Advancements in Hematopoietic Stem Cell Gene Therapy: A Journey of Progress for Viral Transduction
Abstract
1. Introduction
2. Gene Delivery in Hematopoietic Stem Cells
2.1. Overview
2.2. Gammaretroviruses
2.3. Lentiviruses
2.4. Genome Editing
3. Advancements in Transduction Technologies
3.1. Viral Vector Engineering
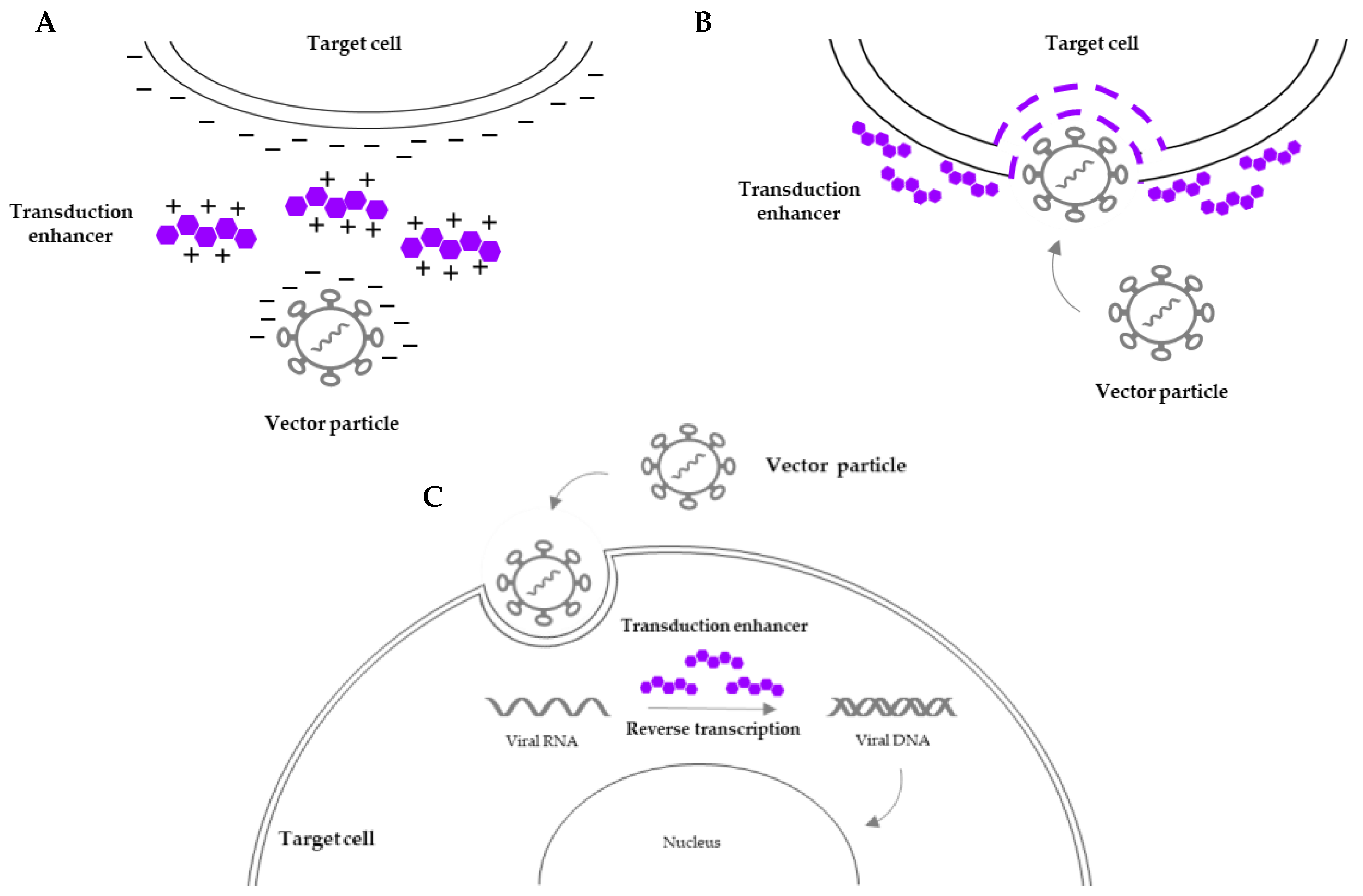
3.2. Transduction Enhancers
| Reagent | Mechanism of Action | Side Effects | Side Effects in Gene Therapy | Clinical Applications in Gene Therapy | References |
|---|---|---|---|---|---|
| Prostaglandin E2 | Improvement of reverse transcription (under investigation) | Nausea, vomiting, diarrhea, abdominal pain | Reduction of HSC clonogenic potential | Hurler syndrome, β-thalassemia | [21,26,28] |
| Protamine sulfate | Lower charge repulsion between the vector and the cell surface | Low blood pressure, allergic reactions, vomiting | Cell toxicity (concentrations higher than 10 µg/mL) | N/A | [33] |
| Poloxamers | Membrane fluidization and reduction in electrostatic barriers | Dehydration, abdominal discomfort | N/A | N/A | [34] |
| LentiBOOST™ | Increased permeability of the target cell surface | N/A | N/A | X-SCID, Artemis-SCID | [35] |
| Vectofusin-1® | Enhanced adhesion and fusion of viral particles to the cell membrane | N/A | N/A | N/A | [38] |
| Rapamycin | Inhibition of mTOR signaling pathway (immunosuppression) | Anemia, increased blood pressure, muscle pain | N/A | N/A | [41,43] |
| Cyclosporin A Cyclosporin H | Inhibition of cyclophilin A (immunosuppression) Inhibition of IFITM3 | Blurred vision, back pain, dizziness, decreased appetite | N/A | N/A | [43,45,46] |
4. Safety Considerations
4.1. Genotoxicity and Leukemias
4.2. Immunogenicity
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Gatti, R.A.; Meuwissen, H.J.; Allen, H.D.; Hong, R.; Good, R.A. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 1968, 292, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.H.; Albertini, R.J.; Joo, P.; Anderson, J.L.; Bortin, M.M. Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet 1968, 2, 1364–1366. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, L.; Manfra, I.; Bonomini, S.; Schifano, C.; Segreto, R.; Monti, A.; Sammarelli, G.; Todaro, G.; Sassi, M.; Bertaggia, I.; et al. Haploidentical hematopoietic stem cell transplantation in adults using the αβTCR/CD19-based depletion of G-CSF-mobilized peripheral blood progenitor cells. Bone Marrow Transplant. 2019, 54, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Hatzimichael, E.; Tuthill, M. Hematopoietic stem cell transplantation. Stem Cells Cloning 2010, 3, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Bartolomae, C.C.; Cesana, D.; Cartier, N.; Aubourg, P.; Ranzani, M.; Cesani, M.; Benedicenti, F.; Plati, T.; Rubagotti, E.; et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood 2011, 117, 5332–5339. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Hanawa, H.; Vandergriff, J.; Kelly, P.; Vanin, E.F.; Nienhuis, A.W. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38− subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000, 7, 183–195. [Google Scholar] [CrossRef][Green Version]
