The Multiple Roles of Lactate in the Skeletal Muscle
Abstract
:1. Lactate as a Metabolite and Signalling Molecule
2. Lactate in Muscle Renewal
3. Protein Lactylation in Skeletal Muscle
4. Lactate and Physical Exercise
5. Lactate in the Skeletal Muscle Diseases
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, A.; Singh, A.; Phogat, J.; Dahuja, A.; Dabur, R. Magnoflorine prevent the skeletal muscle atrophy via Akt/mTOR/FoxO signal pathway and increase slow-MyHC production in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 267, 113510. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate shuttles in Nature. Biochem. Soc. Trans. 2002, 30, 258–264. [Google Scholar] [CrossRef]
- Nalbandian, M.; Radak, Z.; Takeda, M. Lactate Metabolism and Satellite Cell Fate. Front. Physiol. 2020, 11, 610983. [Google Scholar] [CrossRef]
- Everse, J.; Kaplan, N.O. Lactate dehydrogenases: Structure and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1973, 37, 61–133. [Google Scholar]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family—Role and regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hussien, R.; Oommen, S.; Gohil, K.; Brooks, G.A. Lactate sensitive transcription factor network in L6 cells: Activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007, 21, 2602–2612. [Google Scholar] [CrossRef]
- Brooks, G.A. Cell–cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2022, 600, 1229–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Mahieu, N.G.; Huang, X.; Singh, M.; A Crawford, P.; Johnson, S.L.; Gross, R.W.; Schaefer, J.; Patti, G.J. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 2016, 12, 937–943. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hussien, R.; Brooks, G.A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: Evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1237–E1244. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [CrossRef]
- Bergman, B.C.; Tsvetkova, T.; Lowes, B.; Wolfel, E.E. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J. Physiol. 2009, 587, 2087–2099. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; Horning, M.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Brooks, G.A. Lactate: Brain Fuel in Human Traumatic Brain Injury: A Comparison with Normal Healthy Control Subjects. J. Neurotrauma 2015, 32, 820–832. [Google Scholar] [CrossRef]
