Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Clinical Effusion, Serum, and Tissue Specimens
2.2. Origins of the Two Selected Cell Lines
2.3. Immunohistochemistry, Immunocytochemistry, and Immunofluorescence
2.4. Cell Viability Assay
2.5. Western Blot Analysis
2.6. In Vitro Migration and Invasion Assay
2.7. In Vitro Wound-Healing Assay
2.8. Enzyme-Linked Immunosorbent Assay
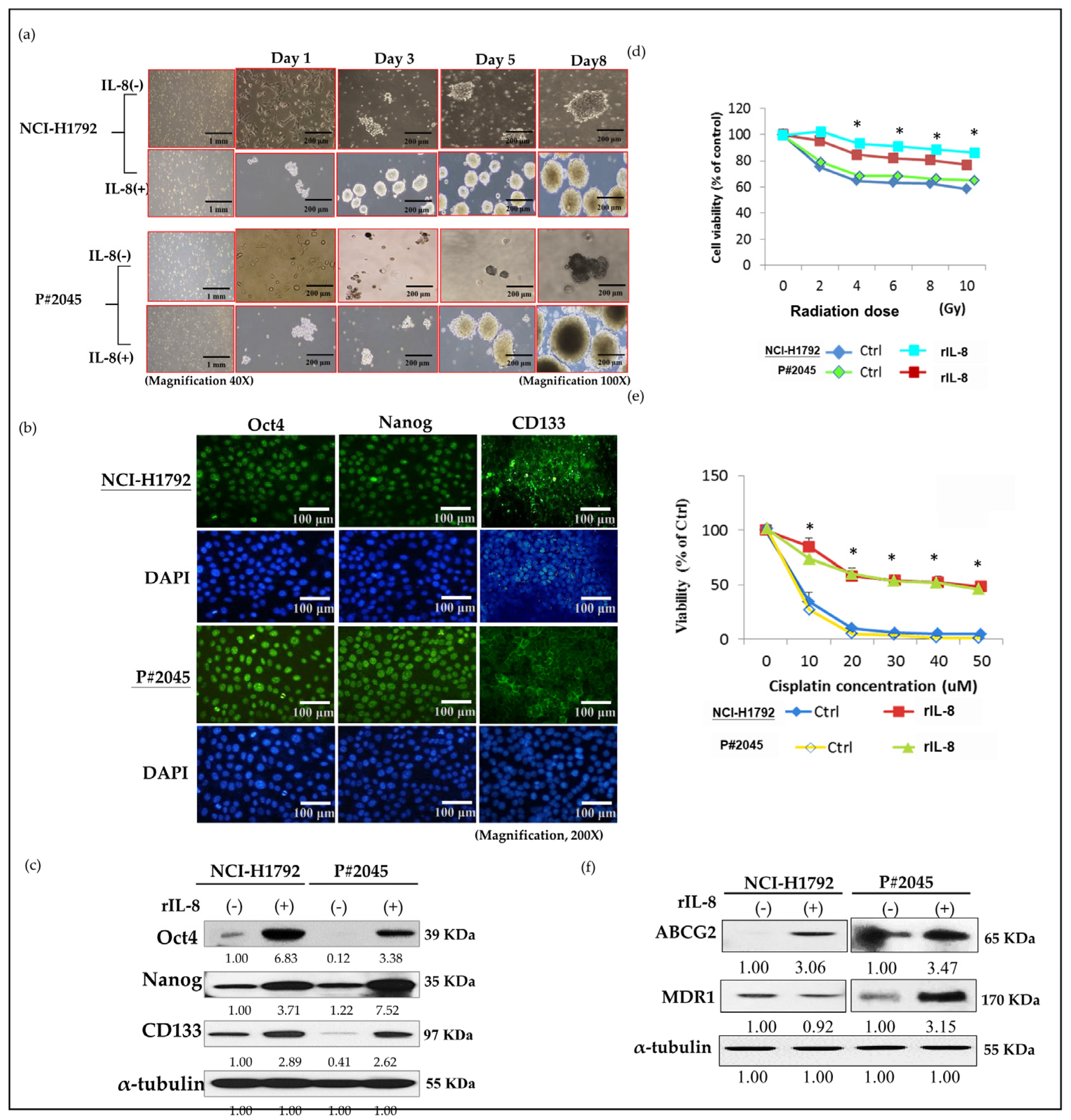
2.9. Sphere Culture
2.10. Assays for Chemosensitivity and Radiosensitivity
2.11. Statistical Analysis
3. Results
3.1. IL-8 Levels in MPE and Serum of PADC Was Higher Than Those in Systemic Disease with Pleural Effusion (SDPE) and IL-8 Levels in Both Representative Cell Lines Were Compared
3.2. IL-8 Promoted Epithelial-Mesenchymal Transition (EMT) and Activities of Migration and Invasion While Increasing the Expression of EMT-Associated Markers
3.3. High Levels of IL-8 in MPE Enhanced CSC Properties, Including Sphere Formation, Increased Expression of CSC Markers, and Resistance to Radiotherapy and Chemotherapy, as Well as Drug Resistance Genes
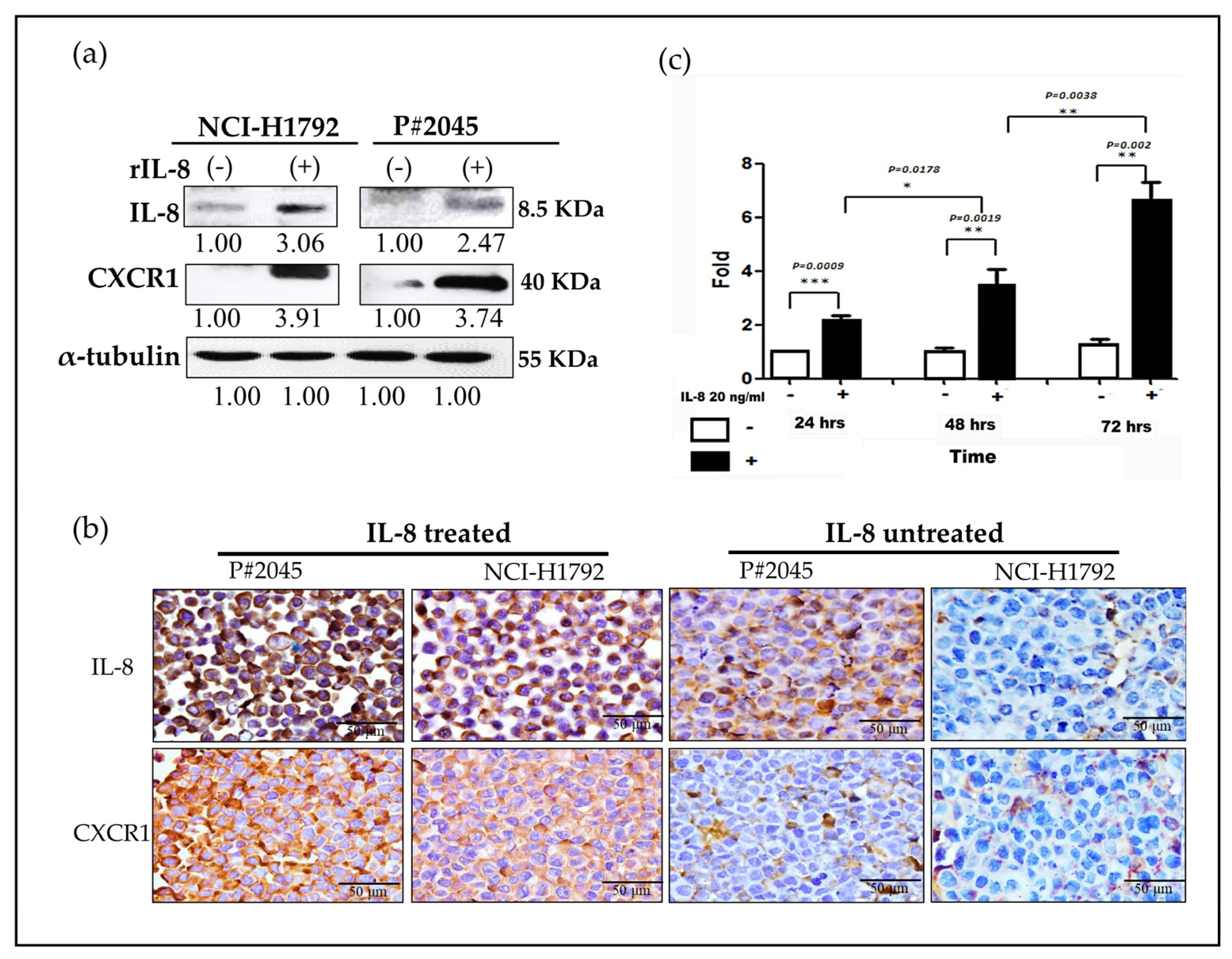
3.4. An Increase in CXCR1 Expression Induced by IL-8 Was Observed via Paracrine and Autocrine Signaling Pathways in MPE
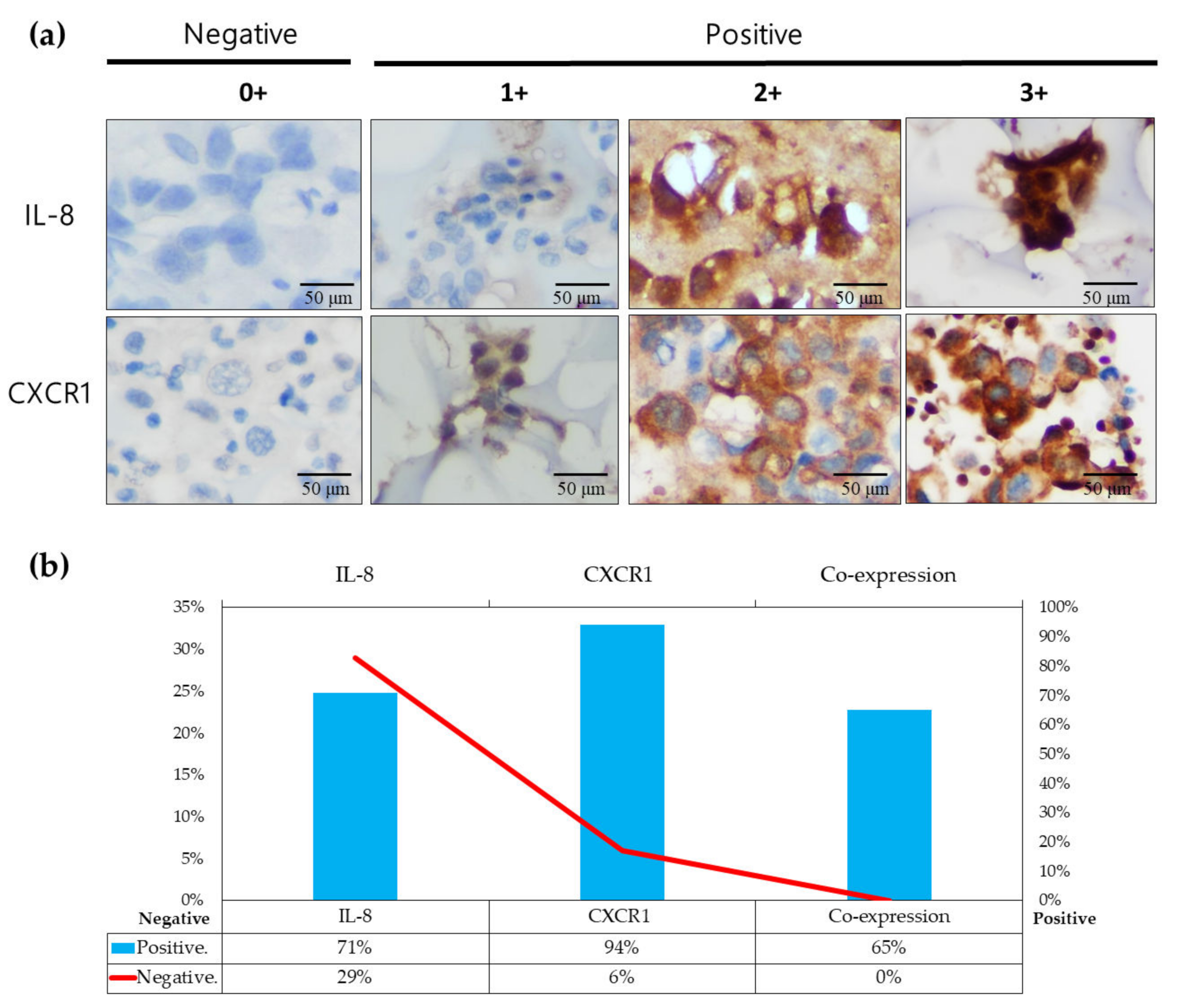
3.5. IL-8/CXCR1 Immunoexpression Correlates with Clinical Outcomes in Patients with PADC and MPE Indicating MPE as an Early Metastatic Indicator for Distant Metastasis Preparation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.F.; Lin, Y.S.; Jao, S.W.; Chang, Y.C.; Liu, C.L.; Lin, Y.J.; Nieh, S. Pulmonary Adenocarcinoma in Malignant Pleural Effusion Enriches Cancer Stem Cell Properties during Metastatic Cascade. PLoS ONE 2013, 8, e54659. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef] [PubMed]
