Bone Marrow Aspirate Concentrate Combined with Ultra-Purified Alginate Bioresorbable Gel Enhances Intervertebral Disc Repair in a Canine Model: A Preclinical Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Animal Experimentation for In Vitro Study
2.1.2. Preparation of BMAC
2.1.3. Measuring Cell Counts and Calculation of Concentration Ratio
2.2. In Vivo Study
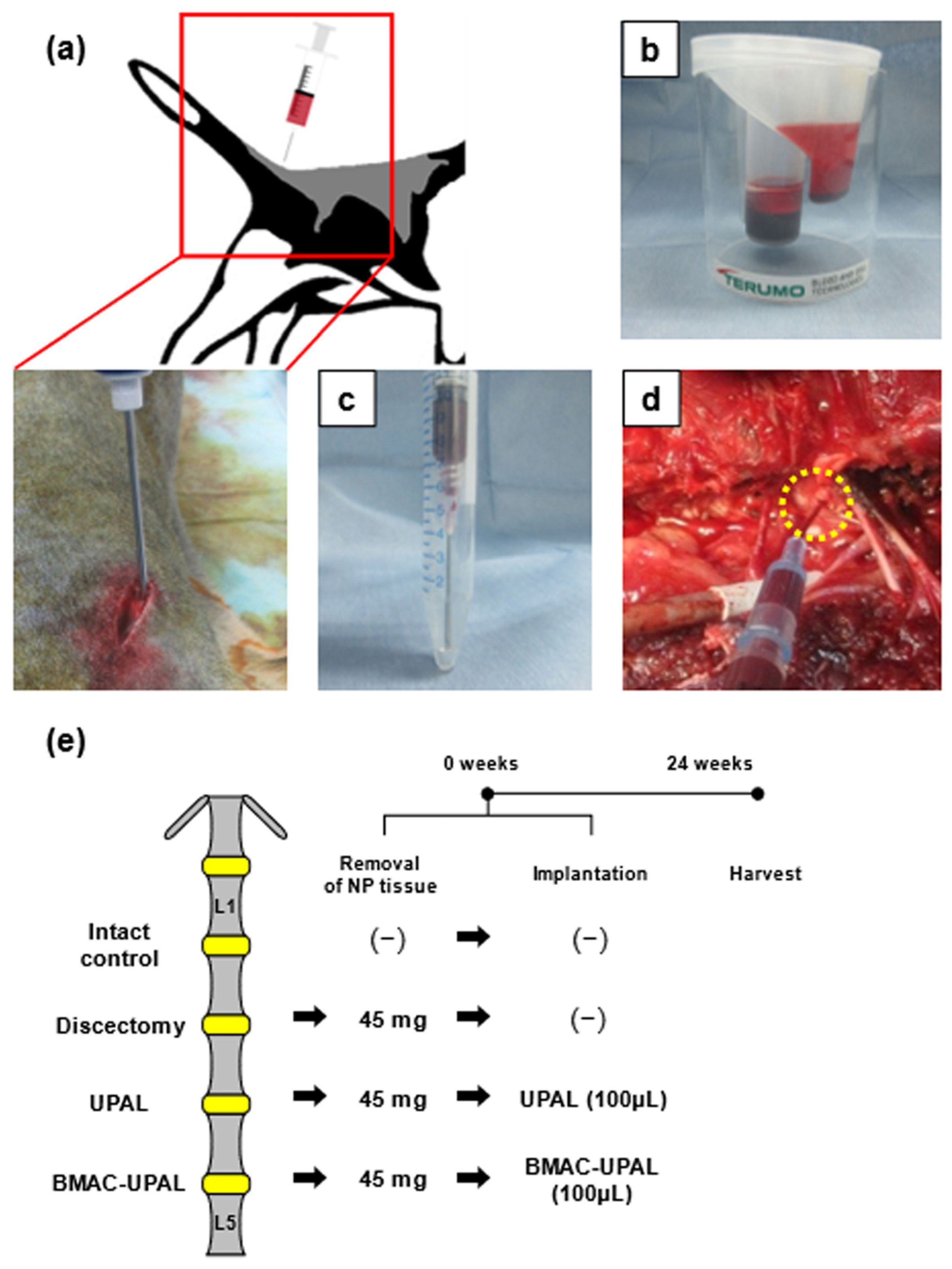
2.2.1. Animal Experimentation for In Vivo Study
2.2.2. Collecting BM and Preparation of BMAC
2.2.3. Discectomy and BMAC-UPAL Implantation
2.2.4. Magnetic Resonance Imaging (MRI) Analysis
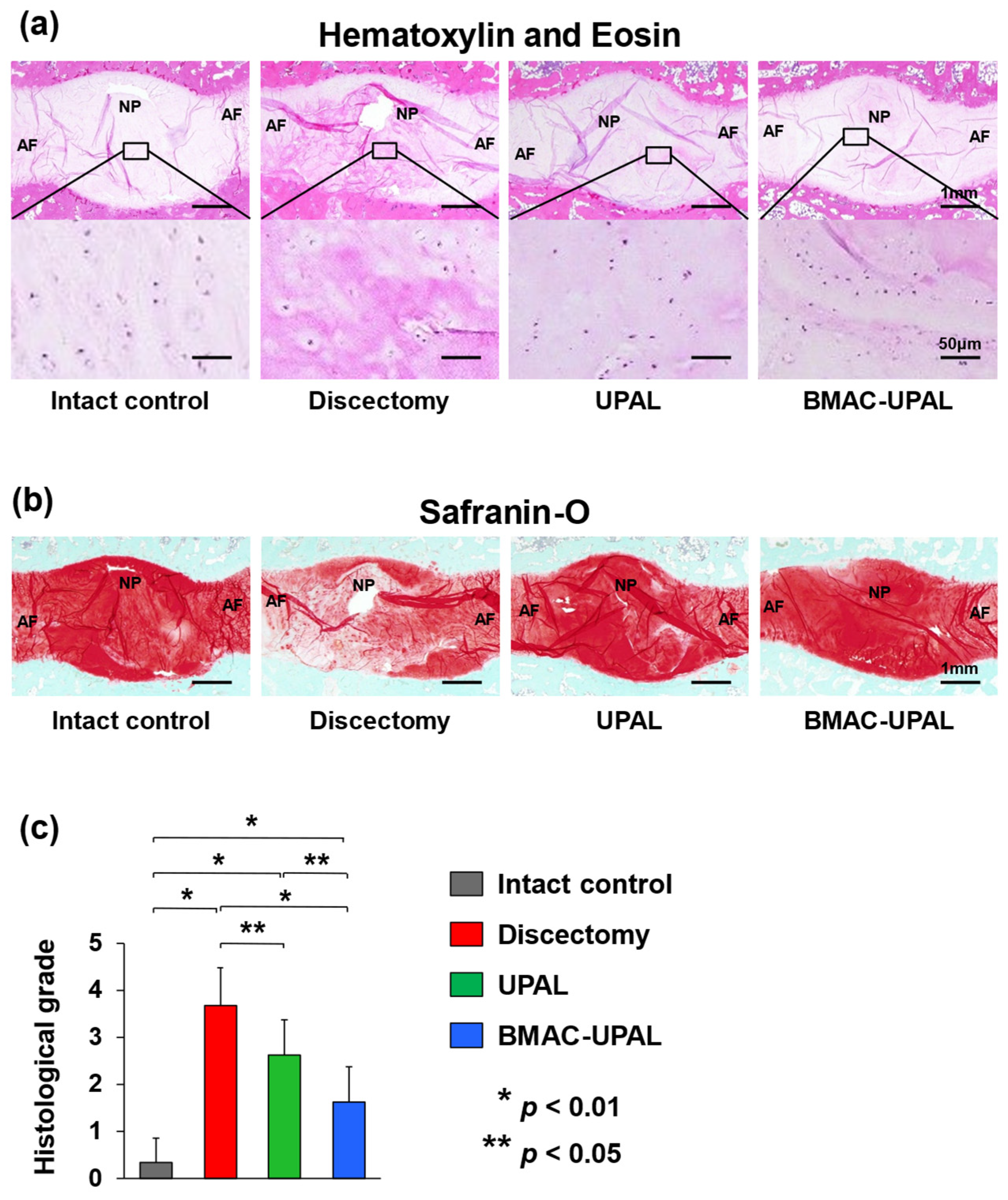
2.2.5. Histological Analysis
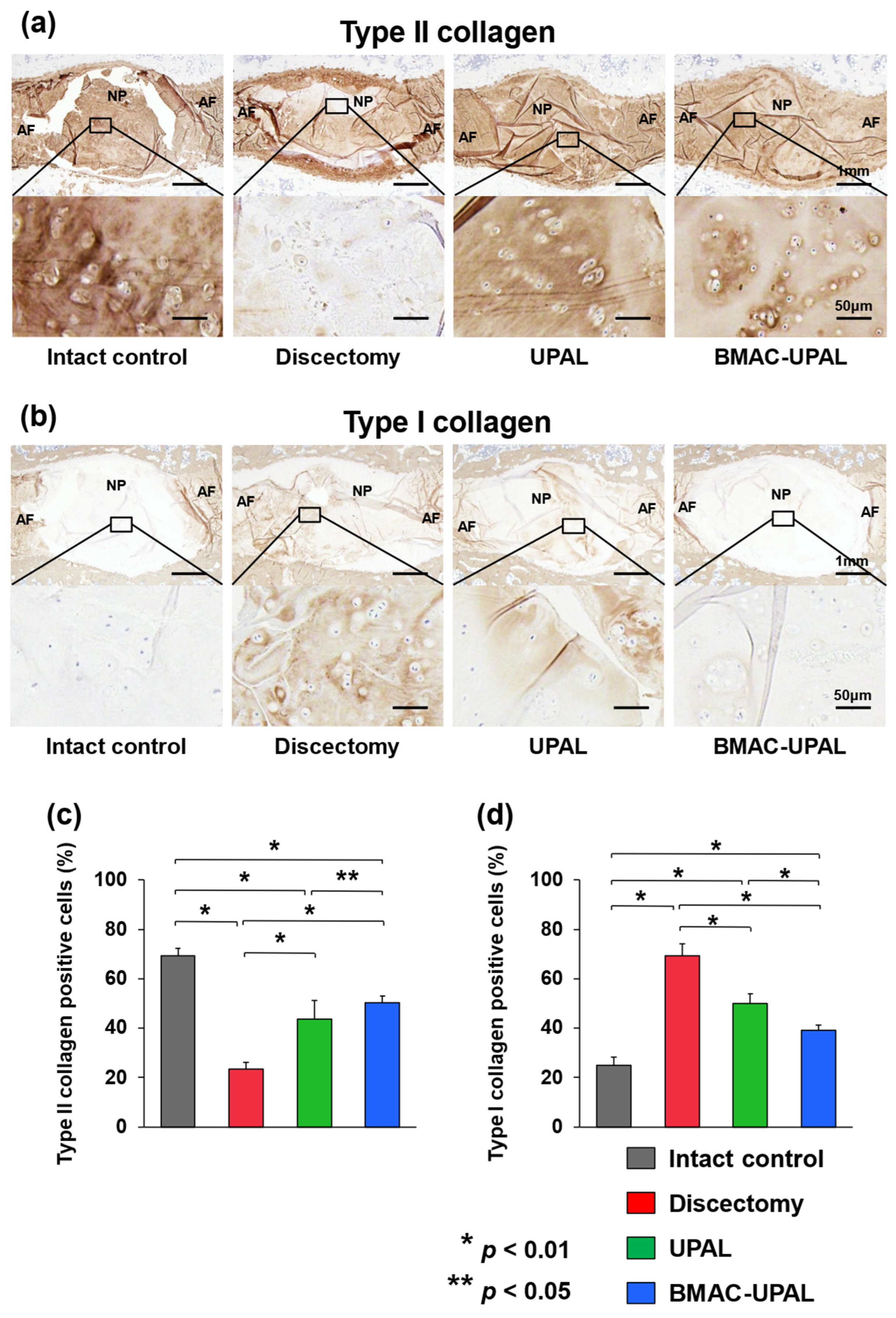
2.2.6. Immunohistochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Comparison of Concentration Ratios among Three Commercially Available BMAC Preparation Kits
3.2. BMAC-UPAL Implantation Suppressed IVD Degeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oehme, D.; Ghosh, P.; Shimmon, S.; Wu, J.; McDonald, C.; Troupis, J.M.; Goldschlager, T.; Rosenfeld, J.V.; Jenkin, G. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: A preliminary study in an ovine model. J. Neurosurg. Spine 2014, 20, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Sudo, H.; Todoh, M.; Yamada, K.; Iwasaki, K.; Ohnishi, T.; Hirohama, N.; Nonoyama, T.; Ukeba, D.; Ura, K.; et al. An acellular bioresorbable ultra-purified alginate gel promotes intervertebral disc repair: A preclinical proof-of-concept study. EBioMedicine 2018, 37, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Buckley, C.T. Differential response of encapsulated nucleus pulposus and bone marrow stem cells in isolation