- Cicalese, M.P.; Ferrua, F.; Castagnaro, L.; Pajno, R.; Barzaghi, F.; Giannelli, S.; Dionisio, F.; Brigida, I.; Bonopane, M.; Casiraghi, M.; et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 2016, 128, 45–54. [Google Scholar] [CrossRef]
- Locatelli, F.; Thompson, A.A.; Kwiatkowski, J.L.; Porter, J.B.; Thrasher, A.J.; Hongeng, S.; Sauer, M.G.; Thuret, I.; Lal, A.; Algeri, M.; et al. Betibeglogene Autotemcel Gene Therapy for Non-β0/β0 Genotype β-Thalassemia. N. Engl. J. Med. 2022, 386, 415–427. [Google Scholar] [CrossRef]
- Magrin, E.; Semeraro, M.; Hebert, N.; Joseph, L.; Magnani, A.; Chalumeau, A.; Gabrion, A.; Roudaut, C.; Marouene, J.; Lefrere, F.; et al. Long-term outcomes of lentiviral gene therapy for the β-hemoglobinopathies: The HGB-205 trial. Nat. Med. 2022, 28, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, J.; Breazzano, S. Investor Outlook: Rising from the Ashes; GSK’s European Approval of Strimvelis for ADA-SCID. Hum. Gene Ther. Clin. Dev. 2016, 27, 57–61. [Google Scholar] [CrossRef]
- Horgan, C.; Watts, K.; Ram, D.; Rust, S.; Hutton, R.; Jones, S.; Wynn, R. A retrospective cohort study of Libmeldy (atidarsagene autotemcel) for MLD: What we have accomplished and what opportunities lie ahead. JIMD Rep. 2023, 64, 346–352. [Google Scholar] [CrossRef]
- Schuessler-Lenz, M.; Enzmann, H.; Vamvakas, S. Regulators’ Advice Can Make a Difference: European Medicines Agency Approval of Zynteglo for Beta Thalassemia. Clin. Pharmacol. Ther. 2020, 107, 492–494. [Google Scholar] [CrossRef]
- Zeng, J.; Nguyen, M.A.; Liu, P.; Ferreira da Silva, L.; Lin, L.Y.; Justus, D.G.; Petri, K.; Clement, K.; Porter, S.N.; Verma, A.; et al. Gene editing without ex vivo culture evades genotoxicity in human hematopoietic stem cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lee, B.C.; Gin, A.; Wu, C.; Singh, K.; Grice, M.; Mortlock, R.; Abraham, D.; Fan, X.; Zhou, Y.; AlJanahi, A.; et al. Impact of CRISPR/HDR editing versus lentiviral transduction on long-term engraftment and clonal dynamics of HSPCs in rhesus macaques. Cell Stem Cell 2024, 31, 455–466. [Google Scholar] [CrossRef]
- Finkelshtein, D.; Werman, A.; Novick, D.; Barak, S.; Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2013, 110, 7306–7311. [Google Scholar] [CrossRef] [PubMed]
- Amirache, F.; Lévy, C.; Costa, C.; Mangeot, P.E.; Torbett, B.E.; Wang, C.X.; Nègre, D.; Cosset, F.L.; Verhoeyen, E. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 2014, 123, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Girard-Gagnepain, A.; Amirache, F.; Costa, C.; Lévy, C.; Frecha, C.; Fusil, F.; Nègre, D.; Lavillette, D.; Cosset, F.L.; Verhoeyen, E. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood 2014, 124, 1221–1231. [Google Scholar] [CrossRef]
- Verhoeyen, E.; Wiznerowicz, M.; Olivier, D.; Izac, B.; Trono, D.; Dubart-Kupperschmitt, A.; Cosset, F.L. Novel lentiviral vectors displaying “early-acting cytokines” selectively promote survival and transduction of NOD/SCID repopulating human hematopoietic stem cells. Blood 2005, 106, 3386–3395. [Google Scholar] [CrossRef]
- Glimm, H.; Oh, I.H.; Eaves, C.J. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G2/M transit and do not reenter G0. Blood 2000, 96, 4185–4193. [Google Scholar] [CrossRef]