- Sainz, B., Jr.; Carron, E.; Vallespinos, M.; Machado, H.L. Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies. Mediat. Inflamm. 2016, 2016, 9012369. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Kotyza, J. Interleukin-8 (CXCL8) in tumor associated non-vascular extracellular fluids: Its diagnostic and prognostic values. A review. Int. J. Biol. Markers 2012, 27, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Laskar, S.; Pandey, B.N. Autocrine IL-8 and VEGF mediate epithelial-mesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells. Cell. Signal. 2013, 25, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Hayama, N.; Hattori, S.; Takahashi, G.; Takahashi, F.; Takeuchi, T.; Tanaka, J.; Horio, Y.; Takiguchi, H.; Tomomatsu, K.; Kitahara, A.; et al. Cytokine/Chemokine/Growth Factor Levels in Malignant Pleural Effusion of Non-small Cell Lung Cancer. Tokai J. Exp. Clin. Med. 2020, 45, 224–229. [Google Scholar] [PubMed]
- Dick, I.M.; Lee, Y.C.G.; Cheah, H.M.; Miranda, A.; Robinson, B.W.S.; Creaney, J. Profile of soluble factors in pleural effusions predict prognosis in mesothelioma. Cancer Biomark. Sect. A Dis. Markers 2022, 33, 159–169. [Google Scholar] [CrossRef]
- Kaanane, H.; Senhaji, N.; Berradi, H.; Benchakroun, N.; Benider, A.; Karkouri, M.; El Attar, H.; Flores, O.; Khyatti, M.; Nadifi, S. The influence of Interleukin-6, Interleukin-8, Interleukin-10, Interleukin-17, TNF-A, MIF, STAT3 on lung cancer risk in Moroccan population. Cytokine 2022, 151, 155806. [Google Scholar] [CrossRef]
- Molczyk, C.; Singh, R.K. CXCR1: A Cancer Stem Cell Marker and Therapeutic Target in Solid Tumors. Biomedicines 2023, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Wang, B.; Wei, J.; Zhang, Y.; Li, Q.; Luan, X.; Cheng, J.W.; Gordon, J.R.; Li, F.; Liu, H. CXCR1/2 antagonism with CXCL8/Interleukin-8 analogue CXCL8(3-72)K11R/G31P restricts lung cancer growth by inhibiting tumor cell proliferation and suppressing angiogenesis. Oncotarget 2015, 6, 21315–21327. