Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity
Abstract
:1. Introduction
2. The Limits of Human Tolerance to Microbiota
3. Factors Influencing the Impact of the Gut Microbiome on Immunity
4. Molecular Mechanisms Encoded by Host and Microbial Genes in Gut Microbiome–Immune System Interactions
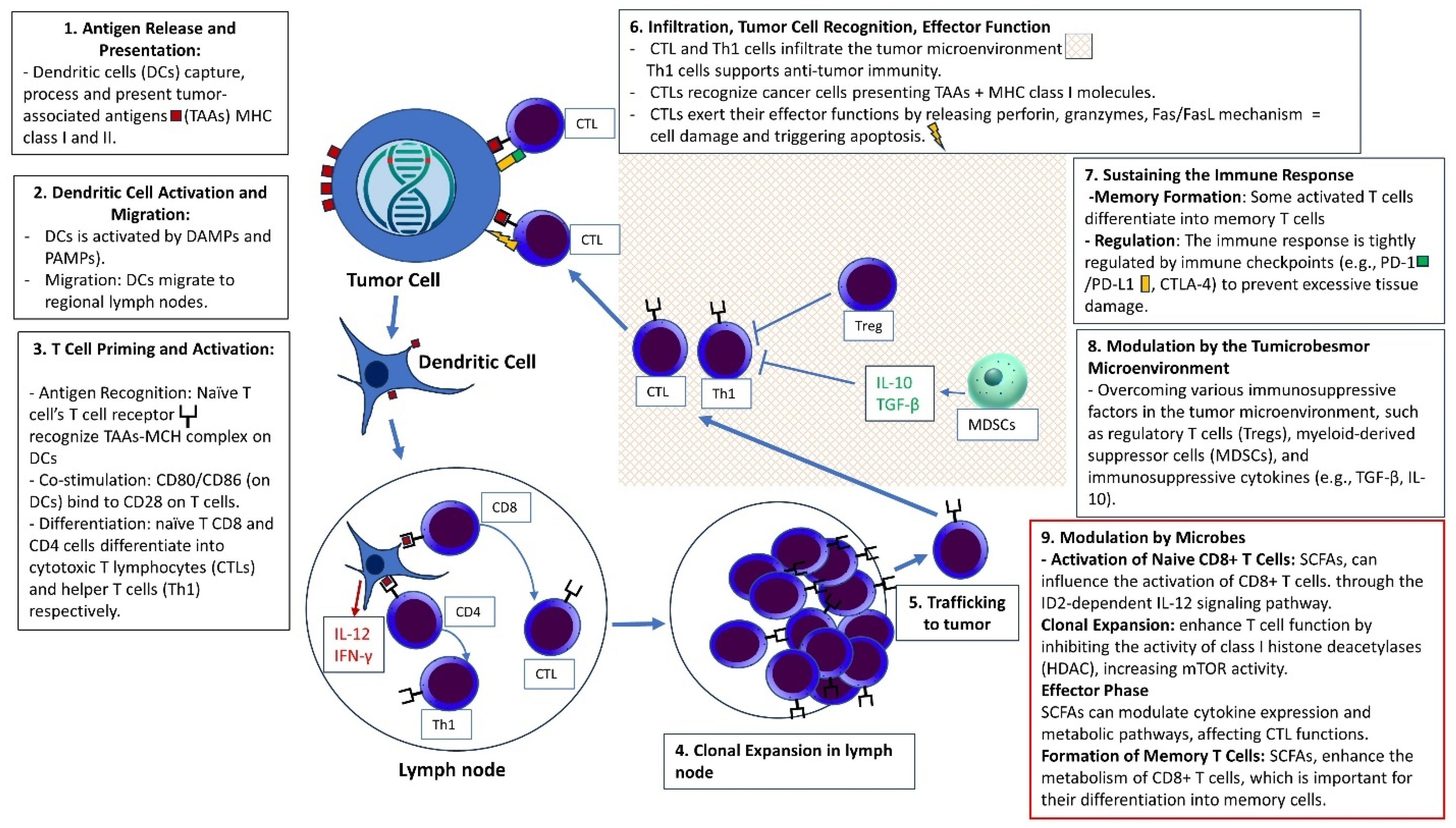
5. Microbiome and Colorectal Cancer Immunity
6. The Influence of Gut Microbiota on Immunotherapy Efficacy in Colorectal Cancer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Shao, J.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.; Liu, Z.; He, D.; Li, C.; Zhang, X. Regulation of Short-Chain Fatty Acids in the Immune System. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut Microbiota Influences the Efficiency of Immune Checkpoint Inhibitors by Modulating the Immune System (Review). Oncol. Lett. 2024, 27, 87. [Google Scholar] [CrossRef]
- Rohatgi, A.; Kirkwood, J.M. Beyond PD-1: The Next Frontier for Immunotherapy in Melanoma. Front. Oncol. 2021, 11, 640314. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Hazra, R.; Chattopadhyay, S.; Mallick, A.; Gayen, S.; Roy, S. Revealing the Therapeutic Properties of Gut Microbiota: Transforming Cancer Immunotherapy from Basic to Clinical Approaches. Med. Oncol. 2024, 41, 175. [Google Scholar] [CrossRef]
- Pietrzak, B.; Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M. Circulating Microbial Cell-Free DNA in Health and Disease. Int. J. Mol. Sci. 2023, 24, 3051. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.; Midtervoll, I.; Samuelsen, S.O.; Kristoffersen, A.K.; Enersen, M.; Håheim, L.L. The Blood Microbiome and Its Association to Cardiovascular Disease Mortality: Case-Cohort Study. BMC Cardiovasc. Disord. 2022, 22, 344. [Google Scholar] [CrossRef]
- Panaiotov, S.; Hodzhev, Y.; Tsafarova, B.; Tolchkov, V.; Kalfin, R. Culturable and Non-Culturable Blood Microbiota of Healthy Individuals. Microorganisms 2021, 9, 1464. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We Be Worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, J.; Zhou, D.; Wang, J. Bacterial Lipopolysaccharide-Induced Endothelial Activation and Dysfunction: A New Predictive and Therapeutic Paradigm for Sepsis. Eur. J. Med. Res. 2023, 28, 339. [Google Scholar] [CrossRef]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions. Front. Cell. Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- He, L.; Zhong, Z.; Wen, S.; Li, P.; Jiang, Q.; Liu, F. Gut Microbiota-Derived Butyrate Restores Impaired Regulatory T Cells in Patients with AChR Myasthenia Gravis via mTOR-Mediated Autophagy. Cell Commun. Signal. CCS 2024, 22, 215. [Google Scholar] [CrossRef]
- Moon, J.; Lee, A.R.; Kim, H.; Jhun, J.; Lee, S.-Y.; Choi, J.W.; Jeong, Y.; Park, M.S.; Ji, G.E.; Cho, M.-L.; et al. Faecalibacterium prausnitzii Alleviates Inflammatory Arthritis and Regulates IL-17 Production, Short Chain Fatty Acids, and the Intestinal Microbial Flora in Experimental Mouse Model for Rheumatoid Arthritis. Arthritis Res. Ther. 2023, 25, 130. [Google Scholar] [CrossRef]
- Touch, S.; Godefroy, E.; Rolhion, N.; Danne, C.; Oeuvray, C.; Straube, M.; Galbert, C.; Brot, L.; Alonso Salgueiro, I.; Chadi, S.; et al. Human CD4+CD8α+ Tregs Induced by Faecalibacterium prausnitzii Protect against Intestinal Inflammation. JCI Insight 2022, 7, e154722. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii Prevents Physiological Damages in a Chronic Low-Grade Inflammation Murine Model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Zheng, L.; Luo, M.; Kuang, G.; Liu, Y.; Liang, D.; Huang, H.; Yi, X.; Wang, C.; Wang, Y.; Xie, Q.; et al. Capsular Polysaccharide From Bacteroides Fragilis Protects Against Ulcerative Colitis in an Undegraded Form. Front. Pharmacol. 2020, 11, 570476. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.A.; Jones, M.B.; Hambor, J.; Cobb, B.A. Characterization of Polysaccharide A Response Reveals Interferon Responsive Gene Signature and Immunomodulatory Marker Expression. Front. Immunol. 