and coculture in alginate and chitosan hydrogels. Tissue Eng. Part A 2015, 21, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Lim, J.J.; Keeter, C.; McCulloch, P.C.; Houck, D.A.; McCarty, E.C.; Frank, R.M.; Kraeutler, M.J. Patients with Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy 2023, 39, 1714–1734. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, B.; Chahla, J.; Korrapati, A.; Lavoie-Gagne, O.; Forlenza, E.; Diaz, C.C.; Chung, C.B.; Bae, W.C.; Bach, B.R.; Cole, B.; et al. Bone Marrow Aspirate Concentrate Augmentation May Accelerate Allograft Ligamentization in Anterior Cruciate Ligament Reconstruction: A Double-Blinded Randomized Controlled Trial. Arthroscopy 2022, 38, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.A.; Suzuki, R.K.; Sand, T.T.; Murphy, M.B. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int. Orthop. 2017, 41, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.A.; Murphy, M.B.; Suzuki, R.K.; Sand, T.T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 2015, 33, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ukeba, D.; Yamada, K.; Tsujimoto, T.; Ura, K.; Nonoyama, T.; Iwasaki, N.; Sudo, H. Bone Marrow Aspirate Concentrate Combined with in Situ Forming Bioresorbable Gel Enhances Intervertebral Disc Regeneration in Rabbits. J. Bone Joint Surg. Am. 2021, 103, e31. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kenichiro, M.; Ito, Y.M.; Inage, F.; Isoe, T.; Yokota, N.; Sugita, O.; Sato, N.; Tha, K.K.; Iwasaki, N.; et al. Exploratory clinical trial on the safety and capability of dMD-001 in lumbar disc herniation: Study protocol for a first-in-human pilot study. Contemp. Clin. Trials Commun. 2021, 23, 100805. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.L.; Ahmad, T.S.; Selvaratnam, L.; Kamarul, T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 2013, 222, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Huang, Y.H.; Chang, N.K.; Lin, W.C.; Chien, P.W.; Su, T.M.; Hsieh, D.J.; Lee, T.C. Characterization and spinal fusion effect of rabbit mesenchymal stem cells. BMC Res. Notes 2013, 6, 528. [Google Scholar] [CrossRef]
- Ukeba, D.; Yamada, K.; Suyama, T.; Lebl, D.R.; Tsujimoto, T.; Nonoyama, T.; Sugino, H.; Iwasaki, N.; Watanabe, M.; Matsuzaki, Y.; et al. Combination of ultra-purified stem cells with an in situ-forming bioresorbable gel enhances intervertebral disc regeneration. EBioMedicine 2022, 76, 103845. [Google Scholar] [CrossRef]
- Ganey, T.; Libera, J.; Moos, V.; Alasevic, O.; Fritsch, K.G.; Meisel, H.J.; Hutton, W.C. Disc chondrocyte transplantation in a canine model: A treatment for degenerated or damaged intervertebral disc. Spine 2003, 28, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Masuda, K. Relevance of in vitro and in vivo models for intervertebral disc degeneration. J. Bone Joint Surg. Am. 2006, 88 (Suppl. S2), 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ganey, T.; Hutton, W.C.; Moseley, T.; Hedrick, M.; Meisel, H.J. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine 2009, 34, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.K.; Xin, H.; Zhang, C.; Wang, C.; Xu, C.; Li, C.; He, Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng. Part A 2010, 16, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Gu, T.; Xin, H.; Wu, J.; Xu, C.; Zhang, C.; He, Q.; Ruan, D. Intervention of rAAV-hTERT-Transducted Nucleus Pulposus