- Emhoff, C.-A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol. 2013, 114, 297–306. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, F.; Afonso, J.; Costa, M.; Granja, S. Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy. Front. Oncol. 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Llibre, A.; Lee, W.; Mauro, C. Understanding lactate sensing and signalling. Trends Endocrinol. Metab. 2022, 33, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology 2017, 32, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate Inhibits Lipolysis in Fat Cells through Activation of an Orphan G-protein-coupled Receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y. Lactate: A multifunctional signaling molecule. Yeungnam Univ. J. Med. 2021, 38, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Ouyang, X.; Farooq, A.; Ghani, A.; Ahsan, K.; Guerra, M.; Mehal, W.Z. Activation of N-methyl-d-aspartate receptor downregulates inflammasome activity and liver inflammation via a β-arrestin-2 pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G732–G740. [Google Scholar] [CrossRef]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate Reduces Liver and Pancreatic Injury in Toll-Like Receptor– and Inflammasome-Mediated Inflammation via GPR81-Mediated Suppression of Innate Immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Tsai, C.-H.; Pucino, V.; Ho, P.-C.; Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 2021, 21, 151–161. [Google Scholar] [CrossRef]
- Nolt, B.; Tu, F.; Wang, X.; Ha, T.; Winter, R.; Williams, D.L.; Li, C. Lactate and Immunosuppression in Sepsis. Shock 2018, 49, 120–125. [Google Scholar] [CrossRef]
- Pucino, V.; Bombardieri, M.; Pitzalis, C.; Mauro, C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur. J. Immunol. 2017, 47, 14–21. [Google Scholar] [CrossRef]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef] [PubMed]
- Nasi, A.; Fekete, T.; Krishnamurthy, A.; Snowden, S.; Rajnavölgyi, E.; Catrina, A.I.; Wheelock, C.E.; Vivar, N.; Rethi, B. Dendritic Cell Reprogramming by Endogenously Produced Lactic Acid. J. Immunol. 2013, 191, 3090–3099. [Google Scholar] [CrossRef]
- Peter, K.; Rehli, M.; Singer, K.; Renner-Sattler, K.; Kreutz, M. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 2015, 457, 412–418. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Husain, Z.; Huang, Y.; Seth, P.; Sukhatme, V.P. Tumor-Derived Lactate Modifies Antitumor Immune Response: Effect on Myeloid-Derived Suppressor Cells and NK Cells. J. Immunol. 2013, 191, 1486–1495. [Google Scholar] [CrossRef]