2020, 11, 556813. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus Spp. and Bifidobacterium Spp. in Gastrointestinal Cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, K.; Dogru, Y.D.; Huang, Q.; Yang, Y.; Tolstikov, V.; Raisingani, A.; Chrudinova, M.; Oh, J.; Kelley, K.; Ludvigsson, J.F.; et al. Dynamics of the Gut Microbiome, IgA Response, and Plasma Metabolome in the Development of Pediatric Celiac Disease. Microbiome 2023, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut Microbiome and Human Health: Exploring How the Probiotic Genus Lactobacillus Modulate Immune Responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The Role of Akkermansia muciniphila in Inflammatory Bowel Disease: Current Knowledge and Perspectives. Front. Immunol. 2023, 13, 1089600. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like Proteins of Akkermansia muciniphila Modulate Host Immune Responses and Gut Barrier Function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Wade, H.; Pan, K.; Duan, Q.; Kaluzny, S.; Pandey, E.; Fatumoju, L.; Saraswathi, V.; Wu, R.; Harris, E.N.; Su, Q. Akkermansia Muciniphila and Its Membrane Protein Ameliorates Intestinal Inflammatory Stress and Promotes Epithelial Wound Healing via CREBH and miR-143/145. J. Biomed. Sci. 2023, 30, 38. [Google Scholar] [CrossRef]
- Molaaghaee-Rouzbahani, S.; Asri, N.; Sapone, A.; Baghaei, K.; Yadegar, A.; Amani, D.; Rostami-Nejad, M. Akkermansia muciniphila Exerts Immunomodulatory and Anti-Inflammatory Effects on Gliadin-Stimulated THP-1 Derived Macrophages. Sci. Rep. 2023, 13, 3237. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L.; et al. Dietary Fiber Intake, the Gut Microbiome, and Chronic Systemic Inflammation in a Cohort of Adult Men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Frame, L.A.; Costa, E.; Jackson, S.A. Current Explorations of Nutrition and the Gut Microbiome: A Comprehensive Evaluation of the Review Literature. Nutr. Rev. 2020, 78, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; He, Q. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-Associated Dysbiosis Affects the Ability of the Gut Microbiota to Control Intestinal Inflammation upon Fecal Microbiota Transplantation in Experimental Colitis Models. Microbiome 2021, 9, 39. [Google Scholar] [CrossRef]
- Xu, F.; Fu, Y.; Sun, T.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.; Yang, J.; Wang, J.; et al. The Interplay between Host Genetics and the Gut Microbiome Reveals Common and Distinct Microbiome Features for Complex Human Diseases. Microbiome 2020, 8, 145. [Google Scholar] [CrossRef]
- Wu, K.; Luo, Q.; Liu, Y.; Li, A.; Xia, D.; Sun, X. Causal Relationship between Gut Microbiota and Gastrointestinal Diseases: A Mendelian Randomization Study. J. Transl. Med. 2024, 22, 92. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, C.; Wang, J.; Cheng, Y.; Wang, L.; Tong, Y.; Yan, D. Identification of Host Gene-Microbiome Associations in Colorectal Cancer Patients Using Mendelian Randomization. J. Transl. Med. 2023, 21, 535. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-Scale Association Analyses Identify Host Factors Influencing Human Gut Microbiome Composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Amemiya, K.; Dankmeyer, J.L.; Bernhards, R.C.; Fetterer, D.P.; Waag, D.M.; Worsham, P.L.; DeShazer, D. Activation of Toll-Like Receptors by Live Gram-Negative Bacterial Pathogens Reveals Mitigation of TLR4 Responses and Activation of TLR5 by Flagella. Front. Cell. Infect. Microbiol. 2021, 11, 745325. [Google Scholar] [CrossRef] [PubMed]