Cells in Early Stage of Intervertebral Disc Degeneration: A Study in Canine Model. Tissue Eng. Part A 2015, 21, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.N.; Kramer, J.S.; Stoker, A.M.; Bozynski, C.C.; Cook, C.R.; Stannard, J.T.; Choma, T.J.; Cook, J.L. Canine models of spine disorders. JOR Spine 2020, 3, e1109. [Google Scholar] [CrossRef] [PubMed]
- Ukeba, D.; Sudo, H.; Tsujimoto, T.; Ura, K.; Yamada, K.; Iwasaki, N. Bone marrow mesenchymal stem cells combined with ultra-purified alginate gel as a regenerative therapeutic strategy after discectomy for degenerated intervertebral discs. EBioMedicine 2020, 53, 102698. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Minami, A. Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheum. 2011, 63, 1648–1657. [Google Scholar] [CrossRef]
- Ura, K.; Sudo, H.; Iwasaki, K.; Tsujimoto, T.; Ukeba, D.; Iwasaki, N. Effects of Intradiscal Injection of Local Anesthetics on Intervertebral Disc Degeneration in Rabbit Degenerated Intervertebral Disc. J. Orthop. Res. 2019, 37, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Mochida, J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine 1998, 23, 1531–1538; discussion 1539. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Mochida, J.; Iwashina, T.; Hiyama, A.; Omi, H.; Imai, M.; Nakai, T.; Ando, K.; Hotta, T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 2006, 27, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Pockert, A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35 Pt 4, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.W.; Park, Y.B. Editorial Commentary: Considering Clinical Application of Bone Marrow Aspirate Concentrate for Restoration of Cartilage Defects in the Knee? Is It a Kind of Stem Cell Therapy? Arthroscopy 2019, 35, 1878–1879. [Google Scholar] [CrossRef] [PubMed]
