Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purpose
2.2. Systematic Review
2.3. Search Strategy
2.4. Eligibility Criteria
2.5. Study Selection
2.6. Data Extraction
2.7. Quality and Risk of Bias Assessments
3. Results
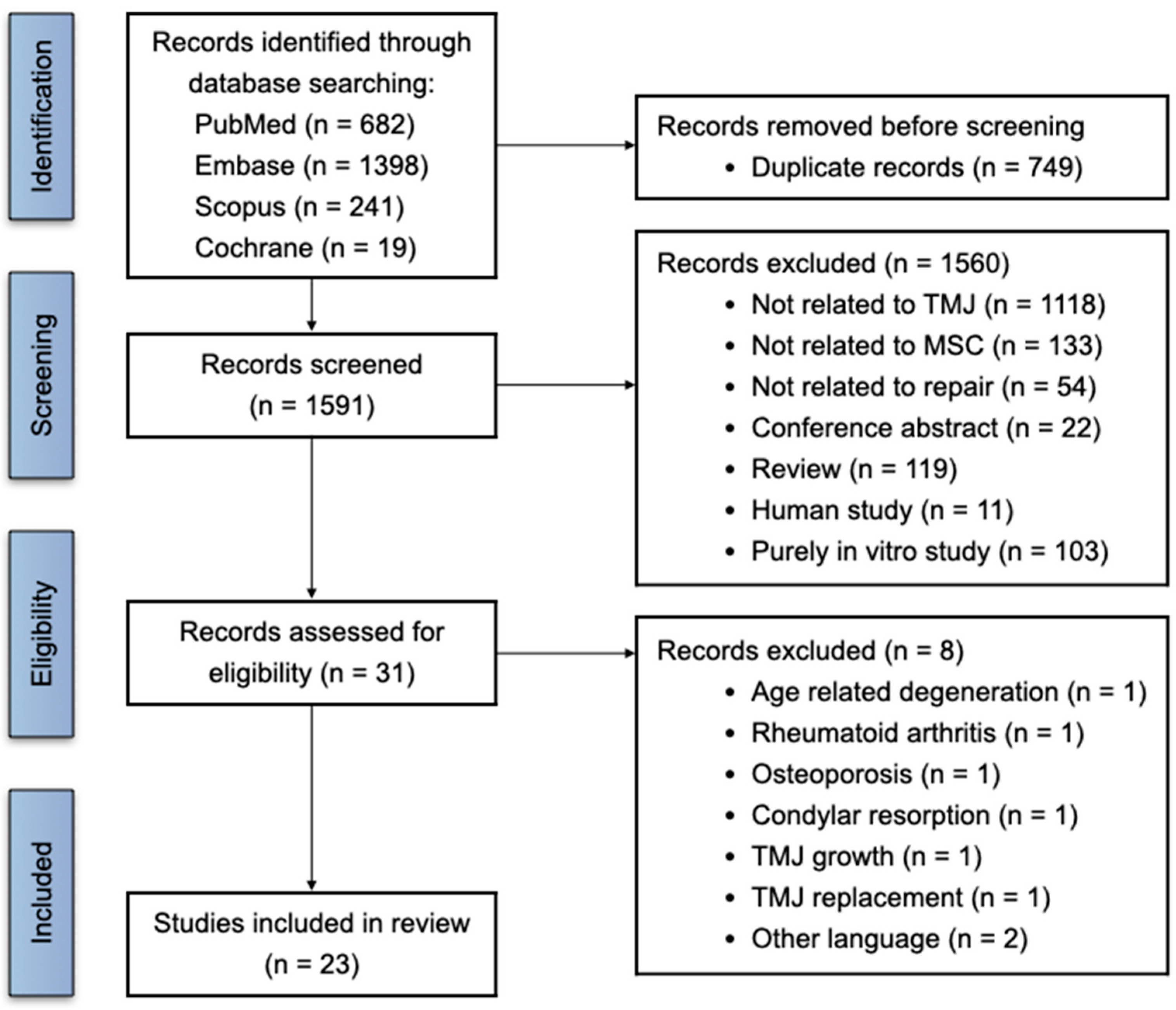
3.1. Search Process and Study Selection
3.2. Basic Characteristics of the Included Studies
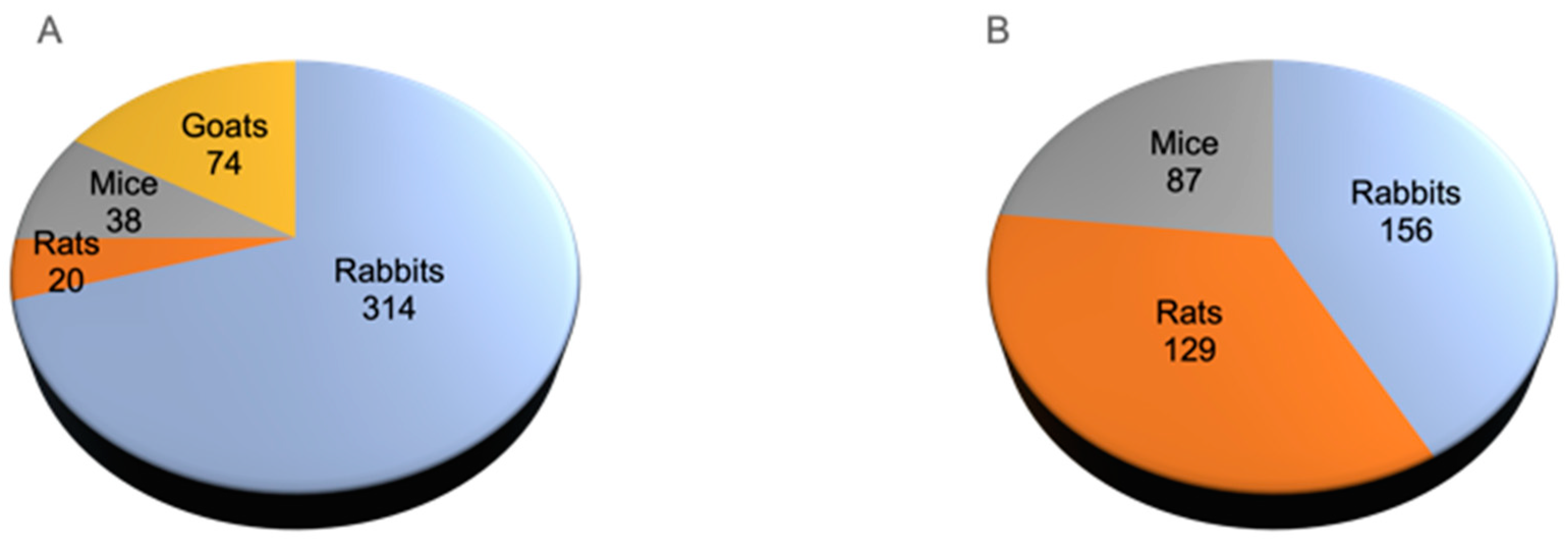
3.3. Animal Models
3.4. Source of MSCs, Their Derived Secretome, or EVs
3.5. Isolation and Characterization of MSCs, Their Derived Secretome, or EVs
3.6. Concentration
3.7. Delivery
3.8. Morphological Outcomes
3.9. Histological Outcomes
3.10. Molecular Outcomes
3.11. Pain Behavioral Outcomes
3.12. Fate and Biodistribution of MSCs, Their Derived Secretome, or EVs
3.13. Cellular Proliferation, Migration, and Matrix Synthesis
3.14. Immunomodulation
3.15. Compliance with the ARRIVE Guidelines
3.16. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, O.; Biffar, R.; Kocher, T.; Meyer, G. Prevalence and clinical signs of degenerative temporomandibular joint changes validated by magnetic resonance imaging in a non-patient group. Ann. Anat. 2007, 189, 342–346. [Google Scholar] [CrossRef]
- Zhang, S.; Yap, A.U.; Toh, W.S. Stem Cells for Temporomandibular Joint Repair and Regeneration. Stem Cell Rev. Rep. 2015, 11, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Teo, A.Q.A.; Wong, K.L.; Shen, L.; Lim, J.Y.; Toh, W.S.; Lee, E.H.; Hui, J.H.P. Equivalent 10-Year Outcomes After Implantation of Autologous Bone Marrow-Derived Mesenchymal Stem Cells Versus Autologous Chondrocyte Implantation for Chondral Defects of the Knee. Am. J. Sports Med. 2019, 47, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.S.; Kwan, Y.T.; Neo, W.J.; Chong, J.Y.; Kuek, T.Y.J.; See, J.Z.F.; Wong, K.L.; Toh, W.S.; Hui, J.H.P. Intra-articular Injections of Mesenchymal Stem Cells Without Adjuvant Therapies for Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 49, 3113–3124. [Google Scholar] [CrossRef] [PubMed]
- De Riu, G.; Vaira, L.A.; Carta, E.; Meloni, S.M.; Sembronio, S.; Robiony, M. Bone marrow nucleated cell concentrate autograft in temporomandibular joint degenerative disorders: 1-year results of a randomized clinical trial. J. Craniomaxillofac. Surg. 2019, 47, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Carboni, A.; Amodeo, G.; Perugini, M.; Arangio, P.; Orsini, R.; Scopelliti, D. Temporomandibular Disorders Clinical and Anatomical Outcomes After Fat-Derived Stem Cells Injection. J. Craniofac. Surg. 2019, 30, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, B.; Man, C.; Ma, Y.; Hu, J. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthr. Cartil. 2011, 19, 743–750. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Y.; Zhang, L.; Li, B.; Wang, X. Co-culture of bone marrow stromal cells and chondrocytes in vivo for the repair of the goat condylar cartilage defects. Exp. Ther. Med. 2018, 16, 2969–2977. [Google Scholar] [CrossRef]
- Putnová, B.; Hurník, P.; Jekl, V.; Žiak, D.; Machoň, V.; Škorič, M.; Stránský, J.; Štembírek, J. Effect of human adipose-derived regenerative cells on temporomandibular joint healing in immunodeficient rabbits. Acta Vet. Brno 2019, 88, 49–56. [Google Scholar] [CrossRef]
- Cheng, B.; Tu, T.; Shi, X.; Liu, Y.; Zhao, Y.; Zhao, Y.; Li, Y.; Chen, H.; Chen, Y.; Zhang, M. A novel construct with biomechanical flexibility for articular cartilage regeneration. Stem Cell Res. Ther. 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Sumarta, N.P.M.; Kamadjaja, D.B.; Hendrijantini, N.; Danudiningrat, C.P.; Rantam, F.A. Human umbilical cord mesenchymal stem cells over platelet rich fibrin scaffold for mandibular cartilage defects regenerative medicine. Pesqui. Bras. Odontopediatria Clínica Integr. 2021, 21, e0034. [Google Scholar] [CrossRef]
- Cheng, M.S.; Yi, X.; Zhou, Q. Overexpression of HIF-1alpha in Bone Marrow Mesenchymal Stem Cells Promote the Repair of Mandibular Condylar Osteochondral Defect in a Rabbit Model. J. Oral Maxillofac. Surg. 2021, 79, 345.e1–345.e15. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, Y.; Zou, L.; Yan, S.; Zhu, H.; Zhang, K.; Liu, W.; He, D.; Yin, J. A bilayered scaffold with segregated hydrophilicity-hydrophobicity enables reconstruction of goat hierarchical temporomandibular joint condyle cartilage. Acta Biomater. 2021, 121, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.; Wang, P.; Ma, J.; Wang, P.; Han, X.; Fan, Y.; Bai, D.; Sun, Y.; Zhang, X. Cell-mediated injectable blend hydrogel-BCP ceramic scaffold for in situ condylar osteochondral repair. Acta Biomater. 2021, 123, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.; Hakim, M.; Liapaki, A.; Lowe, B.; Faquin, W.; Thamm, J. Are stem cells useful in the regeneration and repair of cartilage defects in the TMJ condyle? An In Vivo Study. J. Dent. Oral Disord. 2021, 7, 1159. [Google Scholar]