- Valeri, E.; Unali, G.; Piras, F.; Abou-Alezz, M.; Pais, G.; Benedicenti, F.; Lidonnici, M.R.; Cuccovillo, I.; Castiglioni, I.; Arévalo, S.; et al. Removal of innate immune barriers allows efficient transduction of quiescent human hematopoietic stem cells. Mol. Ther. 2024, 32, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Heffner, G.C.; Bonner, M.; Christiansen, L.; Pierciey, F.J.; Campbell, D.; Smurnyy, Y.; Zhang, W.; Hamel, A.; Shaw, S.; Lewis, G.; et al. Prostaglandin E2 Increases Lentiviral Vector Transduction Efficiency of Adult Human Hematopoietic Stem and Progenitor Cells. Mol. Ther. 2018, 26, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Zonari, E.; Desantis, G.; Petrillo, C.; Boccalatte, F.E.; Lidonnici, M.R.; Kajaste-Rudnitski, A.; Aiuti, A.; Ferrari, G.; Naldini, L.; Gentner, B. Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy. Stem Cell Rep. 2017, 8, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Nassehi, T.; Drysdale, C.M.; Gamer, J.; Yapundich, M.; Demirci, S.; Haro-Mora, J.J.; Leonard, A.; Hsieh, M.M.; Tisdale, J.F. High-Efficiency Lentiviral Transduction of Human CD34+ Cells in High-Density Culture with Poloxamer and Prostaglandin E2. Mol. Ther. Methods Clin. Dev. 2019, 13, 187–196. [Google Scholar] [CrossRef]
- Masiuk, K.E.; Zhang, R.; Osborne, K.; Hollis, R.P.; Campo-Fernandez, B.; Kohn, D.B. PGE2 and Poloxamer Synperonic F108 Enhance Transduction of Human HSPCs with a β-Globin Lentiviral Vector. Mol. Ther. Methods Clin. Dev. 2019, 13, 390–398. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, Y.S.; Wielgosz, M.M.; Ferrara, F.; Ma, Z.; Condori, J.; Palmer, L.E.; Zhao, X.; Kang, G.; Rawlings, D.J.; et al. Optimizing lentiviral vector transduction of hematopoietic stem cells for gene therapy. Gene Ther. 2020, 27, 545–556. [Google Scholar] [CrossRef]
- Poletti, V.; Montepeloso, A.; Pellin, D.; Biffi, A. Prostaglandin E2 as transduction enhancer affects competitive engraftment of human hematopoietic stem and progenitor cells. Mol. Ther. Methods Clin. Dev. 2023, 31, 101131. [Google Scholar] [CrossRef]
- Cutler, C.; Multani, P.; Robbins, D.; Kim, H.T.; Le, T.; Hoggatt, J.; Pelus, L.M.; Desponts, C.; Chen, Y.B.; Rezner, B.; et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 2013, 122, 3074–3081. [Google Scholar] [CrossRef]
- Gentner, B.; Tucci, F.; Galimberti, S.; Fumagalli, F.; De Pellegrin, M.; Silvani, P.; Camesasca, C.; Pontesilli, S.; Darin, S.; Ciotti, F.; et al. Hematopoietic Stem- and Progenitor-Cell Gene Therapy for Hurler Syndrome. N. Engl. J. Med. 2021, 385, 1929–1940. [Google Scholar] [CrossRef]
- Leonard, A.; Tisdale, J.F. A pause in gene therapy: Reflecting on the unique challenges of sickle cell disease. Mol. Ther. 2021, 29, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; DeBaun, M.R. Leukemia after gene therapy for sickle cell disease: Insertional mutagenesis, busulfan, both, or neither. Blood 2021, 138, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: First Regulatory Approvals for CRISPR-Cas9 Therapeutic Gene Editing for Sickle Cell Disease and Transfusion-Dependent β-Thalassemia. Med. Sci. Monit. 2024, 30, e944204. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Rosinski, M.; Morgan, J.R.; Yarmush, M.L. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys. J. 2004, 86, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Cornetta, K.; Anderson, W.F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: Implications for human gene therapy. J. Virol. Methods 1989, 23, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Anastasov, N.; Höfig, I.; Mall, S.; Krackhardt, A.M.; Thirion, C. Optimized Lentiviral Transduction Protocols by Use of a Poloxamer Enhancer, Spinoculation, and scFv-Antibody Fusions to VSV-G. Methods Mol. Biol. 2016, 1448, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Hauber, I.; Beschorner, N.; Schrödel, S.; Chemnitz, J.; Kröger, N.; Hauber, J.; Thirion, C. Improving Lentiviral Transduction of CD34+ Hematopoietic Stem and Progenitor Cells. Hum. Gene Ther. Methods 2018, 29, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.W.; León-Rico, D.; Ferreira, C.B.; Buckland, K.F.; Santilli, G.; Armant, M.A.; Schambach, A.; Cavazza, A.; Thrasher, A.J. Enhancing Lentiviral and Alpharetroviral Transduction of Human Hematopoietic Stem Cells for Clinical Application. Mol. Ther. Methods Clin. Dev. 2019, 14, 134–147. [Google Scholar] [CrossRef]
- Delville, M.; Soheili, T.; Bellier, F.; Durand, A.; Denis, A.; Lagresle-Peyrou, C.; Cavazzana, M.; Andre-Schmutz, I.; Six, E. A Nontoxic Transduction Enhancer Enables Highly Efficient Lentiviral Transduction of Primary Murine T Cells and Hematopoietic Stem Cells. Mol. Ther. Methods Clin. Dev. 2018, 10, 341–347. [Google Scholar] [CrossRef]
- Fenard, D.; Ingrao, D.; Seye, A.; Buisset, J.; Genries, S.; Martin, S.; Kichler, A.; Galy, A. Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells. Mol. Ther. Nucleic Acids 2013, 2, e90. [Google Scholar] [CrossRef]
- Radek, C.; Bernadin, O.; Drechsel, K.; Cordes, N.; Pfeifer, R.; Sträßer, P.; Mormin, M.; Gutierrez-Guerrero, A.; Cosset, F.L.; Kaiser, A.D.; et al. Vectofusin-1 Improves Transduction of Primary Human Cells with Diverse Retroviral and Lentiviral Pseudotypes, Enabling Robust, Automated Closed-System Manufacturing. Hum. Gene Ther. 2019, 30, 1477–1493. [Google Scholar] [CrossRef]
- Piovan, C.; Marin, V.; Scavullo, C.; Corna, S.; Giuliani, E.; Bossi, S.; Galy, A.; Fenard, D.; Bordignon, C.; Rizzardi, G.P.; et al. Vectofusin-1 Promotes RD114-TR-Pseudotyped Lentiviral Vector Transduction of Human HSPCs and T Lymphocytes. Mol. Ther. Methods Clin. Dev. 2017, 5, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Sather, B.D.; Wang, X.; Adair, J.; Khan, I.; Singh, S.; Lang, S.; Adams, A.; Curinga, G.; Kiem, H.P.; et al. Rapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cells. Blood 2014, 124, 913–923. [Google Scholar] [CrossRef]
- Luo, Y.; Li, L.; Zou, P.; Wang, J.; Shao, L.; Zhou, D.; Liu, L. Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence. Transplantation 2014, 97, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, C.; Cesana, D.; Piras, F.; Bartolaccini, S.; Naldini, L.; Montini, E.; Kajaste-Rudnitski, A. Cyclosporin a and rapamycin relieve distinct lentiviral restriction blocks in hematopoietic stem and progenitor cells. Mol. Ther. 2015, 23, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.; Kumkhaek, C.; Hsieh, M.M.; Donahue, R.E.; Tisdale, J.F.; Uchida, N. TRIM5α variations influence transduction efficiency with lentiviral vectors in both human and rhesus CD34+ cells in vitro and in vivo. Mol. Ther. 2014, 22, 348–358. [Google Scholar] [CrossRef]