- Hunt, T.K.; Aslam, R.S.; Beckert, S.; Wagner, S.; Ghani, Q.P.; Hussain, M.Z.; Roy, S.; Sen, C.K. Aerobically Derived Lactate Stimulates Revascularization and Tissue Repair via Redox Mechanisms. Antioxidants Redox Signal. 2007, 9, 1115–1124. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.-P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakatani, M.; Ito, T.; Matsui, Y.; Ando, K.; Suda, Y.; Ohashi, K.; Yokoyama, S.; Goto, K. Activation of Lactate Receptor Positively Regulates Skeletal Muscle Mass in Mice. Physiol. Res. 2023, 72, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Nordström, F.; Liegnell, R.; Apró, W.; Blackwood, S.J.; Katz, A.; Moberg, M. The lactate receptor GPR81 is predominantly expressed in type II human skeletal muscle fibers: Potential for lactate autocrine signaling. Am. J. Physiol. Cell Physiol. 2023, 324, C477–C487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, L.; Sun, J.; Qu, Y.; Chen, M. The Role of cAMP-PKA Pathway in Lactate-Induced Intramuscular Triglyceride Accumulation and Mitochondria Content Increase in Mice. Front. Physiol. 2021, 12, 709135. [Google Scholar] [CrossRef]
- Guilhot, C.; Catenacci, M.; Lofaro, S.; Rudnicki, M.A. The satellite cell in skeletal muscle: A story of heterogeneity. Curr. Top. Dev. Biol. 2024, 158, 15–51. [Google Scholar] [PubMed]
- Pala, F.; Di Girolamo, D.; Mella, S.; Yennek, S.; Chatre, L.; Ricchetti, M.; Tajbakhsh, S. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci. 2018, 131, jcs212977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Muri, J.; Fitzgerald, G.; Gorski, T.; Gianni-Barrera, R.; Masschelein, E.; D’hulst, G.; Gilardoni, P.; Turiel, G.; Fan, Z.; et al. Endothelial Lactate Controls Muscle Regeneration from Ischemia by Inducing M2-like Macrophage Polarization. Cell Metab. 2020, 31, 1136–1153.e7. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Shibasaki, A.; Naka, A.; Saito, H.; Iida, K. Lactate Promotes Myoblast Differentiation and Myotube Hypertrophy via a Pathway Involving MyoD In Vitro and Enhances Muscle Regeneration In Vivo. Int. J. Mol. Sci. 2018, 19, 3649. [Google Scholar] [CrossRef] [PubMed]
- Washington, T.A.; Brown, L.; Smith, D.A.; Davis, G.; Baum, J.; Bottje, W. Monocarboxylate transporter expression at the onset of skeletal muscle regeneration. Physiol. Rep. 2013, 1, e00075. [Google Scholar] [CrossRef] [PubMed]
- Willkomm, L.; Schubert, S.; Jung, R.; Elsen, M.; Borde, J.; Gehlert, S.; Suhr, F.; Bloch, W. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res. 2014, 12, 742–753. [Google Scholar] [CrossRef]