- Godkowicz, M.; Druszczyńska, M. NOD1, NOD2, and NLRC5 Receptors in Antiviral and Antimycobacterial Immunity. Vaccines 2022, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Anwer, S.; Szászi, K. Claudin-2: Roles beyond Permeability Functions. Int. J. Mol. Sci. 2019, 20, 5655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Li, L.; Wang, Y.; Li, Y.; Zhang, S. Claudin Family Participates in the Pathogenesis of Inflammatory Bowel Diseases and Colitis-Associated Colorectal Cancer. Front. Immunol. 2019, 10, 1441. [Google Scholar] [CrossRef]
- Zou, T.; Yang, J.; Guo, X.; He, Q.; Wang, Z.; You, J. Dietary Seaweed-Derived Polysaccharides Improve Growth Performance of Weaned Pigs through Maintaining Intestinal Barrier Function and Modulating Gut Microbial Populations. J. Anim. Sci. Biotechnol. 2021, 12, 28. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Secor, J.D.; Fligor, S.C.; Tsikis, S.T.; Yu, L.J.; Puder, M. Free Fatty Acid Receptors as Mediators and Therapeutic Targets in Liver Disease. Front. Physiol. 2021, 12, 656441. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Guo, S.; Yun, Y.; Cheng, X.; He, X.; Cai, P.; Lan, Y.; Zhou, H.; Jiang, H.; et al. Structural Identification of Lysophosphatidylcholines as Activating Ligands for Orphan Receptor GPR119. Nat. Struct. Mol. Biol. 2022, 29, 863–870. [Google Scholar] [CrossRef]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Kidess, E.; Kleerebezem, M.; Brugman, S. Colonizing Microbes, IL-10 and IL-22: Keeping the Peace at the Mucosal Surface. Front. Microbiol. 2021, 12, 729053. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.; Eri, R. CCR6–CCL20 Axis in IBD: What Have We Learnt in the Last 20 Years? Gastrointest. Disord. 2019, 1, 57–74. [Google Scholar] [CrossRef]
- Daulagala, A.C.; Bridges, M.C.; Kourtidis, A. E-Cadherin Beyond Structure: A Signaling Hub in Colon Homeostasis and Disease. Int. J. Mol. Sci. 2019, 20, 2756. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Bajic, D.; Niemann, A.; Hillmer, A.-K.; Mejias-Luque, R.; Bluemel, S.; Docampo, M.; Funk, M.C.; Tonin, E.; Boutros, M.; Schnabl, B.; et al. Gut Microbiota-Derived Propionate Regulates the Expression of Reg3 Mucosal Lectins and Ameliorates Experimental Colitis in Mice. J. Crohns Colitis 2020, 14, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Engering, A.; Van Kooyk, Y. DC-SIGN, a C-Type Lectin on Dendritic Cells That Unveils Many Aspects of Dendritic Cell Biology. J. Leukoc. Biol. 2002, 71, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Kao, C.-Y. Current Understanding of the Gut Microbiota Shaping Mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef]
- Wang, H.; Yin, J.; Gu, X.; Shao, W.; Jia, Z.; Chen, H.; Xia, W. Immune Regulator Retinoic Acid-Inducible Gene I (RIG-I) in the Pathogenesis of Cardiovascular Disease. Front. Immunol. 2022, 13, 893204. [Google Scholar] [CrossRef]
- Chao, L.; Zhang, W.; Feng, Y.; Gao, P.; Ma, J. Pyroptosis: A New Insight into Intestinal Inflammation and Cancer. Front. Immunol. 2024, 15, 1364911. [Google Scholar] [CrossRef]
- Chevalier, G.; Laveissière, A.; Desachy, G.; Barnich, N.; Sivignon, A.; Maresca, M.; Nicoletti, C.; Di Pasquale, E.; Martinez-Medina, M.; Simpson, K.W.; et al. Blockage of Bacterial FimH Prevents Mucosal Inflammation Associated with Crohn’s Disease. Microbiome 2021, 9, 176. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile Salt Hydrolases: Gatekeepers of Bile Acid Metabolism and Host-Microbiome Crosstalk in the Gastrointestinal Tract. PLoS Pathog. 2019, 15, e1007581. [Google Scholar] [CrossRef] [PubMed]
- Woida, P.J.; Satchell, K.J.F. Bacterial Toxin and Effector Regulation of Intestinal Immune Signaling. Front. Cell Dev. Biol. 2022, 10, 837691. [Google Scholar] [CrossRef] [PubMed]
- Schwidder, M.; Heinisch, L.; Schmidt, H. Genetics, Toxicity, and Distribution of Enterohemorrhagic Escherichia Coli Hemolysin. Toxins 2019, 11, 502. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-Derived Indoles Alleviate Intestinal Inflammation and Modulate Microbiome by Microbial Cross-Feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, R.; Zhang, J.; Li, P. Overexpression of luxS Promotes Stress Resistance and Biofilm Formation of Lactobacillus paraplantarum L-ZS9 by Regulating the Expression of Multiple Genes. Front. Microbiol. 2018, 9, 2628. [Google Scholar] [CrossRef]