- Petrillo, C.; Thorne, L.G.; Unali, G.; Schiroli, G.; Giordano, A.M.S.; Piras, F.; Cuccovillo, I.; Petit, S.J.; Ahsan, F.; Noursadeghi, M.; et al. Cyclosporine H Overcomes Innate Immune Restrictions to Improve Lentiviral Transduction and Gene Editing in Human Hematopoietic Stem Cells. Cell Stem Cell 2018, 23, 820–832.e9. [Google Scholar] [CrossRef] [PubMed]
- Olender, L.; Bujanover, N.; Sharabi, O.; Goldstein, O.; Gazit, R. Cyclosporine H Improves the Multi-Vector Lentiviral Transduction of Murine Haematopoietic Progenitors and Stem Cells. Sci. Rep. 2020, 10, 1812. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.L.; Fraser, C.C.; Cavazzana-Calvo, M.; et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef]
- Marshall, E. Gene therapy. Second child in French trial is found to have leukemia. Science 2003, 299, 320. [Google Scholar] [CrossRef]
- Kaiser, J. RAC Hears a Plea for Resuming Trials, Despite Cancer Risk. Science 2003, 299, 991. Available online: https://www.jstor.org/stable/i371100 (accessed on 15 April 2024). [CrossRef] [PubMed]
- Ott, M.G.; Schmidt, M.; Schwarzwaelder, K.; Stein, S.; Siler, U.; Koehl, U.; Glimm, H.; Kühlcke, K.; Schilz, A.; Kunkel, H.; et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006, 12, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Boztug, K.; Schmidt, M.; Schwarzer, A.; Banerjee, P.P.; Díez, I.A.; Dewey, R.A.; Böhm, M.; Nowrouzi, A.; Ball, C.R.; Glimm, H.; et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010, 363, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Bastone, A.L.; Dziadek, V.; John-Neek, P.; Mansel, F.; Fleischauer, J.; Agyeman-Duah, E.; Schaudien, D.; Dittrich-Breiholz, O.; Schwarzer, A.; Schambach, A.; et al. Development of an in vitro genotoxicity assay to detect retroviral vector-induced lymphoid insertional mutants. Mol. Ther. Methods Clin. Dev. 2023, 30, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Modlich, U.; Bohne, J.; Schmidt, M.; von Kalle, C.; Knöss, S.; Schambach, A.; Baum, C. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 2006, 108, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Modlich, U.; Navarro, S.; Zychlinski, D.; Maetzig, T.; Knoess, S.; Brugman, M.H.; Schambach, A.; Charrier, S.; Galy, A.; Thrasher, A.J.; et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009, 17, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.J.; Boztug, K.; Paruzynski, A.; Witzel, M.; Schwarzer, A.; Rothe, M.; Modlich, U.; Beier, R.; Göhring, G.; Steinemann, D.; et al. Gene therapy for Wiskott-Aldrich syndrome—Long-term efficacy and genotoxicity. Sci. Transl. Med. 2014, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, A.; Talbot, S.R.; Selich, A.; Morgan, M.; Schott, J.W.; Dittrich-Breiholz, O.; Bastone, A.L.; Weigel, B.; Ha, T.C.; Dziadek, V.; et al. Predicting genotoxicity of viral vectors for stem cell gene therapy using gene expression-based machine learning. Mol. Ther. 2021, 29, 3383–3397. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Haapaniemi, E.; Botla, S.; Persson, J.; Schmierer, B.; Taipale, J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018, 24, 927–930. [Google Scholar] [CrossRef]