- Ohno, Y.; Ando, K.; Ito, T.; Suda, Y.; Matsui, Y.; Oyama, A.; Kaneko, H.; Yokoyama, S.; Egawa, T.; Goto, K. Lactate Stimulates a Potential for Hypertrophy and Regeneration of Mouse Skeletal Muscle. Nutrients 2019, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Willkomm, L.; Gehlert, S.; Jacko, D.; Schiffer, T.; Bloch, W. p38 MAPK activation and H3K4 trimethylation is decreased by lactate in vitro and high intensity resistance training in human skeletal muscle. PLoS ONE 2017, 12, e0176609. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Jiang, D.; Dey, T.; Mathews, Q.; Liu, R.-M.; Liu, G. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 115–125. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wu, G.; Liu, K.; Chen, Q.; Tao, J.; Liu, H.; Shen, M. Lactate promotes myogenesis via activating H3K9 lactylation-dependent up-regulation of Neu2 expression. J. Cachex-Sarcopenia Muscle 2023, 14, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yue, X.; Sun, W.; Zhou, Q.; Chang, C.; Gong, W.; Feng, J.; Li, X.; Zhan, R.; Mo, K.; et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci. Adv. 2023, 9, eadg4993. [Google Scholar] [CrossRef] [PubMed]
- Maschari, D.; Saxena, G.; Law, T.D.; Walsh, E.; Campbell, M.C.; A Consitt, L. Lactate-induced lactylation in skeletal muscle is associated with insulin resistance in humans. Front. Physiol. 2022, 13, 951390. [Google Scholar] [CrossRef] [PubMed]
- Bouzakri, K.; Roques, M.; Gual, P.; Espinosa, S.; Guebre-Egziabher, F.; Riou, J.-P.; Laville, M.; Le Marchand-Brustel, Y.; Tanti, J.-F.; Vidal, H. Reduced Activation of Phosphatidylinositol-3 Kinase and Increased Serine 636 Phosphorylation of Insulin Receptor Substrate-1 in Primary Culture of Skeletal Muscle Cells from Patients With Type 2 Diabetes. Diabetes 2003, 52, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Tsiani, E. Attenuation of Free Fatty Acid-Induced Muscle Insulin Resistance by Rosemary Extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Hartogh, D.J.D.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010, 199, 499–508. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Mikami, T. Exercise-Induced Lactate Release Mediates Mitochondrial Biogenesis in the Hippocampus of Mice via Monocarboxylate Transporters. Front. Physiol. 2021, 12, 736905. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, E.; Park, J.; Kim, J.; Tanaka, M.; Choi, J. Physiological significance of elevated levels of lactate by exercise training in the brain and body. J. Biosci. Bioeng. 