- Cheng, X.; Redanz, S.; Treerat, P.; Qin, H.; Choi, D.; Zhou, X.; Xu, X.; Merritt, J.; Kreth, J. Magnesium-Dependent Promotion of H2O2 Production Increases Ecological Competitiveness of Oral Commensal Streptococci. J. Dent. Res. 2020, 99, 847–854. [Google Scholar] [CrossRef]
- Kaur, H.; Ali, S.A.; Yan, F. Interactions between the Gut Microbiota-Derived Functional Factors and Intestinal Epithelial Cells—Implication in the Microbiota-Host Mutualism. Front. Immunol. 2022, 13, 1006081. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Moon, J.A.; Son, D.Y.; Hong, K.W. Transcriptional Responses of Human Intestinal Epithelial HT-29 Cells to Spore-Displayed P40 Derived from Lacticaseibacillus rhamnosus GG. BMC Microbiol. 2022, 22, 316. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti–PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef]
- Chen, F.; Dai, X.; Zhou, C.-C.; Li, K.-X.; Zhang, Y.-J.; Lou, X.-Y.; Zhu, Y.-M.; Sun, Y.-L.; Peng, B.-X.; Cui, W. Integrated Analysis of the Faecal Metagenome and Serum Metabolome Reveals the Role of Gut Microbiome-Associated Metabolites in the Detection of Colorectal Cancer and Adenoma. Gut 2022, 71, 1315–1325. [Google Scholar] [CrossRef]
- Muñoz-Tamayo, R.; Laroche, B.; Walter, E.; Doré, J.; Duncan, S.H.; Flint, H.J.; Leclerc, M. Kinetic Modelling of Lactate Utilization and Butyrate Production by Key Human Colonic Bacterial Species. FEMS Microbiol. Ecol. 2011, 76, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating Taxonomic, Functional, and Strain-Level Profiling of Diverse Microbial Communities with bioBakery 3. eLife 2021, 10, e65088. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, K.; Fang, C.; Yin, X.; Liu, X.; Zhong, J.; Li, K.; Li, J.; Xu, L.; Yang, X. Altered Synthesis of Genes Associated with Short-Chain Fatty Acids in the Gut of Patients with Atrial Fibrillation. BMC Genom. 2021, 22, 634. [Google Scholar] [CrossRef]
- Bihan, D.G.; Rydzak, T.; Wyss, M.; Pittman, K.; McCoy, K.D.; Lewis, I.A. Method for Absolute Quantification of Short Chain Fatty Acids via Reverse Phase Chromatography Mass Spectrometry. PLoS ONE 2022, 17, e0267093. [Google Scholar] [CrossRef]
- Borelli, B.; Antoniotti, C.; Carullo, M.; Germani, M.M.; Conca, V.; Masi, G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability. Cancers 2022, 14, 4974. [Google Scholar] [CrossRef]
- Cohen, R.; Colle, R.; Pudlarz, T.; Heran, M.; Duval, A.; Svrcek, M.; André, T. Immune Checkpoint Inhibition in Metastatic Colorectal Cancer Harboring Microsatellite Instability or Mismatch Repair Deficiency. Cancers 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, J.; Zhi, M. Heterogeneity of Immune Infiltration and Immunotherapy in Colorectal Cancer. Gastroenterol. Rep. 2023, 12, goad079. [Google Scholar] [CrossRef]
- Li, C.; Wirth, U.; Schardey, J.; Ehrlich-Treuenstätt, V.V.; Bazhin, A.V.; Werner, J.; Kühn, F. An Immune-Related Gene Prognostic Index for Predicting Prognosis in Patients with Colorectal Cancer. Front. Immunol. 2023, 14, 1156488. [Google Scholar] [CrossRef]
- Byrd, D.A.; Fan, W.; Greathouse, K.L.; Wu, M.C.; Xie, H.; Wang, X. The Intratumor Microbiome Is Associated with Microsatellite Instability. J. Natl. Cancer Inst. 2023, 115, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut Microbiota-Stimulated Cathepsin K Secretion Mediates TLR4-Dependent M2 Macrophage Polarization and Promotes Tumor Metastasis in Colorectal Cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef]