- Gomez, M.; Wittig, O.; Diaz-Solano, D.; Cardier, J.E. Mesenchymal Stromal Cell Transplantation Induces Regeneration of Large and Full-Thickness Cartilage Defect of the Temporomandibular Joint. Cartilage 2021, 13, 1814S–1821S. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Wang, B.; Dong, Y.; Zhao, C.; Zhao, Y.; Zhang, L.; Liu, X.; Guo, J.; Chen, Y.; et al. Inflammation-Stimulated MSC-Derived Small Extracellular Vesicle miR-27b-3p Regulates Macrophages by Targeting CSF-1 to Promote Temporomandibular Joint Condylar Regeneration. Small 2022, 18, e2107354. [Google Scholar] [CrossRef]
- Chen, K.; Man, C.; Zhang, B.; Hu, J.; Zhu, S.S. Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2013, 42, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, X.; Zhang, M.; Zhang, H.; Liao, L.; Yang, T.; Zhang, J.; Xian, L.; Chen, D.; Wang, M. RANTES and SDF-1 Are Keys in Cell-based Therapy of TMJ Osteoarthritis. J. Dent. Res. 2015, 94, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Zaghloul, M.; Helal, M.E.; Mansour, N.A.; Grawish, M.E. Impact of Autologous Bone Marrow-Derived Stem Cells on Degenerative Changes of Articulating Surfaces Associated with the Arthritic Temporomandibular Joint: An Experimental Study in Rabbits. J. Oral Maxillofac. Surg. 2017, 75, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Lu, L.; Wan, X.; Zhang, J.; Zhang, H.; Liu, X.; Huang, X.; Xiao, G.; Wang, M. Matrix replenishing by BMSCs is beneficial for osteoarthritic temporomandibular joint cartilage. Osteoarthr. Cartil. 2017, 25, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, G.; Park, J.; Choi, J.; Kang, E.; Lee, B.K. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci. Rep. 2019, 9, 13854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Kano, F.; Hashimoto, N.; Mori, H.; Liu, Y.; Xia, L.; Sakamaki, T.; Hibi, H.; Iwamoto, T.; Tanaka, E.; et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthr. Cartil. 2020, 28, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.J.; Zhang, T.; Fu, Y.; Liu, Y.; Gan, Y.H.; Zhou, Y.H.; Yang, R.L.; Wang, X.D. DPSCs Attenuate Experimental Progressive TMJ Arthritis by Inhibiting the STAT1 Pathway. J. Dent. Res. 2020, 99, 446–455. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Li, W.; Yang, Y.; Zhang, Z.; Ma, R.; Wu, M. BMSC-Derived Small Extracellular Vesicles Induce Cartilage Reconstruction of Temporomandibular Joint Osteoarthritis via Autotaxin-YAP Signaling Axis. Front. Cell Dev. Biol. 2021, 9, 656153. [Google Scholar] [CrossRef] [PubMed]
- Kohnke, R.; Ahlers, M.O.; Birkelbach, M.A.; Ewald, F.; Krueger, M.; Fiedler, I.; Busse, B.; Heiland, M.; Vollkommer, T.; Gosau, M.; et al. Temporomandibular Joint Osteoarthritis: Regenerative Treatment by a Stem Cell Containing Advanced Therapy Medicinal Product (ATMP)—An In Vivo Animal Trial. Int. J. Mol. Sci. 2021, 22, 443. [Google Scholar] [CrossRef] [PubMed]
- AbuBakr, N.; Fares, A.E.; Mostafa, A.; Farag, D.B.E. Mesenchymal stem cells-derived microvesicles versus platelet-rich plasma in the treatment of monoiodoacetate-induced temporomandibular joint osteoarthritis in Albino rats. Heliyon 2022, 8, e10857. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, C.; Zheng, H.; Meng, Z.; Heng, B.C.; Zhou, T.; Jiang, S.; Wei, Y. Superwettable and injectable GelMA-MSC microspheres promote cartilage repair in temporomandibular joints. Front. Bioeng. Biotechnol. 2022, 10, 1026911. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.Y.W.; Tan, R.; Wong, K.L.; Hey, D.H.W.; Hui, J.H.P.; Toh, W.S. Small extracellular vesicles from mesenchymal stromal cells: The next therapeutic paradigm for musculoskeletal disorders. Cytotherapy 2023, 25, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [PubMed]



| Author, Year | Animal | Age | Sample Size | Gender | Weight | Animal Model | Method of Induction |
|---|---|---|---|---|---|---|---|
| Zhu, 2011 [19] | Eastern cross goats | 25–28 months | 50 | Male | 32–37 kg | Osteochondral defect | Surgical; 3 mm diameter, 5 mm depth |
| Sun, 2018 [20] | Goats | 6–8 months | 12 | Male | 10–22 kg | Condylar cartilage defect | Surgical; full-thickness condylar cartilage defect |
| Putnova, 2019 [21] | NZ rabbits | 10 months | 17 | Male | NR | Osteochondral defect | Surgical; NR |
| Cheng, 2019 [22] | NZ rabbits | 6 months | 180 | Male | NR | Osteochondral defect | Surgical; 3 mm diameter, 3 mm depth |
| Sumarta, 2021 [23] | Wistar rats | 3 months | 20 | Male | 200–300 g | Osteochondral defect | Surgical; 1 mm diameter |
| Cheng, 2021 [24] | NZ rabbits | 4 months | 45 | Male | 2–3 kg | Osteochondral defect | Surgical; 3 mm diameter, 2 mm depth |
| Yu, 2021 [25] | Goats | 12 months | 12 | NR | 20 kg | Osteochondral defect | Surgical; 5 mm diameter, 5 mm depth |
| Wang, 2021 [26] | NZ rabbits | 7 months | 16 | Male | 3 kg | Osteochondral defect | Surgical; 2 mm diameter, 3 mm depth |
| Guastaldi, 2021 [27] | C57BL/6 mice | 8–10 weeks | 30 | Female | NR | Osteochondral defect | Surgical; linear condylar cartilage defect |
| Gomez, 2021 [28] | C57BL/6 mice | 8–10 weeks | 8 | Female | NR | Osteochondral defect | Surgical; focal cartilage defect |
| Liu, 2022 [29] | NZ rabbits | NR | 56 | NR | 2.2–2.5 kg | Osteochondral defect | Surgical; 2 mm diameter, 2 mm depth |