- Oetgen, M.E.; Litrenta, J. Perioperative Blood Management in Pediatric Spine Surgery. J. Am. Acad. Orthop. Surg. 2017, 25, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Idio, F.G., Jr.; Remer, S.; Hoppenfeld, S. The effects of perioperative blood salvage and autologous blood donation on transfusion requirements in scoliosis surgery. J. Spinal Disord. 1998, 11, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Lagerstrand, K.; Baranto, A.; Hebelka, H. Different disc characteristics between young elite skiers with diverse training histories revealed with a novel quantitative magnetic resonance imaging method. Eur. Spine J. 2021, 30, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Sowa, G.; Hubert, M.; Gilbertson, L.G.; Denaro, V.; Kang, J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 2012, 6, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: A report of two case studies. Spine 2010, 35, E475–E480. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sánchez, A.; García-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Centeno, C.J.; Schultz, J.R.; Lutz, G.; Ichim, T.; Silva, F.J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: A long-term safety and feasibility study. J. Transl. Med. 2016, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Hammer, S.; Fuellerer, J.; Lang, S.; Pfeifer, C.G.; Pattappa, G.; Weber, J.; Loibl, M.; Nerlich, M.; Angele, P.; et al. Bone Marrow Aspirate Concentrate for the Treatment of Avascular Meniscus Tears in a One-Step Procedure-Evaluation of an In Vivo Model. Int. J. Mol. Sci. 