- Hale, V.L.; Jeraldo, P.; Chen, J.; Mundy, M.; Yao, J.; Priya, S.; Keeney, G.; Lyke, K.; Ridlon, J.; White, B.A.; et al. Distinct Microbes, Metabolites, and Ecologies Define the Microbiome in Deficient and Proficient Mismatch Repair Colorectal Cancers. Genome Med. 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.; Li, Q.; Ji, F.; Liang, W.; Lau, H.C.H.; Kang, X.; Liu, W.; To, K.K.-W.; Zuo, Z.; Li, X.; et al. Lactobacillus gallinarum-Derived Metabolites Boost Anti-PD1 Efficacy in Colorectal Cancer by Inhibiting Regulatory T Cells through Modulating IDO1/Kyn/AHR Axis. Gut 2023, 72, 2272–2285. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Z.; Wei, J.; Zhao, L. Effects of Gut Microbiota on Immune Responses and Immunotherapy in Colorectal Cancer. Front. Immunol. 2022, 13, 1030745. [Google Scholar] [CrossRef]
- Li, T.; Han, L.; Ma, S.; Lin, W.; Ba, X.; Yan, J.; Huang, Y.; Tu, S.; Qin, K. Interaction of Gut Microbiota with the Tumor Microenvironment: A New Strategy for Antitumor Treatment and Traditional Chinese Medicine in Colorectal Cancer. Front. Mol. Biosci. 2023, 10, 1140325. [Google Scholar] [CrossRef]
- Cremonesi, E.; Governa, V.; Garzon, J.F.G.; Mele, V.; Amicarella, F.; Muraro, M.G.; Trella, E.; Galati-Fournier, V.; Oertli, D.; Däster, S.R.; et al. Gut Microbiota Modulate T Cell Trafficking into Human Colorectal Cancer. Gut 2018, 67, 1984–1994. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Wu, F.; Wang, X. Alterations in the Gut Microbiota and Their Metabolites in Colorectal Cancer: Recent Progress and Future Prospects. Front. Oncol. 2022, 12, 841552. [Google Scholar] [CrossRef]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Jin, X.; Jiang, X.; Guo, S.; Xu, F.; Su, X.; Wang, G.; Zhao, Z.; Gu, X. Identification of Prognostic Immune-Related Gene Signature Associated with Tumor Microenvironment of Colorectal Cancer. BMC Cancer 2021, 21, 905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, J.; Fang, D.; Lv, L.; Wu, W.; Shi, D.; Li, Y.; Yang, L.; Bian, X.; Wu, J.; et al. Multi-Omic Profiling Reveals Associations between the Gut Mucosal Microbiome, the Metabolome, and Host DNA Methylation Associated Gene Expression in Patients with Colorectal Cancer. BMC Microbiol. 2020, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Yang, C.; Zhang, J.; Zhong, D.; Meng, M.; Zhang, L.; Chen, H.; Fang, L. Multi-Omic Profiling Reveals Associations between the Gut Microbiome, Host Genome and Transcriptome in Patients with Colorectal Cancer. J. Transl. Med. 2024, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xiong, Y.; Fu, B.; Guo, D.; Sha, Z.; Lin, X.; Wu, H. Bacteria and Macrophages in the Tumor Microenvironment. Front. Microbiol. 2023, 14, 1115556. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fan, L.; Qi, Y.; Xu, C.; Jia, D.; Jiang, Y.; Chen, S.; Wang, L. Bifidobacterium Adolescentis Induces Decorin+ Macrophages via TLR2 to Suppress Colorectal Carcinogenesis. J. Exp. Clin. Cancer Res. 2023, 42, 172. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, C.; Qin, Y.; Zhu, H.; Guo, H.; Ji, B.; Li, X.; Sun, N.; Li, R.; Wu, Y.; et al. Blockade of C5aR1 Resets M1 via Gut Microbiota-Mediated PFKM Stabilization in a TLR5-Dependent Manner. Cell Death Dis. 2024, 15, 120. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial Short-Chain Fatty Acids Modulate CD8+ T Cell Responses and Improve Adoptive Immunotherapy for Cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut Microbial Metabolites Facilitate Anticancer Therapy Efficacy by Modulating Cytotoxic CD8+ T Cell Immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Mirji, G.; Worth, A.; Bhat, S.A.; El Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.-Y.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The Microbiome-Derived Metabolite TMAO Drives Immune Activation and Boosts Responses to Immune Checkpoint Blockade in Pancreatic Cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef]
- Gut Microbiota Modulates Adoptive Cell Therapy via CD8α Dendritic Cells and IL-12—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29467322/ (accessed on 21 July 2024).
