Mecp2 Deficiency in Peripheral Sensory Neuron Improves Cognitive Function by Enhancing Hippocampal Dendritic Spine Densities in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Mecp2fl/flAdvillincre Mice Breeding
2.3. Sensory Analysis
2.3.1. Cold-Water Tail-Flick Latency Reflex Test
2.3.2. Hot-Plate Test
2.4. Behavioral Analysis
2.4.1. Open Field Test
2.4.2. Marble-Burying and Nestlet-Shredding Test
2.4.3. Three-Chamber Social Approach and Social Novelty Test
2.4.4. Object Recognition Test
2.4.5. Elevated Plus-Maze
2.4.6. Morris Water Maze (MWM) Task
2.4.7. Pole-Climbing Test
2.4.8. Grip Strength Test
2.5. Western Blot Analysis
2.6. Hematoxylin-Eosin (HE), Immunohistochemistry (IHC), and Nissl Staining
2.7. Golgi Staining
2.8. Statistical Analysis
3. Results
3.1. Mecp2 Deficiency in Peripheral Sensory Neuron Modifies Superficial Sensation in Mice
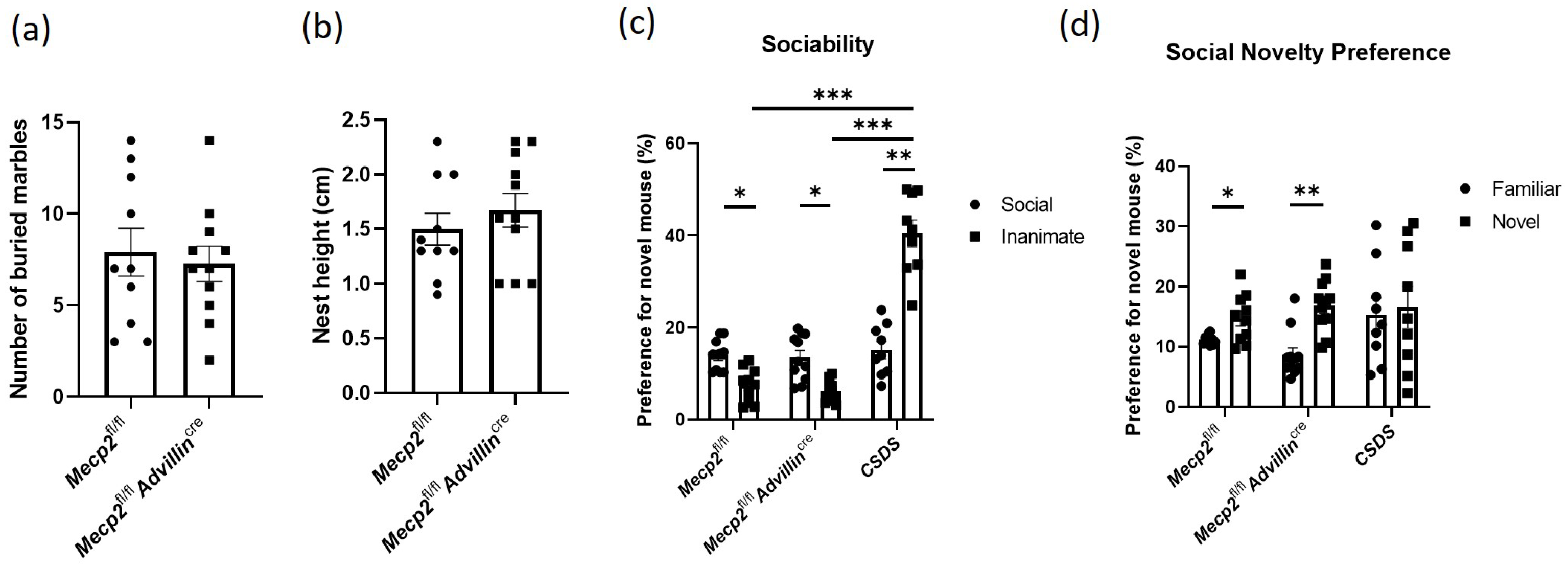
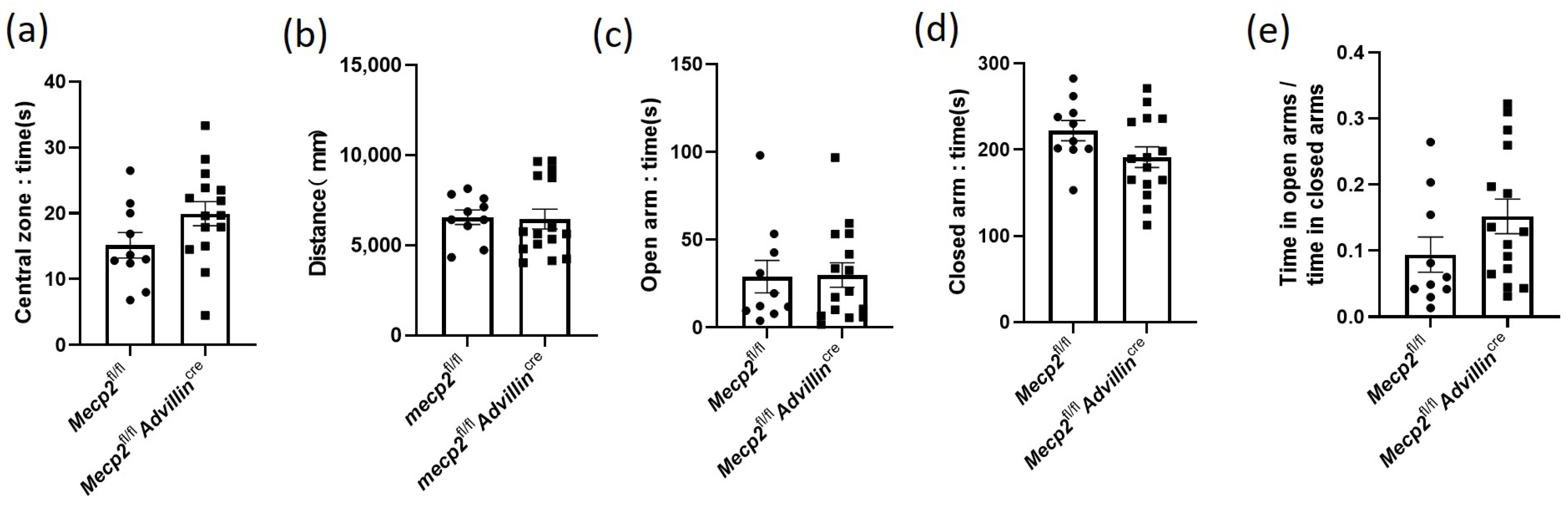
3.2. Lack of Mecp2 in Peripheral Sensory Neuron Does Not Affect Social Behaviors, Exploratory Activities, and Anxiety-like Behavior in Mice
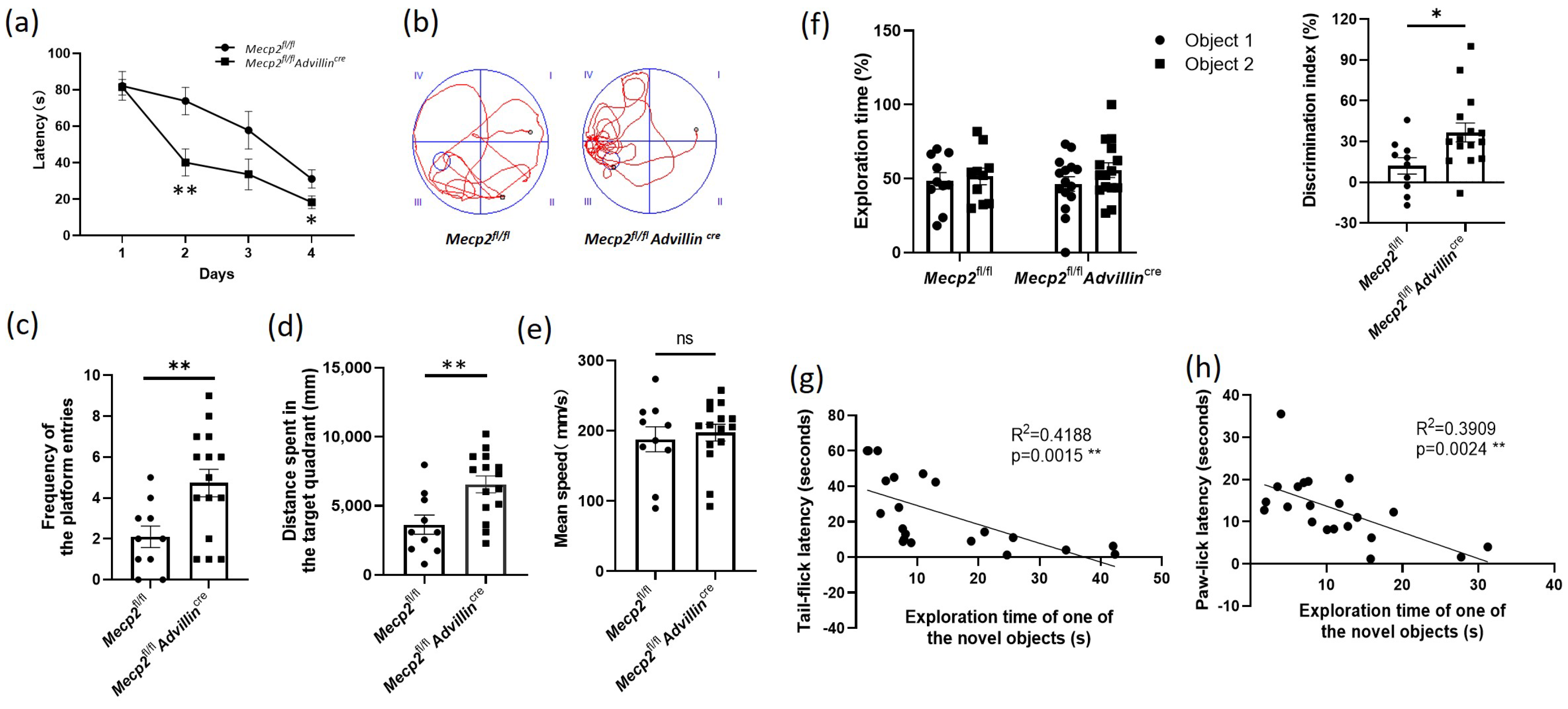
3.3. Peripheral Sensory Neuron Deletion of Mecp2 Leads to Improve Cognitive Function in Mice
3.4. Peripheral Sensory Neuron Deletion of Mecp2 Induces Modification Synaptic Phenotypes in Hippocampus
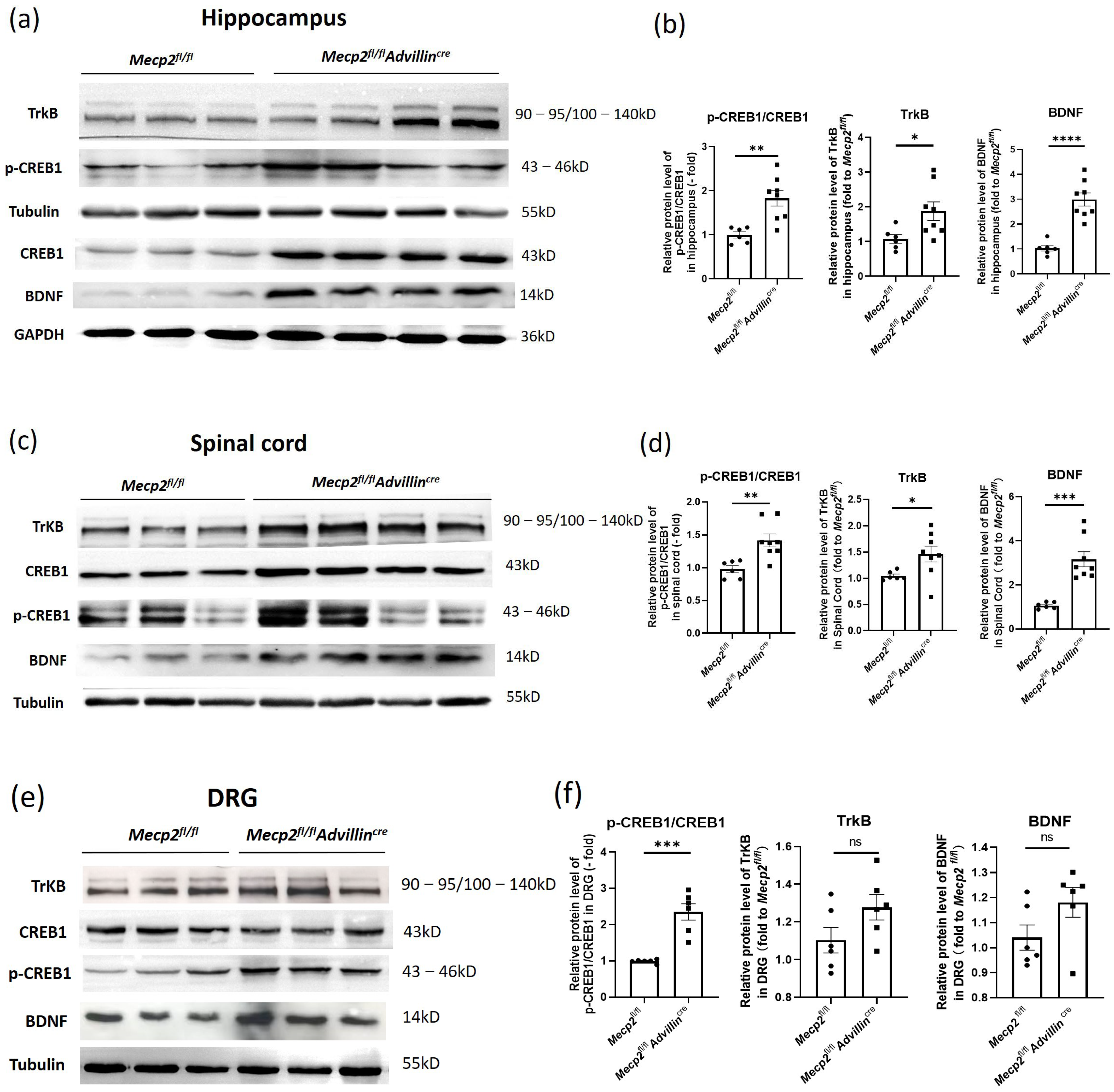
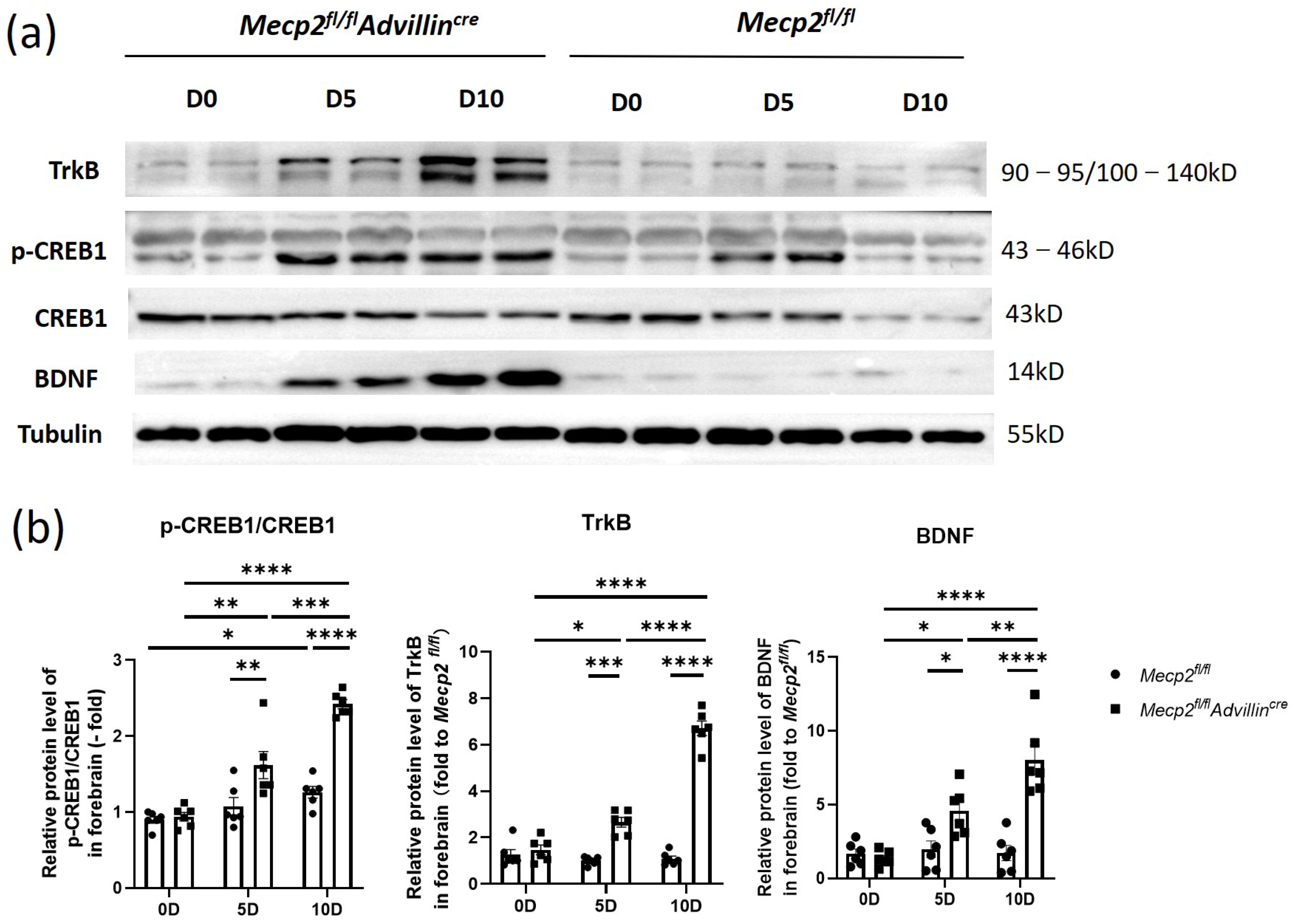