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Webster, S.J.; Flower, D.; Woll, P.J. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br. J. Cancer 2004, 91, 1970–1976. [Google Scholar] [CrossRef]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A. Paget's "Seed and Soil" Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [PubMed]
- Witz, I.P. Yin-yang activities and vicious cycles in the tumor microenvironment. Cancer Res. 2008, 68, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Yang, M.H.; Tsai, M.L.; Lan, H.Y.; Su, S.H.; Chang, S.C.; Teng, H.W.; Yang, S.H.; Lan, Y.T.; Chiou, S.H.; et al. SNAIL Regulates Interleukin-8 Expression, Stem Cell–Like Activity, and Tumorigenicity of Human Colorectal Carcinoma Cells. Gastroenterology 2011, 141, 279–291.e5. [Google Scholar] [CrossRef]
- Infanger, D.W.; Cho, Y.; Lopez, B.S.; Mohanan, S.; Liu, S.C.; Gursel, D.; Boockvar, J.A.; Fischbach, C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013, 73, 7079–7089. [Google Scholar] [CrossRef]
- Chen, S.F.; Chang, Y.C.; Nieh, S.; Liu, C.L.; Yang, C.Y.; Lin, Y.S. Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS ONE 2012, 7, e31864. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, A.; Perrone, G.; Orlando, N.; Citarella, F.; Ciccozzi, M.; Scarlata, S.; Tonini, G. A close relationship between HIF-1α expression and bone metastases in advanced NSCLC, a retrospective analysis. Oncotarget 2019, 10, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Huang, W.Y.; Lee, T.Y.; Chang, Y.M.; Chen, S.F.; Lin, Y.S.; Nieh, S. Interleukin-33-Enhanced CXCR4 Signaling Circuit Mediated by Carcinoma-Associated Fibroblasts Promotes Invasiveness of Head and Neck Cancer. Cancers 2021, 13, 3442. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lin, Y.S.; Lin, Y.C.; Nieh, S.; Chang, Y.M.; Lee, T.Y.; Chen, S.F.; Yang, K.D. Cancer-Associated Fibroblasts Promote Tumor Aggressiveness in Head and Neck Cancer through Chemokine Ligand 11 and C-C Motif Chemokine Receptor 3 Signaling Circuit. Cancers 2022, 14, 3141. [Google Scholar] [CrossRef] [PubMed]