2019, 20, 1120. [Google Scholar] [CrossRef]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Xu, L.; Urita, A.; Onodera, T.; Hishimura, R.; Nonoyama, T.; Hamasaki, M.; Liang, D.; Homan, K.; Gong, J.P.; Iwasaki, N. Ultrapurified Alginate Gel Containing Bone Marrow Aspirate Concentrate Enhances Cartilage and Bone Regeneration on Osteochondral Defects in a Rabbit Model. Am. J. Sports Med. 2021, 49, 2199–2210. [Google Scholar] [CrossRef]
- Cavinatto, L.; Hinckel, B.B.; Tomlinson, R.E.; Gupta, S.; Farr, J.; Bartolozzi, A.R. The Role of Bone Marrow Aspirate Concentrate for the Treatment of Focal Chondral Lesions of the Knee: A Systematic Review and Critical Analysis of Animal and Clinical Studies. Arthroscopy 2019, 35, 1860–1877. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Scotti, C.; Mahajan, V.; Mazzucco, L.; Grigolo, B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-Up. Cartilage 2011, 2, 286–299. [Google Scholar] [CrossRef]
- Gobbi, A.; Whyte, G.P. Long-Term Clinical Outcomes of One-Stage Cartilage Repair in the Knee with Hyaluronic Acid-Based Scaffold Embedded with Mesenchymal Stem Cells Sourced from Bone Marrow Aspirate Concentrate. Am. J. Sports Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef]
- Grassmann, J.P.; Schneppendahl, J.; Sager, M.; Hakimi, A.R.; Herten, M.; Loegters, T.T.; Wild, M.; Hakimi, M.; Windolf, J.; Jungbluth, P. The effect of bone marrow concentrate and hyperbaric oxygen therapy on bone repair. J. Mater. Sci. Mater. Med. 2015, 26, 5331. [Google Scholar] [CrossRef]
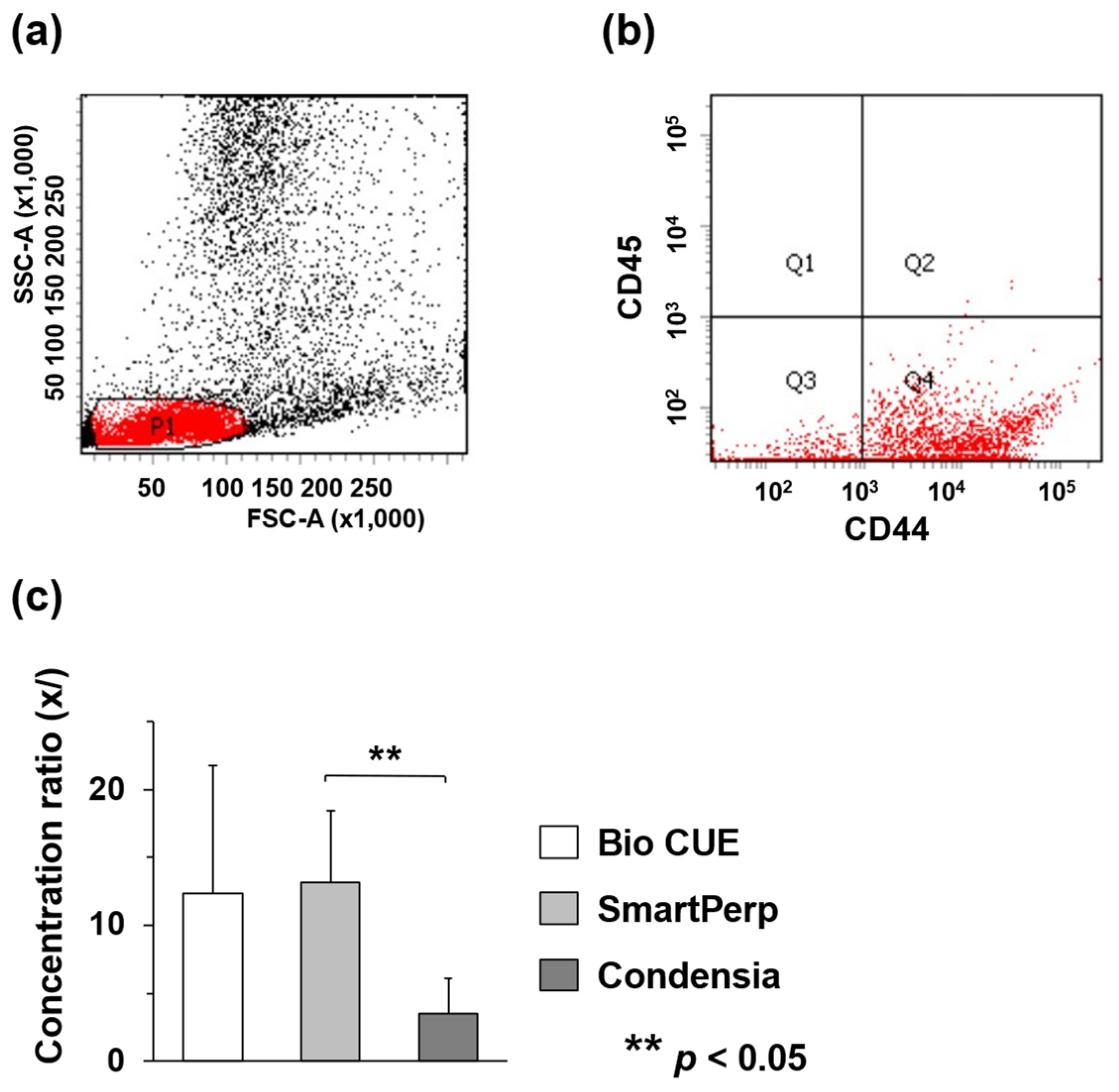

| Number of CD44-Positive and CD45-Negative Cells Pre-Concentration (×105/mL) | Number of CD44-Positive and CD45-Negative Cells Post-Concentration (×106/mL) | Concentration Ratio | |