3.5. Peripheral Sensory Neuron Deletion of Mecp2 Activates BDNF-TrkB-CREB1 Signaling Pathway in Hippocampus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Papin, C.; Mohideen-Abdul, K.; Le Gras, S.; Stoll, I.; Bronner, C.; Dimitrov, S.; Klaholz, B.P.; Hamiche, A. MeCP2 is a microsatellite binding protein that protects CA repeats from nucleosome invasion. Science 2021, 372, eabd5581. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, Y.; Liu, C.; Huang, Y.; Petersen, R.B.; Zheng, L.; Huang, K. Emerging physiological and pathological roles of MeCP2 in non-neurological systems. Arch. Biochem. Biophys. 2021, 700, 108768. [Google Scholar] [CrossRef] [PubMed]
- Ramocki, M.B.; Tavyev, Y.J.; Peters, S.U. The MECP2 duplication syndrome. Am. J. Med. Genet. A 2010, 152A, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.P.K.; Mellios, N.; Sur, M. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci. 2018, 19, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.T.; Zoghbi, H.Y. MeCP2: Only 100% will do. Nat. Neurosci. 2012, 15, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Sandweiss, A.J.; Brandt, V.L.; Zoghbi, H.Y. Advances in understanding of Rett syndrome and MECP2 duplication syndrome: Prospects for future therapies. Lancet Neurol. 2020, 19, 689–698. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R., 3rd. MECP2 and the biology of MECP2 duplication syndrome. J. Neurochem. 2021, 159, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Laurvick, C.L.; de Klerk, N.; Bower, C.; Christodoulou, J.; Ravine, D.; Ellaway, C.; Williamson, S.; Leonard, H. Rett syndrome in Australia: A review of the epidemiology. J. Pediatr. 2006, 148, 347–352. [Google Scholar] [CrossRef]