| Chen, 2013 [30] | NZ rabbits | 6 months | 46 | NR | 2.5–3.2 kg | TMJ-OA | Surgical; disc resection |
| Lu, 2015 [31] | C57BL/6J mice | 6 weeks | 27 | Female | 17–19 g | TMJ-OA | Mechanical; UAC |
| Zaki, 2017 [32] | NZ rabbits | NR | 50 | Male | 1–1.5 kg | TMJ-OA | Chemical; bovine collagen II injection |
| Zhang, 2017 [33] | C57BL/6J mice | 6 weeks | 40 | Female | 17–19 g | TMJ-OA | Mechanical; UAC |
| Kim, 2019 [34] | NZ rabbits | NR | 25 | Male | 2.5–2.8 kg | TMJ-OA | Chemical; MIA injection |
| Zhang, 2019 [35] | SD rats | 8 weeks | 48 | Female | 198–271 g | TMJ-OA | Chemical; MIA injection |
| Ogasawara, 2020 [36] | Mice | 11 weeks | 20 | Male | NR | TMJ-OA | Mechanical; forced mouth opening |
| Cui, 2020 [37] | SD rats | 7 weeks | 15 | Female | 180–200 g | TMJ-OA | Chemical; CFA and MIA injection |
| Wang, 2021 [38] | NZ rabbits | 12–18 weeks | 7 | Male and female | NR | TMJ-OA | Chemical; Collagenase II injection |
| Köhnke, 2021 [39] | Rabbits | 12 weeks | 28 | Female | 800 g | TMJ-OA | Chemical; Collagenase II injection |
| AbuBakr, 2022 [40] | Albino rats | 3–4 months | 48 | Male | 150–170 g | TMJ-OA | Chemical; MIA injection |
| Yang, 2022 [41] | SD rats | 6 weeks | 18 | Male | NR | TMJ-OA | Chemical; CFA combined with IL-1β injection |
| Author, Year | Source | Isolation Method | Characterization | Size Distribution | Marker Expression |
|---|---|---|---|---|---|
| Zhu, 2011 [19] | Goat BMSCs | Density gradient centrifugation, adherence to tissue culture flask | NR | NA | ND |
| Sun, 2018 [20] | Goat BMSCs and auricular chondrocytes | Centrifugation, adherence to tissue culture flask | Phase contrast microscopy | NA | ND |
| Putnova, 2019 [21] | Human ADRCs | Celution 800/CRS system | NR | NA | CD146+, CD34+, CD31+, CD45+ |
| Cheng, 2019 [22] | Rabbit BMSCs | Centrifugation, adherence to tissue culture flask | Flow cytometry, adipogenic and osteogenic differentiation, ARS, ALP, oil red O staining, TEM, SEM | NA | CD34−, CD45−, CD29+ and CD44+ |
| Sumarta, 2021 [23] | Human UCMSCs | NR | Immunocytochemical staining, flow cytometry | NA | CD45−, CD73+, CD90+, CD105+ |
| Cheng, 2021 [24] | Rabbit BMSCs | Centrifugation, adherence to tissue culture flask | Immunofluorescence, WB analysis for HIF-1α expression | NA | HIF-1α |
| Yu, 2021 [25] | Goat BMSCs | Centrifugation, adherence to tissue culture flask | Chondrogenic induction, CLSM, SEM, gene expression analysis | NA | Col I, Col II, Sox9 |
| Wang, 2021 [26] | Rabbit BMSCs and articular chondrocytes | Centrifugation, adherence to tissue culture flask | Live/dead, osteogenic and chondrogenic differentiation, ALP, ARS, SEM, GAG/DNA, gene expression analysis | NA | ACAN, Sox9, Col1a2, Col2a1, Col10a1 |
| Guastaldi, 2021 [27] | Mouse TMJ condyle-derived MSCs | NR | Live/dead, flow cytometry | NA | NR |
| Gomez, 2021 [28] | Human BMSCs | Ficoll-Hypaque isolation, adherence to tissue culture flask | Flow cytometry, osteogenic and chondrogenic differentiation, ARS, AB staining | NA | CD90+, CD105+ |
| Liu, 2022 [29] | Human ADSC-sEVs | Ultracentrifugation | Human ADSCs: flow cytometry human ADSC-sEVs: TEM, NTA, nanoflow cytometry | ~130 nm | Human ADSCs: CD73+, CD90+, CD105+, CD14−, CD19−, CD34−, CD45−, HLA-DR- Human ADSC-sEVs: CD9+, CD81+ |
| Chen, 2013 [30] | Rabbit BMSCs | Centrifugation, adherence to tissue culture flask | ND | NA | ND |
| Lu, 2015 [31] | GFP-labeled mouse BMSCs | NR | NR | NA | ND |
| Zaki, 2017 [32] | Rabbit BMSCs | Centrifugation, adherence to tissue culture flask | Flow cytometry | NA | CD90+, CD105+, CD106+, CD45− |
| Zhang, 2017 [33] | GFP-labeled mouse BMSCs | NR | NR | NA | ND |
| Kim, 2019 [34] | Human UCMSCs | Density gradient centrifugation, adherence to tissue culture flask | Flow cytometry, proliferation, chondrogenic differentiation, HE, AB staining, gene expression analysis | NA | CD34−, CD45−, CD90+, CD105+ FGF-2, TGF-β1, IGF-1, Col1α1, Col2α1, ACAN, OCT4, NANOG, Sox2 |
| Zhang, 2019 [35] | Human ESC-MSC exosomes | Size fractionation | WB, NTA, protein concentration | 100–200 nm | CD81, ALIX, TSG101 |
| Ogasawara, 2020 [36] | Human SHED-CM | Supernatants after centrifugation | LC-MS/MS analysis | NA | SPON2, IGF2, SDC4, SDC1, SFRP1, PTN, MDK, TGFb2, PDGFD, HGF |
| Cui, 2020 [37] | Human DPSCs | Centrifugation, adherence to tissue culture flask | NR | NA | NR |
| Wang, 2021 [38] | Human BMSC-derived sEVs | Centrifugation, microfiltration, ultrafiltration | NTA, TEM, immunoblotting | ~106 nm | CD81+, CD63+, Rab5+, ALIX+, GRP94− |
| Köhnke, 2021 [39] | Human adipose-derived MSCs | NR | Flow cytometry, adipogenic, chondrogenic and osteogenic differentiation | NA | CD13+, CD44+, CD73+, CD90+, CD105+, CD31−, CD45−, CD235a−, HLA-II− |
| AbuBakr, 2022 [40] | Rat BMSC-derived MVs | Ultracentrifugation | TEM, FACS, ELISA | ~79 nm | BMSCs: CD90+, CD105+, CD14− BMSC-derived MVs: CD63+, CD81+ |
| Yang, 2022 [41] | Rat BMSCs | Centrifugation, adherence to tissue culture flask | SEM, immunocytochemical staining, ELISA, gene expression analysis | NA | Sox9, Col2a1, ACAN |