- Garcia, A.B.; Ruiz, S.; Navarro, P.; Rodriguez-Moreno, J.F.; Sevillano, E.; Yague, M.; Amarilla, S.; Garcia-Donas, J. 770 Analysis of Gut Microbiome in Patients Receiving Adoptive T-Cell Therapy (ACT) across Different Solid Tumour Types. J. Immunother. Cancer 2020, 8 (Suppl. 3), A461. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Hou, W.; Ding, Y.; Zhu, Y.; Zheng, J.; Huang, Q.; Cao, Z.; Xie, R.; Wei, Q.; et al. Colorectal Cancer-Associated T Cell Receptor Repertoire Abnormalities Are Linked to Gut Microbiome Shifts and Somatic Cell Mutations. Gut Microbes 2023, 15, 2263934. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.-M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic Short Chain Fatty Acids Limit Antitumor Effect of CTLA-4 Blockade in Hosts with Cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Shoji, F.; Yamaguchi, M.; Okamoto, M.; Takamori, S.; Yamazaki, K.; Okamoto, T.; Maehara, Y. Gut Microbiota Diversity and Specific Composition during Immunotherapy in Responders with Non-Small Cell Lung Cancer. Front. Mol. Biosci. 2022, 9, 1040424. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.; Xie, M.; Huang, P.; Yu, Y.; Sun, Q.; Shangguan, W.; Li, W.; Zhu, Z.; Xue, J.; et al. Gut Microbiota Parabacteroides distasonis Enchances the Efficacy of Immunotherapy for Bladder Cancer by Activating Anti-Tumor Immune Responses. BMC Microbiol. 2024, 24, 237. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Zhu, R.; Wang, S.; Xue, J.; Zhang, D.; Lan, Z.; Zhang, C.; Liang, Y.; Zhang, N.; et al. Gut Microbiota and Metabolites Signatures of Clinical Response in Anti-PD-1/PD-L1 Based Immunotherapy of Biliary Tract Cancer. Biomark. Res. 2024, 12, 56. [Google Scholar] [CrossRef]
- Hexun, Z.; Miyake, T.; Maekawa, T.; Mori, H.; Yasukawa, D.; Ohno, M.; Nishida, A.; Andoh, A.; Tani, M. High Abundance of Lachnospiraceae in the Human Gut Microbiome Is Related to High Immunoscores in Advanced Colorectal Cancer. Cancer Immunol. Immunother. CII 2023, 72, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Gazzaniga, F.S.; Wu, M.; Luthens, A.K.; Gillis, J.; Zheng, W.; LaFleur, M.W.; Johnson, S.B.; Morad, G.; Park, E.M.; et al. Targeting PD-L2-RGMb Overcomes Microbiome-Related Immunotherapy Resistance. Nature 2023, 617, 377–385. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yu, B.; Zhou, J.; Zhang, J.; Zhang, R.; Xie, J.; Wang, Q.; Zhao, S. Lysates of Lactobacillus Acidophilus Combined with CTLA-4-Blocking Antibodies Enhance Antitumor Immunity in a Mouse Colon Cancer Model. Sci. Rep. 2019, 9, 20128. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, X.; Kang, W.; Hao, H.; Mao, Y.; Zhang, H.; Chen, Y.; Tan, Y.; He, Y.; Zhao, W.; et al. Metagenomic and Metabolomic Analyses Reveal Synergistic Effects of Fecal Microbiota Transplantation and Anti-PD-1 Therapy on Treating Colorectal Cancer. Front. Immunol. 2022, 13, 874922. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-Derived Inosine Modulates Response to Checkpoint Inhibitor Immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Wang, F.; He, M.-M.; Yao, Y.-C.; Zhao, X.; Wang, Z.-Q.; Jin, Y.; Luo, H.-Y.; Li, J.-B.; Wang, F.-H.; Qiu, M.-Z.; et al. Regorafenib plus Toripalimab in Patients with Metastatic Colorectal Cancer: A Phase Ib/II Clinical Trial and Gut Microbiome Analysis. Cell Rep. Med. 2021, 2, 100383. [Google Scholar] [CrossRef]
- Huang, X.; Hu, M.; Sun, T.; Li, J.; Zhou, Y.; Yan, Y.; Xuan, B.; Wang, J.; Xiong, H.; Ji, L.; et al. Multi-Kingdom Gut Microbiota Analyses Define Bacterial-Fungal Interplay and Microbial Markers of Pan-Cancer Immunotherapy across Cohorts. Cell Host Microbe 2023, 31, 1930–1943.e4. [Google Scholar] [CrossRef]
- Martini, G.; Ciardiello, D.; Dallio, M.; Famiglietti, V.; Esposito, L.; Corte, C.M.D.; Napolitano, S.; Fasano, M.; Gravina, A.G.; Romano, M.; et al. Gut Microbiota Correlates with Antitumor Activity in Patients with mCRC and NSCLC Treated with Cetuximab plus Avelumab. Int. J. Cancer 2022, 151, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, J.; Gong, L.; Dong, Y.; Shou, J.; Pan, H.; Yu, Z.; Fang, Y. Composition of the Gut Microbiota Associated with the Response to Immunotherapy in Advanced Cancer Patients: A Chinese Real-World Pilot Study. J. Clin. Med. 2022, 11, 5479. [Google Scholar] [CrossRef] [PubMed]