- Louise, S.; Fyfe, S.; Bebbington, A.; Bahi-Buisson, N.; Anderson, A.; Pineda, M.; Percy, A.; Ben Zeev, B.; Wu, X.R.; Bao, X.; et al. InterRett, a model for international data collection in a rare genetic disorder. Res. Autism Spectr. Disord. 2009, 3, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Geranton, S.M.; Bebbington, A.; Jacoby, P.; Bahi-Buisson, N.; Ravine, D.; Leonard, H. Linking MECP2 and pain sensitivity: The example of Rett syndrome. Am. J. Med. Genet. A 2010, 152A, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Chen, Y.; Liu, J.; Zhang, D.; Yang, Y.; Shen, J.; Miao, C.; Tang, S.J.; Liu, X.; Mulkey, D.K.; et al. Spinal astrocytic MeCP2 regulates Kir4.1 for the maintenance of chronic hyperalgesia in neuropathic pain. Prog. Neurobiol. 2023, 224, 102436. [Google Scholar] [CrossRef] [PubMed]
- Orefice, L.L.; Mosko, J.R.; Morency, D.T.; Wells, M.F.; Tasnim, A.; Mozeika, S.M.; Ye, M.; Chirila, A.M.; Emanuel, A.J.; Rankin, G.; et al. Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell 2019, 178, 867–886.e24. [Google Scholar] [CrossRef] [PubMed]
- Orefice, L.L.; Zimmerman, A.L.; Chirila, A.M.; Sleboda, S.J.; Head, J.P.; Ginty, D.D. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 2016, 166, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lafuente, C.L.; Kalynchuk, L.E.; Caruncho, H.J.; Ausio, J. The Role of MeCP2 in Regulating Synaptic Plasticity in the Context of Stress and Depression. Cells 2022, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Zhang, F.; Su, M.; Li, J.; Yi, W.; Hou, L.X.; Yang, S.M.; Liu, J.Y.; Zhang, H.A.; Ma, T.; et al. MeCP2 prevents age-associated cognitive decline via restoring synaptic plasticity in a senescence-accelerated mouse model. Aging Cell 2021, 