| Author, Year | Groups | Study Timepoints | Conc. | Volume/ Frequency | Delivery | Method of Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gross | Imaging | Histology | IHC | Molecular | Pain | ||||||
| Zhu, 2011 [19] | PLGA scaffold with NELL-1-modified BMSCs group, BMSCs group, PLGA group, empty defect group | 6, 24 weeks | 7.5 × 107 cells/mL | 40 μL/ one time | Implantation/PLGA scaffold | Gross morphology | Micro-CT (BV/TV) | SO, histological scoring | Col II | ND | ND |
| Sun, 2018 [20] | Gel-cell group, gel group | 4, 8, 12 weeks | 5 × 107 cells/mL | NR/ one time | Implantation/Pluronic F-127 gel | Gross morphology | X-ray | HE, Wakitani scoring | Col II | ND | ND |
| Putnova, 2019 [21] | Human ADRC group, control group | 11, 28 days | NR | 1 mL/ one time | Flushing/ ADRC solution | Gross morphology | ND | HE, AB, Histomorphometry | ND | ND | ND |
| Cheng, 2019 [22] | Blank control group, PRF group, BMSC sheet group, BMSC/PRF construct group, pressure-pretreated BMSC/PRF construct group | 2, 4, 8 weeks | NR | NR/ one time | Implantation/PRF scaffold | Gross morphology | ND | HE, TB, histological scoring | ND | Sox9, Col II, ACAN | ND |
| Sumarta, 2021 [23] | Untreated defects, defects treated with UCMSCs, defects treated with PRF scaffold, defects treated with UCMSCs and PRF scaffold | 6 weeks | 2 × 106 cells | NR/ one time | Implantation/PRF | ND | ND | HE | Ki67, FGF18, Sox9, Col II, ACAN | ND | ND |
| Cheng, 2021 [24] | Empty group, collagen scaffold group, collagen scaffold with BMSCs group, collagen scaffold with HIF-1α overexpressing BMSCs group, sham group | 12 weeks | 2 × 105 cells/mL | NR/ one time | Implantation/rat tail collagen scaffold | Gross morphology | Micro-CT (BV/TV, Tb.Th, Tb.Sp, Tb.N) | SO/FG, histological scoring | Col II, HIF-1α | ND | ND |
| Yu, 2021 [25] | Healthy control, defect group, bi-layered scaffold group, bi-layered scaffold with induced 14-day BMSCs group | 2 months | 5 × 107 cells/mL | 200 μL/ one time | Implantation/PEG crosslinked-PLGA-g-PCL scaffold | Gross morphology | ND | HE, SO/FG | Col I, Col II | ND | ND |
| Wang, 2021 [26] | Empty defect group, bi-layer scaffold group, bi-layer scaffold with BMSCs/chondrocyte group | 6, 24 weeks | 5 × 106 cells/mL | NR/ one time | Implantation/HA-SH-Col I hydrogel-BCP ceramic scaffold | Gross morphology | Micro-CT (BMD, BV/TV, Tb.Th, Tb.N) | HE, Masson, SO/FG, O’Driscoll scoring | Col I, Col II, Col X | ND | ND |
| Guastaldi, 2021 [27] | Sham group, defect group, defect treated with MSCs + hydrogel + biosilica group | 4, 8 weeks | 1 × 106 cells/mL | 20 μL/ one time | Implantation/gelatine/biosilica-based hydrogel scaffold | ND | ND | HE, SO/FG | ND | ND | ND |
| Gomez, 2021 [28] | BMSC/PRP treated group, control group (untreated), sham group | 6 weeks | 105 cells/mL | 100 µL/ one time | Implantation/PRP | ND | ND | HE, AB, Mankin scoring | ND | ND | ND |
| Liu, 2022 [29] | Non-implanted group, scaffold group, scaffold with AE group, scaffold with IAE group | 4, 8 weeks | 5 µg sEV/scaffold | NR/ one time | Implantation/SF scaffold | Gross morphology (ICRS macro-scopic scoring) | Micro-CT (BV/TV, Tb.Th, Tb.Sp, Tb.N) | HE, SO/FG | Col I, Col II | ND | ND |
| Author, Year | Groups | Study Timepoints | Conc. | Volume/ Frequency | Delivery | Method of Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gross | Imaging | Histology | IHC | Molecular | Pain | ||||||
| Chen, 2013 [30] | Non-chondrogenic MSCs group, chondrogenic differentiated MSCs group, vehicle (hylartin) group, normal control group | 4, 12, 24 weeks | 2 × 106 cells/mL | 100 µL/ one time | I.A. injection/ hylartin solution | ND | Micro-CT (BV/TV, Tb.Th, Tb.Sp) | SO, TB, histological grading | Col II | Sox9, Col2a1, ACAN, MMP13 | ND |
| Lu, 2015 [31] | Control group, BMSCs group, UAC group, UAC with BMSCs group | 4, 8, 12 weeks | 105 cells/mL | 20 µL/ weekly | I.A. injection/DMEM with 10% FCS | ND | Micro-CT (BV/TV, Tb.Th, Tb.Sp, Tb.N) | HE, SO, TRAP | Col II, SDF-1, RANTES, GFP | Col1a1,2a1,10a1, ACAN, OCN, MMP13, TNF-α, IL-1β | ND |
| Zaki, 2017 [32] | Untreated group, PBS group, BMSCs group, Arthritis with PBS group, Arthritis with BMSCs group | 3 weeks | NR | 200 µL/ one time | I.A. injection/ PBS | ND | ND | HE, SO, histomorphometry | ND | ND | ND |
| Zhang, 2017 [33] | Control group, UAC group, UAC with BMSCs group | 4, 8, 12 weeks | 105 cells/mL | 20 µL/ weekly | I.A. injection/ DMEM with 10% FCS | ND | TEM | HE, SO | Col I, Col II, Ki67, DAP3, CD163, GFP | ND | ND |
| Kim, 2019 [34] | Healthy control group; TMJ-OA group; TMJ-OA treated with DEX group; TMJ-OA treated with low, medium, or high dose of human UCMSCs groups | 8 weeks | 5 × 105, 2.5 × 106, 5 × 106 cells/mL | 200 µL/ one time | I.A. injection/ saline | ND | Micro-CT | HE, SO/FG, Mankin scoring | ACAN, Col I | TNF-α, IL-1β,-6,-10,-17, TGF-β1, IGF-1, FGF-2, Col1a1,2a1, ACAN | ND |