- Goc, J.; Lv, M.; Bessman, N.J.; Flamar, A.-L.; Sahota, S.; Suzuki, H.; Teng, F.; Putzel, G.G.; JRI Live Cell Bank; Eberl, G.; et al. Dysregulation of ILC3s Unleashes Progression and Immunotherapy Resistance in Colon Cancer. Cell 2021, 184, 5015–5030.e16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Fidelle, M.; Routy, B.; Kroemer, G.; Wargo, J.A.; Segata, N.; Zitvogel, L. Gut OncoMicrobiome Signatures (GOMS) as next-Generation Biomarkers for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 583–603. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Saini, N.Y.; Zamir, E.; Blumenberg, V.; Schubert, M.-L.; Mor, U.; Fante, M.A.; Schmidt, S.; Hayase, E.; Hayase, T.; et al. A Non-Antibiotic-Disrupted Gut Microbiome Is Associated with Clinical Responses to CD19-CAR-T Cell Cancer Immunotherapy. Nat. Med. 2023, 29, 906–916. [Google Scholar] [CrossRef]
- Fidelle, M.; Rauber, C.; Alves Costa Silva, C.; Tian, A.-L.; Lahmar, I.; de La Varende, A.-L.M.; Zhao, L.; Thelemaque, C.; Lebhar, I.; Messaoudene, M.; et al. A Microbiota-Modulated Checkpoint Directs Immunosuppressive Intestinal T Cells into Cancers. Science 2023, 380, eabo2296. [Google Scholar] [CrossRef]
- Holder, A.M.; Dedeilia, A.; Sierra-Davidson, K.; Cohen, S.; Liu, D.; Parikh, A.; Boland, G.M. Defining Clinically Useful Biomarkers of Immune Checkpoint Inhibitors in Solid Tumours. Nat. Rev. Cancer 2024, 24, 498–512. [Google Scholar] [CrossRef]
- Tito, R.Y.; Verbandt, S.; Aguirre Vazquez, M.; Lahti, L.; Verspecht, C.; Lloréns-Rico, V.; Vieira-Silva, S.; Arts, J.; Falony, G.; Dekker, E.; et al. Microbiome Confounders and Quantitative Profiling Challenge Predicted Microbial Targets in Colorectal Cancer Development. Nat. Med. 2024, 30, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Rynazal, R.; Fujisawa, K.; Shiroma, H.; Salim, F.; Mizutani, S.; Shiba, S.; Yachida, S.; Yamada, T. Leveraging Explainable AI for Gut Microbiome-Based Colorectal Cancer Classification. Genome Biol. 2023, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front. Immunol. 2022, 12, 807648. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-Y.; Cui, Z.; Yan, Y.-Q.; Ning, L.-J.; Wang, Z.-H.; Hong, J. Microbe-Based Management for Colorectal Cancer. Chin. Med. J. 2021, 134, 2922. [Google Scholar] [CrossRef] [PubMed]
- Kalasabail, S.; Engelman, J.; Zhang, L.Y.; El-Omar, E.; Yim, H.C.H. A Perspective on the Role of Microbiome for Colorectal Cancer Treatment. Cancers 2021, 13, 4623. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, D.-X.; Zhang, Y.-N. Does the Microbiota Composition Influence the Efficacy of Colorectal Cancer Immunotherapy? Front. Oncol. 2022, 12, 852194. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Ge, W.; Shi, C.; Xiao, G.; Wang, X.; Lü, X. The Probiotic Companilactobacillus crustorum MN047 Alleviates Colitis-Associated Tumorigenesis via Modulating the Intestinal Microenvironment. Food Funct. 2021, 12, 11331–11342. [Google Scholar] [CrossRef]
- Hey, D.V.D.; Payne, S.; Pasch, C.; Clipson, L.; Matkowskyj, K.; Deming, D. Abstract 4929: Cooperation between the Microbiota and Oncogenic Mutations Leading to the Development of Colon Cancer. Cancer Res. 2017, 77, 4929. [Google Scholar] [CrossRef]

| Factor | Influence on Gut Microbiome | Impact on Immunity | Key Points | References |
|---|---|---|---|---|
| Microbial Diversity | High diversity linked with robust immune health | Enhances immune responses, modulates immune cells | Promote SCFA production, crucial for immune regulation and barrier integrity | [17,18,19,20,21,22] |
| Dietary Influences | Fiber-rich, polyphenol-rich diets support a healthy microbiome; high-fat, high-sugar diets disrupt balance | Fiber and polyphenols enhance anti-inflammatory responses; fats and sugars may increase inflammation | Diet affects microbiome composition, influencing systemic inflammation and immune response | [32,33] |
