify warfarin-induced regulation of porcine CYP1A2 and CYP3A expression and activity in vitro. Xenobiotica 2022, 52, 435–441. [Google Scholar] [CrossRef]
- Tan, C.S.S.; Lee, S.W.H. Warfarin and food, herbal or dietary supplement interactions: A systematic review. Br. J. Clin. Pharmacol. 2021, 87, 352–374. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.B.; Luis, P.B.; Alfafara, C.; Billheimer, D.; Schneider, C.; Funk, J.L. Curcuminoid Content and Safety-Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States. Mol. Nutr. Food Res. 2018, 62, e1800143. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Patel, A.R.; Srivastav, P.P.; Singh, S.; Gupta, A.K.; Chávez-González, M.L.; Aguilar, C.N.; Chakravorty, N.; et al. Curcumin Extraction, Isolation, Quantification and Its Application in Functional Foods: A Review With a Focus on Immune Enhancement Activities and COVID-19. Front. Nutr. 2021, 8, 747956. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Lacerda, J.F.; Lagos, A.C.; Carolino, E.; Silva-Herdade, A.S.; Silva, M.; Sousa Guerreiro, C. Functional Food Components, Intestinal Permeability and Inflammatory Markers in Patients with Inflammatory Bowel Disease. Nutrients 2021, 13, 642. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiveti, C.K.; Kumar, B.; Singh, A.D.; Biradar, S.K.; Ahmad, R.; Singh, A.B.; Tummala, H. Stable Dietary Ora-Curcumin Formulation Protects from Experimental Colitis and Colorectal Cancer. Cells 2024, 13, 957. https://doi.org/10.3390/cells13110957
Valiveti CK, Kumar B, Singh AD, Biradar SK, Ahmad R, Singh AB, Tummala H. Stable Dietary Ora-Curcumin Formulation Protects from Experimental Colitis and Colorectal Cancer. Cells. 2024; 13(11):957. https://doi.org/10.3390/cells13110957
Chicago/Turabian StyleValiveti, Chaitanya K., Balawant Kumar, Anuj D. Singh, Sham K. Biradar, Rizwan Ahmad, Amar B. Singh, and Hemachand Tummala. 2024. "Stable Dietary Ora-Curcumin Formulation Protects from Experimental Colitis and Colorectal Cancer" Cells 13, no. 11: 957. https://doi.org/10.3390/cells13110957