| Zhang, 2019 [35] | OA + PBS group, OA + exosome group, sham group | 2, 4, 8 weeks | 2 mg/mL | 50 µL/ weekly | I.A. injection/ PBS | ND | Micro-CT (BV/TV Tb.Th Tb.Sp Tb.N) | HE, TB, Mankin scoring, histomorphometry | α-SMA, MMP13, IL-1β, iNOS, PCNA, CCP3 | IL-1β,-4,-10 iNOS, TNF-α, TGF-β1, Col1a1,2a1, Sox9, COMP, ACAN, MMP3,9,13 TIMP1,2,3, ADAMTS4,5, BAX, Casp3,8,9, Survivin, PCNA, α-SMA, SP, CGRP, NGF, P75NTR, TrkA | HWT |
| Ogasawara, 2020 [36] | Sham group, pre-treatment group, DMEM group, SHED-CM group, | 12 days | 3 μg/mL | 0.5 mL/ 5 days | Tail vein injection/ serum-free DMEM | ND | Micro-CT (BV/TV, Tb.Th, Tb.Sp) | HE, TB, Mankin scoring, TUNEL, TRAP | MMP13, iNOS, IL-1β, PCNA | ND | HWT |
| Cui, 2020 [37] | Control group, TMJ-OA with saline group, TMJ-OA with DPSCs group | 2, 4 weeks | 4 × 106 cells/mL | 50 µL/ one time | I.A. injection/ saline | ND | Micro-CT (BV/TV BS/BV Tb.Sp Tb.N) | HE, TB, Mankin scoring | CD4, IFN-γ, TNF-α, MMP3, MMP13 | ND | HWT |
| Wang, 2021 [38] | Control group, OA group, sEV group, OA + sEV group | 4, 6, 8 weeks | (2–4) × 109 particles/mL | 200 µL/ one time | I.A. injection/ PBS | Gross morphology | Micro-CT (BV/TV BS/BV BMD Tb.Th) Tb.Sp | HE, SO/FG, AB, Wakitani scoring | PCNA, Col I, Col II, ACAN, Sox9, MMP13, RUNX2 | ND | ND |
| Köhnke, 2021 [39] | AB serum group, HA group, MSCs group, MSCs + HA group | 4 weeks | 106 cells/mL | 150 µL/ one time | I.A. injection/ HA | ND | CT, SEM | SO, Picrosirius red | ND | ND | ND |
| AbuBakr, 2022 [40] | OA group, OA + MVs group, OA + PRP group | 2, 4 weeks. | 2 mg/mL | 50 µL/ one time | I.A. injection/ PBS | ND | ND | HE | ND | IL-1β, TNF-α, NF-κB, MMP3,13, Col II | ND |
| Yang, 2022 [41] | BMSC-coated microspheres group, microspheres group, control group | 1, 2 weeks | NR | 200 µL/ one time | I.A. injection/ GelMA microspheres | ND | SEM, Micro-CT (BMD, BV/TV) | HE | Sox9 | ND | ND |
| Author, Year | Key Outcomes (In Vitro) | Key Outcomes (In Vivo) |
|---|---|---|
| Zhu, 2011 [19] | ND | NELL-1-modified BMSCs/PLGA composite rapidly repaired large osteochondral defect in the mandibular condyle with regeneration of fibrocartilage and subchondral bone. |
| Sun, 2018 [20] | ND | Co-culture of goat BMSCs and chondrocytes at 7:3 ratio in hydrogel induced chondrogenic differentiation of BMSCs to enhance TMJ repair. |
| Putnova, 2019 [21] | ND | Human ADRCs supported soft tissue repair and promoted bone remodeling in hard tissues. |
| Cheng, 2019 [22] | Hydrostatic pressure pre-treatment (120 kPa/1 h for 4 days) optimally promoted BMSC proliferation and chondrogenic differentiation in the BMSC/PRF construct. | Pressure-pretreated BMSC/PRF construct enhanced cartilage regeneration with improved mechanical properties and integration of the neocartilage. |
| Sumarta, 2021 [23] | ND | Human UCMSCs in PRF scaffold proved capable of regenerating mandibular cartilage defect through increased expression of FGF-18, Sox9, Ki67, Col II, ACAN, and cartilage thickness |
| Cheng, 2021 [24] | ND | Transplantation of HIF-1α overexpressed BMSCs combined with a collagen scaffold promoted cartilaginous repair of condylar cartilage and inhibited subchondral bone sclerosis in TMJ osteochondral defect. |
| Yu, 2021 [25] | The bi-layered PLGA-g-PCL scaffold with segregated hydrophilicity–hydrophobicity facilitated chondrogenic differentiation of BMSCs toward top fibrocartilage layer and bottom hyaline cartilage layer. | The bi-layered PLGA-g-PCL-PEG scaffold with segregated hydrophilicity–hydrophobicity carrying induced 14-day BMSCs enabled reconstruction of goat hierarchical TMJ condylar cartilage. |
| Wang, 2021 [26] | The HA-SH/Col I blend hydrogel-BCP ceramic bi-layered scaffold enhanced proliferation and matrix synthesis of rabbit BMSCs and chondrocytes, as well as osteogenic differentiation of BMSCs. | Rabbit BMSCs/chondrocytes-loaded bi-layer scaffold could effectively promote the regeneration of both fibrocartilage and subchondral bone. |
| Guastaldi, 2021 [27] | ND | The MSCs + hydrogel + biosilica was effective in promoting TMJ condylar cartilage regeneration, as evidenced by intact articular surfaces, maturation, and distribution of chondrocytes along the condyle. |
| Gomez, 2021 [28] | ND | BMSC/PRP implantation promoted repair of the articular surface with the presence of cartilage-like tissue and subchondral bone filling the defect area. |
| Liu, 2022 [29] | Both IAE and AE showed comparable effects on proliferation, whereas IAE outperformed AE on BMSC migration and M2 macrophage polarization in vitro. RNA sequencing identified high miR-27b-3p expression levels in IAE that may regulate macrophage polarization by targeting CSF-1. | IAE loaded onto SF scaffold outperformed AE-loaded scaffold in TMJ osteochondral regeneration, with newly formed cartilage stained for GAG, collagen I and II, and reconstituted subchondral bone. |
| Author, Year | Key Outcomes (In Vitro) | Key Outcomes (In Vivo) |
|---|---|---|
| Chen, 2013 [30] | ND | I.A injection of MSCs could delay the progression of TMJ-OA, and in vitro chondrogenic differentiation of MSCs could enhance the therapeutic effects. |