20, e13451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hong, E.J.; Cohen, S.; Zhao, W.N.; Ho, H.Y.; Schmidt, L.; Chen, W.G.; Lin, Y.; Savner, E.; Griffith, E.C.; et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 2006, 52, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.Y.; Shen, S.; Liu, M.; Xia, C.; Kay, J.C.; Zhang, Q.L. Inflammation and activity augment brain-derived neurotrophic factor peripheral release. Neuroscience 2016, 318, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Wang, Z.; Liao, Q.; Zhu, Y.; Zhou, W.H.; Xu, W.; Qiu, Z. MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev. Cell 2014, 28, 547–560. [Google Scholar] [CrossRef]
- Chen, H.H.; Mohsin, M.; Ge, J.Y.; Feng, Y.T.; Wang, J.G.; Ou, Y.S.; Jiang, Z.J.; Hu, B.Y.; Liu, X.J. Optogenetic Activation of Peripheral Somatosensory Neurons in Transgenic Mice as a Neuropathic Pain Model for Assessing the Therapeutic Efficacy of Analgesics. ACS Pharmacol. Transl. Sci. 2024, 7, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.; Baker, A.L.; Moron, J.A.; Carlton, S.M. Systemic morphine treatment induces changes in firing patterns and responses of nociceptive afferent fibers in mouse glabrous skin. Pain 2013, 154, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Miyakawa, T. Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacol. Rep. 2019, 39, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miao, J.; Jiang, Y.; Dai, C.L.; Iqbal, K.; Liu, F.; Chu, D. Passive immunization inhibits tau phosphorylation and improves recognition learning and memory in 3xTg-AD mice. Exp. Neurol. 2023, 362, 114337. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Perez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Xu, D.W.; Ji, C.H.; Wang, C.N.; Liu, Y.; Tang, W.Q.; Gu, J.H.; Chen, Y.M.; Huang, J.; Liu, J.F.; et al. Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF. Pharmacol. Res. 2021, 174, 105932. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess Folic Acid Supplementation Before and During Pregnancy and Lactation Activates Fos Gene Expression and Alters Behaviors in Male Mouse Offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, W.; Wu, Q.; Lin, H.; Lu, Z.; Shen, X.; Chen, Y.; Zhou, Y.; Huang, L.; Wu, F.; et al. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients 2021, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, H.; Zhao, X.L.; Zeng, X.H. Hydrogen sulfide protects Sertoli cells against toxicant Acrolein-induced cell injury. Food Chem. Toxicol. 2023, 176, 113784. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Chen, J.; Cheng, K.; Dou, Z.; Leavenworth, J.D.; Yang, H.; Xu, D.; Luo, L. Nrf2-Dependent Protective Effect of Paeoniflorin on alpha-Naphthalene Isothiocyanate-Induced Hepatic Injury. Am. J. Chin. Med. 2022, 50, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, Y.; Wu, D.; Shou, J.; Wang, S.; Ye, J.; Tang, X.; Wang, X.J. Butylated hydroxyanisole induces distinct expression patterns of Nrf2 and detoxification enzymes in the liver and small intestine of C57BL/6 mice. Toxicol. Appl. Pharmacol. 2015, 288, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bayram-Weston, Z.; Olsen, E.; Harrison, D.J.; Dunnett, S.B.; Brooks, S.P. Optimising Golgi-Cox staining for use with perfusion-fixed brain tissue validated in the zQ175 mouse model of Huntington’s disease. J. Neurosci. Methods 