|---|---|---|---|
| Bio CUE | 4.5 ± 1.1 | 5.1 ± 3.4 | 12.4 |
| SmartPrep | 2.1 ± 1.5 | 2.2 ± 0.9 | 13.2 |
| Condensia | 7.0 ± 2.7 | 2.0 ± 0.6 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukeba, D.; Ishikawa, Y.; Yamada, K.; Ohnishi, T.; Tachi, H.; Tha, K.K.; Iwasaki, N.; Sudo, H. Bone Marrow Aspirate Concentrate Combined with Ultra-Purified Alginate Bioresorbable Gel Enhances Intervertebral Disc Repair in a Canine Model: A Preclinical Proof-of-Concept Study. Cells 2024, 13, 987. https://doi.org/10.3390/cells13110987
Ukeba D, Ishikawa Y, Yamada K, Ohnishi T, Tachi H, Tha KK, Iwasaki N, Sudo H. Bone Marrow Aspirate Concentrate Combined with Ultra-Purified Alginate Bioresorbable Gel Enhances Intervertebral Disc Repair in a Canine Model: A Preclinical Proof-of-Concept Study. Cells. 2024; 13(11):987. https://doi.org/10.3390/cells13110987
Chicago/Turabian StyleUkeba, Daisuke, Yoko Ishikawa, Katsuhisa Yamada, Takashi Ohnishi, Hiroyuki Tachi, Khin Khin Tha, Norimasa Iwasaki, and Hideki Sudo. 2024. "Bone Marrow Aspirate Concentrate Combined with Ultra-Purified Alginate Bioresorbable Gel Enhances Intervertebral Disc Repair in a Canine Model: A Preclinical Proof-of-Concept Study" Cells 13, no. 11: 987. https://doi.org/10.3390/cells13110987
APA StyleUkeba, D., Ishikawa, Y., Yamada, K., Ohnishi, T., Tachi, H., Tha, K. K., Iwasaki, N., & Sudo, H. (2024). Bone Marrow Aspirate Concentrate Combined with Ultra-Purified Alginate Bioresorbable Gel Enhances Intervertebral Disc Repair in a Canine Model: A Preclinical Proof-of-Concept Study. Cells, 13(11), 987. https://doi.org/10.3390/cells13110987