| Lu, 2015 [31] | SDF-1 and RANTES were significantly increased in the UAC cartilage compared to the control cartilage. Migration of BMSCs was enhanced when cocultured with a UAC TMJ condyle and was attenuated in the presence of AMD3100 (CXCR4 antagonist) or BX471 (CCR1 antagonist). | BMSC injections improved cartilage repair and subchondral bone restoration in TMJ-OA mice induced by UAC. The locally injected BMSCs were found to implant and differentiate into chondrocytes in OA cartilage. These effects of BMSCs were inhibited by AMD3100 and BX471. |
| Zaki, 2017 [32] | ND | Rabbit BMSCs could safely and effectively repair degenerative changes of rabbit TMJs with bovine collagen II-induced arthritis. |
| Zhang, 2017 [33] | Fluid flow shear stress (FFSS) stimulation induced cell death of superficial and deep zone chondrocytes. Genes associated with chondrocyte hypertrophy and fibrosis were upregulated in deep zone chondrocytes with FFSS stimulation. | BMSCs rescued the damaged cartilage by increasing matrix production and scavenging activity. |
| Kim, 2019 [34] | Human UCMSC lines isolated from different donors showed comparable proliferation ability but varying in vitro capacities for chondrogenesis and expression of marker genes for growth factors and ECM compared to that of BMSCs. | Human UCMSCs exerted anti-inflammatory effects and promoted cartilage regeneration in a rabbit model of TMJ-OA. Medium dose of MSCs was most effective in regenerating the articular cartilage with the highest gene expression levels of growth factors. |
| Zhang, 2019 [35] | Human ESC-MSC exosomes suppressed inflammation and restored matrix synthesis in IL-1β-treated chondrocytes via adenosine receptor activation of AKT, ERK, and AMPK pathways. | Human ESC-MSC exosomes suppressed pain and inflammation and reduced cell apoptosis and matrix degradation while enhancing cell proliferation and matrix synthesis to promote overall TMJ repair and regeneration. |
| Ogasawara, 2020 [36] | LC-MS/MS analysis identified several factors present in SHED-CM that could be involved in processes such as anti-fibrosis, anti-apoptosis, anti-inflammation, proliferation, differentiation, and migration of chondrocytes. | SHED-CM contained multiple therapeutic factors with the potential to promote the regeneration and repair of mechanical-stress-induced mouse TMJ-OA. |
| Cui, 2020 [37] | DPSCs downregulated the expression of MMP3 and MMP13 in fibroblast-like synoviocytes by suppressing STAT1 activation under the inflammatory condition. | DPSC local injection relieved pain, suppressed synovial inflammation, and reduced cartilage degradation and subchondral bone destruction in rats. |
| Wang, 2021 [38] | BMSC-sEVs enhanced proliferation and migration of mandibular condylar chondrocytes, possibly through activation of the Hippo pathway. | BMSC-sEVs promoted cartilage reconstruction in TMJ-OA via the autotaxin–YAP signaling axis. |
| Köhnke, 2021 [39] | ND | Human adipose-derived MSCs with or without HA were more effective than serum and HA alone in restoring the cartilage thickness in a rabbit TMJ-OA model. |
| AbuBakr. 2022 [40] | ND | BMSC-derived MVs restored damaged condylar structure by suppressing inflammation and matrix degradation in a rat model of TMJ-OA. |
| Yang, 2022 [41] | GelMA microspheres loaded with TGF-β enhanced chondrogenic differentiation of BMSCs coated on the microspheres. | Rat BMSC-coated GelMA microspheres endowed with superwettable properties and sustained TGF-β release, can efficiently colonize the bone defect site, release cytokine, and promote cartilage healing. |
| Item/Item Number | Zhu, 2011 [19] | Sun, 2018 [20] | Putnova, 2019 [21] | Cheng, 2019 [22] | Sumarta, 2021 [23] | Cheng, 2021 [24] | Yu, 2021 [25] | Wang, 2021 [26] | Guastaldi, 2021 [27] | Gomez, 2021 [28] | Liu, 2022 [29] | Cat. Score | Total Score | Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Title (0, inaccurate/not concise; 1, accurate/concise) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | 11 | 1 |
| 2. Abstract Summary of the background; research objectives, including details of the species or strain of animal used; key methods; principal findings; and conclusions of the study (0, clearly inaccurate; 1, possibly accurate; 2, clearly accurate) | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 18 | 22 | 0.82 |
| 3. Introduction Background: objectives, experimental approach and rationale, and relevance to human biology (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 13 | 22 | 0.59 |
| 4. Introduction Objectives: primary and secondary (0, not clear; 1, clear) | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 7 | 11 | 0.64 |
| 5. Methods Ethical statement: nature of the review permission, relevant licenses, and national and institutional guidelines for the care and use of animals (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 16 | 22 | 0.73 |
| 6. Methods Study design: number of experimental and control groups and any steps taken to minimize bias (i.e., allocation concealment, randomization, blinding) (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 0 | 15 | 22 | 0.68 |