- Solari, E.; Marcozzi, C.; Ottaviani, C.; Negrini, D.; Moriondo, A. Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task. Biology 2022, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Kassis, J.; Klominek, J.; Kohn, E.C. Tumor microenvironment: What can effusions teach us? Diagn. Cytopathol. 2005, 33, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Han, L.; Zhao, R.; Fatima, S.; Zhao, L.; Gao, F. Prognosis value of IL-6, IL-8, and IL-1β in serum of patients with lung cancer: A fresh look at interleukins as a biomarker. Heliyon 2022, 8, e09953. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ye, J.; Xie, K.; Hu, W.; Xu, N.; Yang, H. Exosomal IL-8 derived from Lung Cancer and Colon Cancer cells induced adipocyte atrophy via NF-κB signaling pathway. Lipids Health Dis. 2022, 21, 147. [Google Scholar] [CrossRef]
- Geiger, T.R.; Peeper, D.S. Metastasis mechanisms. Biochim. Biophys. Acta 2009, 1796, 293–308. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Liotta, L.A.; Kohn, E. Anoikis: Cancer and the homeless cell. Nature 2004, 430, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Maiuthed, A.; Chantarawong, W.; Chanvorachote, P. Lung Cancer Stem Cells and Cancer Stem Cell-targeting Natural Compounds. Anticancer Res. 2018, 38, 3797–3809. [Google Scholar] [CrossRef] [PubMed]
- Budna, J.; Kaczmarek, M.; Kolecka-Bednarczyk, A.; Spychalski, L.; Zawierucha, P.; Gozdzik-Spychalska, J.; Nowicki, M.; Batura-Gabryel, H.; Sikora, J. Enhanced Suppressive Activity of Regulatory T Cells in the Microenvironment of Malignant Pleural Effusions. J. Immunol. Res. 2018, 2018, 9876014. [Google Scholar] [CrossRef] [PubMed]