2016, 265, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.H.; Jin, S.; Gao, D.; Liu, N.; Chen, S.P.; Zhang, S.; Liu, Q.; Liu, E.; Wang, X.; et al. Opposite monosynaptic scaling of BLP-vCA1 inputs governs hopefulness- and helplessness-modulated spatial learning and memory. Nat. Commun. 2016, 7, 11935. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Perez, A.; Holloway, L.P.; Barbaro, R.P.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.W.; Lauder, J.M.; et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.E.; Bundle, S.F.; Yaylaoglu, M.B.; Carson, J.P.; Thaller, C.; Zoghbi, H.Y. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 18267–18272. [Google Scholar] [CrossRef]
- Schule, B.; Armstrong, D.D.; Vogel, H.; Oviedo, A.; Francke, U. Severe congenital encephalopathy caused by MECP2 null mutations in males: Central hypoxia and reduced neuronal dendritic structure. Clin. Genet. 2008, 74, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, M.D.; Middleton, L.J.; Abdus-Saboor, I. Mechanisms of Tactile Sensory Phenotypes in Autism: Current Understanding and Future Directions for Research. Curr. Psychiatry Rep. 2019, 21, 134. [Google Scholar] [CrossRef] [PubMed]
- Ausio, J.; Martinez de Paz, A.; Esteller, M. MeCP2: The long trip from a chromatin protein to neurological disorders. Trends Mol. Med. 2014, 20, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.Y.; Lim, Z.H.; Korzh, V.; Pietri, T.; Goh, E.L. Methyl-CpG Binding Protein 2 (Mecp2) Regulates Sensory Function Through Sema5b and Robo2. Front. Cell. Neurosci. 