| 7. Methods Experimental procedure: precise details (i.e., how, when, where, why) (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 15 | 22 | 0.68 |
| 8. Methods Experimental animals: species, strain, sex, developmental stage, weight, and source of animals (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | 22 | 0.5 |
| 9. Methods Housing and husbandry: conditions and welfare-related assessments and interventions (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 9 | 22 | 0.41 |
| 10. Methods Sample size: total number of animals used in each experimental group, details of calculation (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 12 | 22 | 0.55 |
| 11. Methods Allocation animals to experimental groups: randomization or matching, order in which animals were treated and assessed (0, no; 1, yes) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 7 | 11 | 0.64 |
| 12. Methods Experimental outcomes: definition of primary and secondary outcomes (0, no; 1, unclear/not complete; 2, yes) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0 |
| 13. Methods Statistical methods: details and unit of analysis (0, no; 1, unclear/ not complete; 2, yes) | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | 2 | 16 | 22 | 0.73 |
| 14. Results Baseline data: characteristics and health status of animals (0, no; 1, yes) | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 | 11 | 0.45 |
| 15. Results Numbers analyzed: absolute numbers in each group included in each analysis, explanation for exclusion (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 8 | 22 | 0.36 |
| 16. Results Outcomes and estimation: results for each analysis with a measure of precision (0, no; 1, unclear/not complete; 2, yes) | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 14 | 22 | 0.64 |
| 17. Results Adverse events: details and modifications for reduction (0, no; 1, unclear/not complete; 2, yes) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | 22 | 0.14 |
| 18. Discussion Interpretation/scientific implications: study limitations including animal model, and implications for the 3Rs (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 10 | 22 | 0.45 |
| 19. Discussion Generalizability/translation: relevance to human biology (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 17 | 22 | 0.77 |
| 20. Discussion Funding: resources and role of the funders (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 21 | 22 | 0.95 |
| Item/Item Number | Chen, 2013 [30] | Lu, 2015 [31] | Zaki, 2017 [32] | Zhang, 2017 [33] | Kim, 2019 [34] | Zhang, 2019 [35] | Ogasawara, 2020 [36] | Cui, 2020 [37] | Wang, 2021 [38] | Köhnke, 2021 [39] | AbuBakr, 2022 [40] | Yang, 2022 [41] | Cat. Score | Total Score | Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Title (0, inaccurate/not concise; 1, accurate/concise) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 12 | 12 | 1 |
| 2. Abstract Summary of the background; research objectives, including details of the species or strain of animal used; key methods; principal findings; and conclusions of the study (0, clearly inaccurate; 1, possibly accurate; 2, clearly accurate) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 14 | 24 | 0.58 |
| 3. Introduction Background: objectives, experimental approach and rationale, and relevance to human biology (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | 24 | 0.58 |
| 4. Introduction Objectives: primary and secondary (0, not clear; 1, clear) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 5 | 12 | 0.42 |
| 5. Methods Ethical statement: nature of the review permission, relevant licenses, and national and institutional guidelines for the care and use of animals (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 20 | 24 | 0.83 |
| 6. Methods Study design: number of experimental and control groups and any steps taken to minimize bias (i.e., allocation concealment, randomization, blinding) (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 19 | 24 | 0.79 |
| 7. Methods Experimental procedure: precise details (i.e., how, when, where, why) (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 16 | 24 | 0.67 |
| 8. Methods Experimental animals: species, strain, sex, developmental stage, weight, and source of animals (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | 24 | 0.58 |
| 9. Methods Housing and husbandry: conditions and welfare-related assessments and interventions (0, clearly insufficient; 1, possibly sufficient; 2, clearly sufficient) | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 2 | 2 | 0 | 11 | 24 | 0.46 |
| 10. Methods Sample size: total number of animals used in each experimental group and details of calculation (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 2 | 0 | 2 | 0 | 2 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 12 | 24 | 0.5 |