- Saraya, T.; Ohkuma, K.; Watanabe, T.; Mikura, S.; Kobayashi, F.; Aso, J.; Nunokawa, H.; Honda, K.; Ogawa, Y.; Tamura, M.; et al. Diagnostic Value of Vascular Endothelial Growth Factor, Transforming Growth Factor-beta, Interleukin-8, and the Ratio of Lactate Dehydrogenase to Adenosine Deaminase in Pleural Effusion. Lung 2018, 196, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, L.; Wang, L.; Wang, F.; Zhang, Z.; Li, J.; Yue, D.; Chen, X.; Ping, Y.; Huang, L.; et al. Impaired T cell function in malignant pleural effusion is caused by TGF-beta derived predominantly from macrophages. Int. J. Cancer 2016, 139, 2261–2269. [Google Scholar] [CrossRef]
- Chen, Y.; Mathy, N.W.; Lu, H. The role of VEGF in the diagnosis and treatment of malignant pleural effusion in patients with nonsmall cell lung cancer (Review). Mol. Med. Rep. 2018, 17, 8019–8030. [Google Scholar] [CrossRef]
- Hsu, I.L.; Su, W.C.; Yan, J.J.; Chang, J.M.; Lai, W.W. Angiogenetic biomarkers in non-small cell lung cancer with malignant pleural effusion: Correlations with patient survival and pleural effusion control. Lung Cancer Amst. Neth. 2009, 65, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int. J. Oncol 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Parte, S.; Are, A.C.; Batra, S.K.; Ponnusamy, M.P. Tumor microenvironment enriches the stemness features: The architectural event of therapy resistance and metastasis. Mol. Cancer 2022, 21, 225. [Google Scholar] [CrossRef]
- Kiss, E.; Abdelwahab, E.; Steib, A.; Papp, E.; Torok, Z.; Jakab, L.; Smuk, G.; Sarosi, V.; Pongracz, J.E. Cisplatin treatment induced interleukin 6 and 8 production alters lung adenocarcinoma cell migration in an oncogenic mutation dependent manner. Respir. Res. 2020, 21, 120. [Google Scholar] [CrossRef]