- Charlesworth, C.T.; Hsu, I.; Wilkinson, A.C.; Nakauchi, H. Immunological barriers to haematopoietic stem cell gene therapy. Nat. Rev. Immunol. 2022, 22, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K. With the Pricing Situation ‘Untenable’ in Europe, Bluebird Will Wind down Its Operations in the ‘Broken’ Market. Fierce Pharma. 2021. Available online: https://www.fiercepharma.com/pharma/situation-untenable-bluebird-will-wind-down-its-operations-broken-europe (accessed on 16 April 2024).
- Cruz, L.J.; Rezaei, S.; Grosveld, F.; Philipsen, S.; Eich, C. Nanoparticles targeting hematopoietic stem and progenitor cells: Multimodal carriers for the treatment of hematological diseases. Front. Genome Ed. 2022, 4, 1030285. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, V.; Ferrari, S.; Beretta, S.; Asperti, C.; Albano, L.; Annoni, A.; Gaddoni, C.; Varesi, A.; Soldi, M.; Cuomo, A.; et al. Lipid nanoparticles allow efficient and harmless ex vivo gene editing of human hematopoietic cells. Blood 2023, 142, 812–826. [Google Scholar] [CrossRef] [PubMed]
| Product Name: Generic (Trade) | Applications | Manufacturer | Mechanism of Action | Approval Agency (Year) |
|---|---|---|---|---|
| STRIMVELIS® | ADA-SCID | Orchard Therapeutics | ADA gene addition via gamma retrovirus | EMA (2016) |
| Betibeglogene autotemcel (ZYNTEGLO™) | Transfusion-dependent Β-thalassemia (TDT) | bluebird bio, Inc. | βA-T87Q-globin gene addition via lentivirus | EMA (2019) * FDA (2022) |
| Atidarsagene autotemcel (LIBMELDY®) | Metachromatic leukodystrophy (MLD) | Orchard Therapeutics | ARSA gene addition via lentivirus | EMA (2020) FDA (2024) |
| Lovotibeglogene autotemcel (LYFGENIA™) | Sickle cell disease (SCD) | bluebird bio, Inc. | βA-T87Q-globin gene addition via lentivirus | FDA (2023) |
| Exagamglogene autotemcel (CASGEVY™) | TDT, SCD | Vertex Pharmaceuticals CRISPR Therapeutics | CRISPR/Cas9 technology | EMA (2023) FDA (2024) |
| Elivaldogene autotemcel (SKYSONA®) | CALD | bluebird bio, Inc. | ABCD1 gene addition via lentivirus | EMA (2021) * FDA (2022) |
| Clinical Trial Registry Number | Disease | Intervention | Sponsor | Phase |
|---|---|---|---|---|
| NCT04797260 | RAG1-SCID | Autologous CD34+ cells transduced with the pCCL.MND.coRAG1.wpre LV | Leiden University Medical Center | I/II |
| NCT05071222 | Artemis-SCID | Autologous CD34+ cells transduced with the G2ARTE LV expressing the DCLRE1C cDNA | Assistance Publique—Hôpitaux de Paris/Genethon | I/II |
| NCT02559830 | MLD, ALD | Autologous CD34+ cells transduced with a LV encoding the human ARSA(for MLD)/ABCD1(for ALD) cDNA | Shenzhen Second People’s Hospital | I/II |
| NCT05860595 | TDT | Autologous CD34+ cells transduced with the βA-T87Q-globin gene LV (KL003) | Institute of Hematology and Blood Diseases Hospital, China/Kanglin Biotech | N/A |
| NCT05762510 | TDT | Autologous CD34+ cells transduced with the GMCN-508B (LentiRed) LV | First Affiliated Hospital of Guangxi Medical University | Early I |
| NCT05432310 | ADA-SCID | Autologous CD34+ cells transduced with the EFS-ADA LV encoding the ADA enzyme | University of California, Los Angeles | I/II |