| 11. Methods Allocation of animals to experimental groups: randomization or matching and order in which animals were treated and assessed (0, no; 1, yes) | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 12 | 0.75 |
| 12. Methods Experimental outcomes: definition of primary and secondary outcomes (0, no; 1, unclear/not complete; 2, yes) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 |
| 13. Methods Statistical methods: details and unit of analysis (0, no; 1, unclear/ not complete; 2, yes) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 21 | 24 | 0.88 |
| 14. Results Baseline data: characteristics and health status of animals (0, no; 1, yes) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 8 | 12 | 0.67 |
| 15. Results Numbers analyzed: absolute numbers in each group included in each analysis and explanation for exclusion (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 8 | 24 | 0.33 |
| 16. Results Outcomes and estimation: results of each analysis with a measure of precision (0, no; 1, unclear/not complete; 2, yes) | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 15 | 24 | 0.63 |
| 17. Results Adverse events: details and modifications for reduction (0, no; 1, unclear/not complete; 2, yes) | 0 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 0 | 0 | 10 | 24 | 0.42 |
| 18. Discussion Interpretation/scientific implications: study limitations including animal model, and implications for the 3Rs (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 11 | 24 | 0.46 |
| 19. Discussion Generalizability/translation: relevance to human biology (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 17 | 24 | 0.71 |
| 20. Discussion Funding: resources and role of the funders (0, clearly inadequate; 1, possibly inadequate; 2, clearly adequate) | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 22 | 24 | 0.92 |
| Author, Year | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence Generation | Baseline Characte-ristics | Allocation Conceal-ment | Random Housing | Blinding | Random Outcome Assessment | Gross Blinding | Imaging Blinding | Histology Blinding | IHC Blinding | Molecular Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias | |
| Zhu, 2011 [19] | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Sun, 2018 [20] | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Putnova, 2019 [21] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | ND | ND | Unclear Risk | Low Risk | Low Risk |
| Cheng, 2019 [22] | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | ND | Low Risk | ND | Unclear Risk | Unclear Risk | Low Risk | Low Risk |
| Sumarta, 2021 [23] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | ND | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Cheng, 2021 [24] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Yu, 2021 [25] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Wang, 2021 [26] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Guastaldi, 2021 [27] | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | ND | Unclear Risk | ND | ND | Low Risk | Low Risk | Low Risk |
| Gomez, 2021 [28] | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | ND | ND | Low Risk | Low Risk | Low Risk |
| Liu, 2022 [29] | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Chen, 2013 [30] | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk |
| Lu, 2015 [31] | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk |
| Zaki, 2017 [32] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | ND | Unclear Risk | ND | ND | Low Risk | Low Risk | Low Risk |
| Zhang, 2017 [33] | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Kim, 2019 [34] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Zhang, 2019 [35] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Ogasawara, 2020 [36] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Unclear Risk | Unclear Risk | ND | Low Risk | Low Risk | Low Risk |
| Cui, 2020 [37] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
| Wang, 2021 [38] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | ND | Low Risk | Low Risk | Low Risk |
| Köhnke, 2021 [39] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | ND | ND | Low Risk | Low Risk | Low Risk |
| AbuBakr, 2022 [40] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | ND | Unclear Risk | ND | Unclear Risk | Unclear Risk | Low Risk | Low Risk |
| Yang, 2022 [41] | Unclear Risk | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Unclear Risk | Unclear Risk | ND | Unclear Risk | Low Risk | Low Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Shi, J.; Di, W.; Teo, K.Y.W.; Toh, W.S. Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies. Cells 2024, 13, 990. https://doi.org/10.3390/cells13110990
Jiang Y, Shi J, Di W, Teo KYW, Toh WS. Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies. Cells. 2024; 13(11):990. https://doi.org/10.3390/cells13110990
Chicago/Turabian StyleJiang, Yuanyuan, Jiajun Shi, Wenjun Di, Kristeen Ye Wen Teo, and Wei Seong Toh. 2024. "Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies" Cells 13, no. 11: 990. https://doi.org/10.3390/cells13110990