| Antibiotics and Medications | Reduce microbial diversity, impact on metabolic functions | Can trigger inflammatory responses or disrupt immune homeostasis | Long-term use of antibiotics linked to negative impacts on microbiome diversity and immune function | [34,35] |
| Host Genetics | Host genetic factors can determine microbiome composition and diversity | Host genetic factors can affect immune responses mediated by the microbiome | Some genetic markers, such as those for lactase production, correlate with microbiome composition | [36,37,38,39] |
| Pro-Immunotherapy | Anti-Immunotherapy |
|---|---|
| Microbes | |
| -Faecalibacterium prausnitzii [104] | -Fusobacterium nucleatum [103,113] |
| -Lachnospiraceae family [108] | -Escherichia coli [103] |
| -Lactobacillus gallinarum [84] | -Prevotella [103] |
| -Coprobacillus cateniformis [84] | -Bacteroides spp. [104,117] |
| -Erysipelatoclostridium ramosum [84] | -Bacillus class [107] |
| -Bacteroides thetaiotaomicron [111] | -Clostridiaceae [116] |
| -Bacteroides fragilis [111] | -Proteobacteria [110] |
| -Bacteroides cellulosilyticus [111] | -Lactobacillus murinus [111] |
| -Bifidobacterium pseudolongum [112] | -Lactobacillales order [107] |
| -Lactobacillus johnsonii [112] | |
| -Olsenella species [112] | |
| -Blautia genus [112] | |
| -Parabacteroides distasonis [106] | |
| -Alistipes genus [107] | |
| -Eubacterium rectale [118] | |
| -Roseburia spp. [118] | |
| -Methanobrevibacter [118] | |
| -Akkermansia muciniphila [119] | |
| -Ruminococcus lactaris [119] | |
| -Schizosaccharomyces octosporus [114] | |
| -Agathobacter [115] | |
| Metabolites | |
| -Indole-3-carboxylic acid (ICA) [84] | -Pyrrolidine [107] |
| -Inosine [112] | |
| -Pentanoate [97] | |
| -Short-chain fatty acids (SCFAs) [98,99,108,118] | |
| -Punicic acid [111] | |
| -Trimethylamine N-oxide (TMAO) [100] | |
| Molecular and Cell Mechanisms | |
| -Upregulation of CD80/CD86 on dendritic cells [104] | -Activation of nuclear factor-κB (NF-κB) pathway [103] |
| -Increased ICOS gene expression in T cells [104] | -Upregulation of cytokines IL-6 and TNF-α [103] |
| -Accumulation of tumor-specific T cells and memory T cells [105] | -Downregulation of miRNA18a via TLR-4/MYD88 pathway [103] |
| -High clonality and low diversity in TCR repertoires [103] | -Decreased Th1 cells and T-bet+ CD8 T cells [117] |
| -Downregulation of PD-L2 expression on dendritic cells [84] | |
| -Increased systemic CD8α+ dendritic cells and IL-12 levels [101] | |
| -Enhanced Th1 differentiation and anti-tumor immunity via A2A receptor signaling pathway on T cells [117] | |
| -Enhanced metabolism and memory potential of CD8+ T cells [98] | |
| -Increased activity of mTOR pathway [97] | |
| -Enhanced systemic CD8α+ dendritic cells and IL-12 levels through vancomycin-induced gut microbiome alterations [101] | |
| -Increased CD3+ and CD8+ tumor-infiltrating lymphocytes (TILs) density [108] | |
| -Positive modulation by Schizosaccharomyces octosporus of inhibitory receptors PD-1 and CTLA-4 [114] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhpazyan, N.K.; Mikhaleva, L.M.; Bedzhanyan, A.L.; Gioeva, Z.V.; Mikhalev, A.I.; Midiber, K.Y.; Pechnikova, V.V.; Biryukov, A.E. Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity. Cells 2024, 13, 1437. https://doi.org/10.3390/cells13171437
Shakhpazyan NK, Mikhaleva LM, Bedzhanyan AL, Gioeva ZV, Mikhalev AI, Midiber KY, Pechnikova VV, Biryukov AE. Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity. Cells. 2024; 13(17):1437. https://doi.org/10.3390/cells13171437
Chicago/Turabian StyleShakhpazyan, Nikolay K., Liudmila M. Mikhaleva, Arkady L. Bedzhanyan, Zarina V. Gioeva, Alexander I. Mikhalev, Konstantin Y. Midiber, Valentina V. Pechnikova, and Andrey E. Biryukov. 2024. "Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity" Cells 13, no. 17: 1437. https://doi.org/10.3390/cells13171437