2015, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, A.; Mu, Y.; Winter, M.K.; Knapp, J.R.; Eggimann, L.S.; Gunewardena, S.S.; Kobayashi, K.; Kato, S.; Krizsan-Agbas, D.; Smith, P.G. Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E6952–E6961. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Akbarian, S.; Tudor, M.; Jaenisch, R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001, 27, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Hendrich, B.; Holmes, M.; Martin, J.E.; Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001, 27, 322–326. [Google Scholar] [CrossRef]
- Na, E.S.; Nelson, E.D.; Kavalali, E.T.; Monteggia, L.M. The impact of MeCP2 loss- or gain-of-function on synaptic plasticity. Neuropsychopharmacology 2013, 38, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Gulmez Karaca, K.; Brito, D.V.C.; Oliveira, A.M.M. MeCP2: A Critical Regulator of Chromatin in Neurodevelopment and Adult Brain Function. Int. J. Mol. Sci. 2019, 20, 4577. [Google Scholar] [CrossRef] [PubMed]
- Berger-Sweeney, J. Cognitive deficits in Rett syndrome: What we know and what we need to know to treat them. Neurobiol. Learn. Mem. 2011, 96, 637–646. [Google Scholar] [CrossRef]
- Verma, V.; Paul, A.; Amrapali Vishwanath, A.; Vaidya, B.; Clement, J.P. Understanding intellectual disability and autism spectrum disorders from common mouse models: Synapses to behaviour. Open Biol. 2019, 9, 180265. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Kim, R.; Sharry, S.; Matsushima, A.; Takemoto-Kimura, S.; Bito, H. Towards a better understanding of cognitive behaviors regulated by gene expression downstream of activity-dependent transcription factors. Neurobiol. Learn. Mem. 2014, 115, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Guo, H.; Liu, Y.Y.; Zhang, J.L.; Hu, W.C.; Yu, M.J.; Zhao, R.; Xie, R.G.; Zhang, H.; Cong, R. Peripheral BDNF Regulates Somatosensory-Sympathetic Coupling in Brachial Plexus Avulsion-Induced Neuropathic Pain. Neurosci. Bull. 2023, 39, 1789–1806. [Google Scholar] [CrossRef] [PubMed]
- Marin, O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat. Med. 2016, 22, 1229–1238. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Wang, J.; Liu, J.; Zhou, Y.; Jiang, Y.; Zhou, W.; Wu, F.; Liu, X.; Luo, L. Mecp2 Deficiency in Peripheral Sensory Neuron Improves Cognitive Function by Enhancing Hippocampal Dendritic Spine Densities in Mice. Cells 2024, 13, 988. https://doi.org/10.3390/cells13110988
Feng Y, Wang J, Liu J, Zhou Y, Jiang Y, Zhou W, Wu F, Liu X, Luo L. Mecp2 Deficiency in Peripheral Sensory Neuron Improves Cognitive Function by Enhancing Hippocampal Dendritic Spine Densities in Mice. Cells. 2024; 13(11):988. https://doi.org/10.3390/cells13110988
Chicago/Turabian StyleFeng, Yuting, Jingge Wang, Jun Liu, Yinwei Zhou, Ying Jiang, Wenhui Zhou, Feng Wu, Xingjun Liu, and Lin Luo. 2024. "Mecp2 Deficiency in Peripheral Sensory Neuron Improves Cognitive Function by Enhancing Hippocampal Dendritic Spine Densities in Mice" Cells 13, no. 11: 988. https://doi.org/10.3390/cells13110988
APA StyleFeng, Y., Wang, J., Liu, J., Zhou, Y., Jiang, Y., Zhou, W., Wu, F., Liu, X., & Luo, L. (2024). Mecp2 Deficiency in Peripheral Sensory Neuron Improves Cognitive Function by Enhancing Hippocampal Dendritic Spine Densities in Mice. Cells, 13(11), 988. https://doi.org/10.3390/cells13110988