| NCT06149403 | Hurler syndrome | Autologous CD34+ cells transduced with LV encoding the human IDUA gene | Orchard Therapeutics | III |
| NCT05265767 | Hemophilia A | Autologous CD34+ cells transduced with LV encoding a novel coagulation factor VIII transgene | Christian Medical College, Vellore, India | I |
| NCT03818763 | Hemophilia A | Autologous CD34+ cells transduced with LV encoding the ITGA2B gene promoter for ectopic expression of human B-domain-deleted factor VIII | Medical College of Wisconsin | I |
| NCT06155500 | SCD | Observational: long-term follow-up of patients treated with CRISPR/Cas9-edited HSPCs from NCT04443907 | Novartis Pharmaceuticals | I |
| NCT01306019 | X-SCID | Autologous CD34+ HSC with VSV-G pseudotyped LV CL20- 4i-EF1alpha-hgammac-OPT | National Institute of Allergy and Infectious Diseases (NIAID) | I/II |
| NCT03538899 | Artemis-SCID | Autologous CD34+ cells transduced with LV (AProArt) encoding the corrected DCLRE1C gene | University of California, San Francisco | I/II |
| NCT05757245 | TDT | Autologous CD34+ cells transduced with GMCN-508A LV | First Affiliated Hospital of Guangxi Medical University | I |
| 2014-000274-20 | WAS | Observational: long-term follow-up of patients treated with w1.6_hWASP_WPRE (VSVg) LV transduced autologous HSCs | Genethon | II |
| 2019-004266-18 | TDT | Observational: long-term follow-up of patients treated with βA-T87Q LV (LentiGlobin BB305) transduced autologous HSCs | bluebird bio, Inc. | III |
| 2020-000517-33 | Leukocyte adhesion deficiency I | Autologous CD34+ cells transduced with LV encoding the ITGB2 gene | Rocket Pharmaceuticals, Inc. | I/II |
| 2017-001366-14 | TDT | Observational: long-term follow-up of patients treated with GSK2696277 | GlaxoSmithKline Research and Development | II |
| 2017-002430-23 | Hurler syndrome | Autologous CD34+ cells transduced with IDUA LV encoding the human α-L-iduronidase gene | Ospedale San Raffaele | I/II |
| 2018-001404-11 | Glioblastoma multiforme | Autologous CD34+ cells transduced with LV encoding the interferon-α2 gene | Genenta Science S.r.l | I/IIa |
| 2013-002245-11 | Hemoglobinopathies | Observational: long-term follow-up of patients treated with LentiGlobin BB305 Drug Product | bluebird bio, Inc. | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giommetti, A.; Papanikolaou, E. Advancements in Hematopoietic Stem Cell Gene Therapy: A Journey of Progress for Viral Transduction. Cells 2024, 13, 1039. https://doi.org/10.3390/cells13121039
Giommetti A, Papanikolaou E. Advancements in Hematopoietic Stem Cell Gene Therapy: A Journey of Progress for Viral Transduction. Cells. 2024; 13(12):1039. https://doi.org/10.3390/cells13121039
Chicago/Turabian StyleGiommetti, Aurora, and Eleni Papanikolaou. 2024. "Advancements in Hematopoietic Stem Cell Gene Therapy: A Journey of Progress for Viral Transduction" Cells 13, no. 12: 1039. https://doi.org/10.3390/cells13121039
APA StyleGiommetti, A., & Papanikolaou, E. (2024). Advancements in Hematopoietic Stem Cell Gene Therapy: A Journey of Progress for Viral Transduction. Cells, 13(12), 1039. https://doi.org/10.3390/cells13121039


.png)