- Jiang, H.; Cui, J.; Chu, H.; Xu, T.; Xie, M.; Jing, X.; Xu, J.; Zhou, J.; Shu, Y. Targeting IL8 as a sequential therapy strategy to overcome chemotherapy resistance in advanced gastric cancer. Cell Death Discov. 2022, 8, 235. [Google Scholar] [CrossRef] [PubMed]



| PADC | Percentage | Metastasis | Metastatic Site | Percentage |
|---|---|---|---|---|
| With MPE | 53% (9/17) | 100% (9/9) | Lung | 66% (6/9) |
| Pleura | 55% (5/9) | |||
| Bone | 44% (4/9) | |||
| Brain | 34% (3/9) | |||
| Pericardium | 11% (1/9) | |||
| Peritoneum | 11% (1/9) | |||
| Without MPE | 47% (8/17) | 25% (2/8) | Lung | 100% (2/2) |
| Pleura | 50% (1/2) |
| Pleural Invasion | PL1/PL2 | PL0 |
| 47% (8/17) * | 41% (7/17) * | |
| MPE | 88% (7/8) | 0% (0/7) |
| Metastasis | 75% (6/8) | 28% (2/7) |
| Metastatic sites | Lung 50% (4/8) Pleura 50% (4/8) Brain 38% (3/8) Bone 25% (2/8) | Lung 28% (2/7) Pleura 14% (1/7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-M.; Huang, W.-Y.; Yang, S.-H.; Jan, C.-I.; Nieh, S.; Lin, Y.-S.; Chen, S.-F.; Lin, Y.-C. Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion. Cells 2024, 13, 968. https://doi.org/10.3390/cells13110968
Chang Y-M, Huang W-Y, Yang S-H, Jan C-I, Nieh S, Lin Y-S, Chen S-F, Lin Y-C. Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion. Cells. 2024; 13(11):968. https://doi.org/10.3390/cells13110968
Chicago/Turabian StyleChang, Yi-Ming, Wen-Yen Huang, Shih-Hsien Yang, Chia-Ing Jan, Shin Nieh, Yaoh-Shiang Lin, Su-Feng Chen, and Yu-Chun Lin. 2024. "Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion" Cells 13, no. 11: 968. https://doi.org/10.3390/cells13110968
APA StyleChang, Y.-M., Huang, W.-Y., Yang, S.-H., Jan, C.-I., Nieh, S., Lin, Y.-S., Chen, S.-F., & Lin, Y.-C. (2024). Interleukin-8/CXCR1 Signaling Contributes to the Progression of Pulmonary Adenocarcinoma Resulting in Malignant Pleural Effusion. Cells, 13(11), 968. https://doi.org/10.3390/cells13110968