2023, 135, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.M.; Rajasekaran, S.; Thomsen, T.W.; Peterson, A.R. Lactate: Friend or Foe. PM&R 2016, 8, S8–S15. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I.; Kowalchuk, J.M.; Heigenhauser, G.J.F. Applying physicochemical principles to skeletal muscle acid-base status. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R891-4–R904-10. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef]
- Yang, W.H.; Park, H.; Grau, M.; Heine, O. Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes? Int. J. Environ. Res. Public Health 2020, 17, 5470. [Google Scholar] [CrossRef] [PubMed]
- Iepsen, U.W.; Plovsing, R.R.; Tjelle, K.; Foss, N.B.; Meyhoff, C.S.; Ryrsø, C.K.; Berg, R.M.G.; Secher, N.H. The role of lactate in sepsis and COVID-19: Perspective from contracting skeletal muscle metabolism. Exp. Physiol. 2022, 107, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef]
- Mächler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; Von Faber-Castell, A.; Kaelin, V.; Zuend, M.; San Martín, A.; Romero-Gómez, I.; et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016, 23, 94–102. [Google Scholar] [CrossRef]
- Kasparov, S. Are Astrocytes the Pressure-Reservoirs of Lactate in the Brain? Cell Metab. 2016, 23, 1–2. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, M.; Wang, S.; Chen, L.; Li, Z.; Li, C.; Cao, P.; Chen, Y. Lactate Is a Key Mediator That Links Obesity to Insulin Resistance via Modulating Cytokine Production From Adipose Tissue. Diabetes 2022, 71, 637–652. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Kim, Y.-B.; Lee, F.N.; Zabolotny, J.M.; Kahn, B.B.; Youn, J.H. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E233–E240. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Rossiter, H.B.; Khamoui, A.V. Distinct glycolytic pathway regulation in liver, tumour and skeletal muscle of mice with cancer cachexia. Cell Biochem. Funct. 2021, 39, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, M.; Gamberi, T.; Magherini, F.; Fiaschi, T. A Metabolic Change towards Fermentation Drives Cancer Cachexia in Myotubes. Biomedicines 2021, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, M.; Gamberi, T.; Garella, R.; Magherini, F.; Squecco, R.; Fiaschi, T. Pyruvate prevents the onset of the cachectic features and metabolic alterations in myotubes downregulating STAT3 signaling. FASEB J. 2022, 36, e22598. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, R.; Wazir, J.; Lin, K.; Song, S.; Li, L.; Pu, W.; Zhao, C.; Wang, Y.; Su, Z.; et al. 2-Deoxy-D-glucose Alleviates Cancer Cachexia-Induced Muscle Wasting by Enhancing Ketone Metabolism and Inhibiting the Cori Cycle. Cells 2022, 11, 2987. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Cui, Q.; Guo, B.; Ding, W.; Liu, J.; Quan, L.; Li, X.; Xie, P.; Jin, L.; et al. Activation of GPR81 by lactate drives tumour-induced cachexia. Nat. Metab. 2024, 6, 708–723. [Google Scholar] [CrossRef]
- Wu, Y.; Weber, J.; Vladutiu, G.; Tarnopolsky, M. Six novel mutations in the myophosphorylase gene in patients with McArdle disease and a family with pseudo-dominant inheritance pattern. Mol. Genet. Metab. 2011, 104, 587–591. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Ogborn, D.I.; Mocellin, N.J.; Schlattner, U.; Tarnopolsky, M.A. Monocarboxylate transporters and mitochondrial creatine kinase protein content in McArdle disease. Mol. Genet. Metab. 2013, 108, 259–262. [Google Scholar] [CrossRef]
- Cuff, M.A.; Shirazi-Beechey, S.P. The Human Monocarboxylate Transporter, MCT1: Genomic Organization and Promoter Analysis. Biochem. Biophys. Res. Commun. 2002, 292, 1048–1056. [Google Scholar] [CrossRef]
- van Hasselt, P.M.; Ferdinandusse, S.; Monroe, G.R.; Ruiter, J.P.; Turkenburg, M.; Geerlings, M.J.; Duran, K.; Harakalova, M.; van der Zwaag, B.; Monavari, A.A.; et al. Monocarboxylate Transporter 1 Deficiency and Ketone Utilization. N. Engl. J. Med. 2014, 371, 1900–1907. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Lewis, B.; Greed, L.; Meili, D.; Flier, A.; Yamamoto, R.; Bilić, K.; Till, C.; Sass, J.O. Heterozygous Monocarboxylate Transporter 1 (MCT1, SLC16A1) Deficiency as a Cause of Recurrent Ketoacidosis. JIMD Rep. 2016, 29, 33–38. [Google Scholar]
- Merezhinskaya, N.; Fishbein, W.N.; Davis, J.I.; Foellmer, J.W. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 2000, 23, 90–97. [Google Scholar] [CrossRef]
- Ulrich, C.M.; Robien, K.; McLeod, H.L. Cancer pharmacogenetics: Polymorphisms, pathways and beyond. Nat. Rev. Cancer 2003, 3, 912–920. [Google Scholar] [CrossRef]
- Fei, F.; Guo, X.; Chen, Y.; Liu, X.; Tu, J.; Xing, J.; Chen, Z.; Ji, J.; He, X. Polymorphisms of monocarboxylate transporter genes are associated with clinical outcomes in patients with colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 1095–1102. [Google Scholar] [CrossRef]
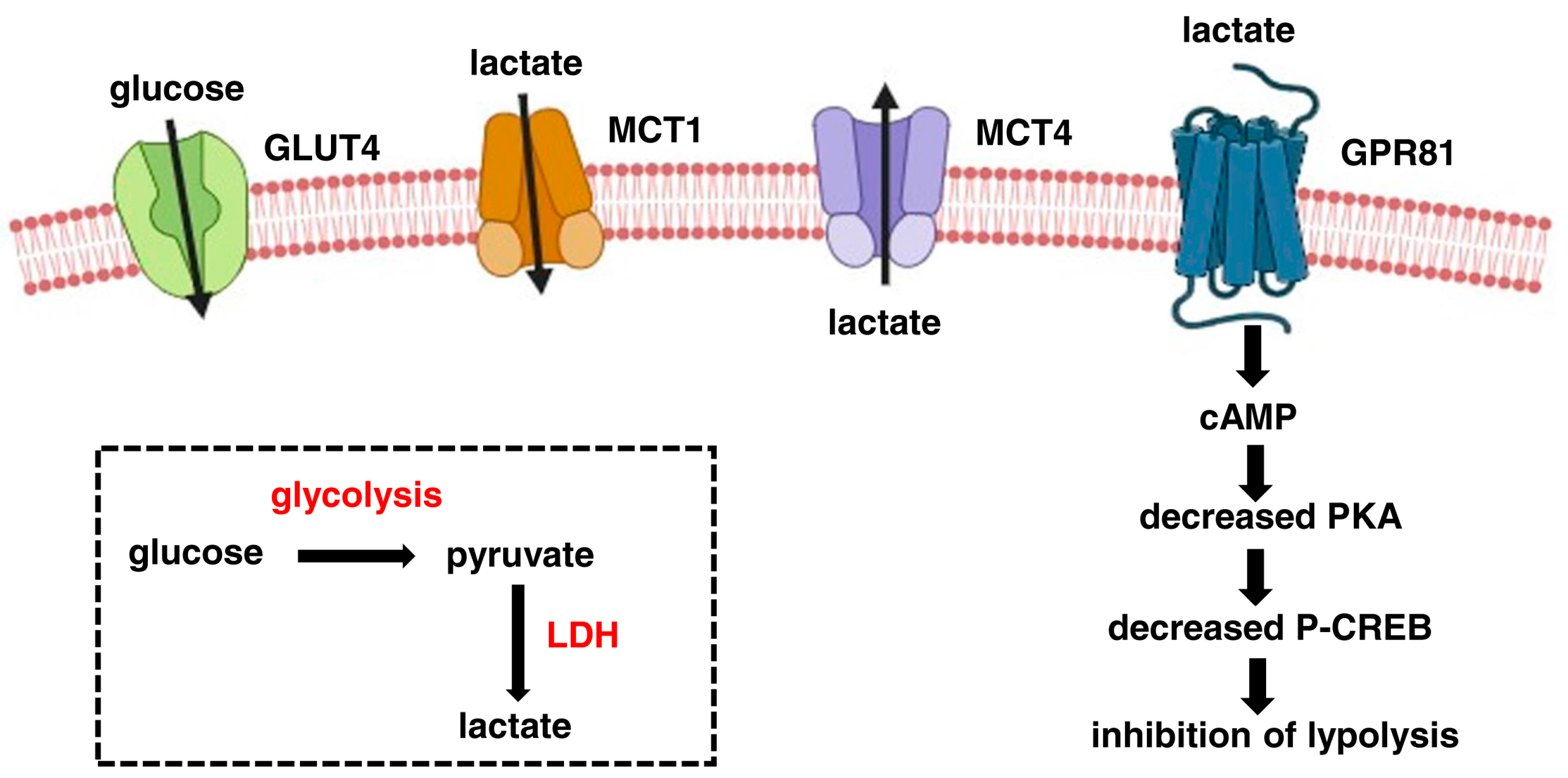
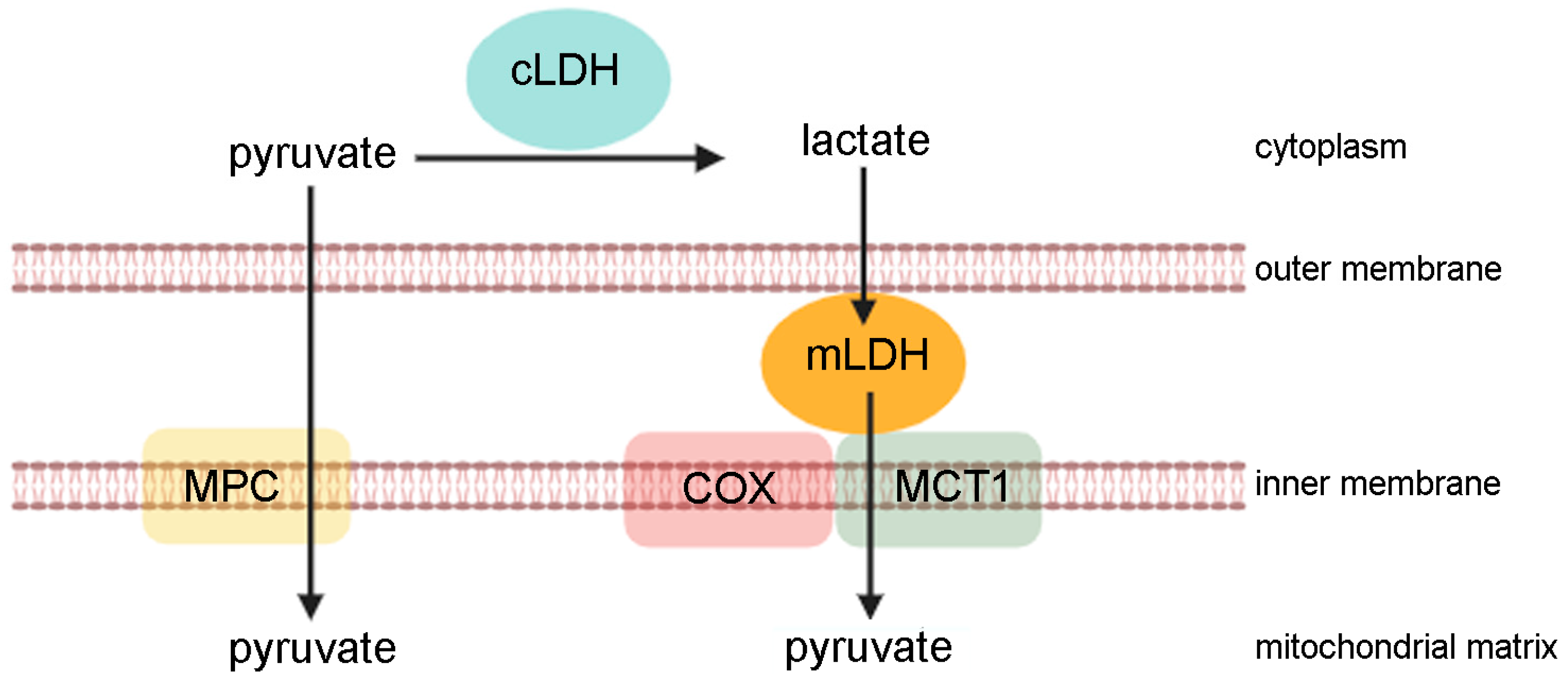
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoloni, B.; Mannelli, M.; Gamberi, T.; Fiaschi, T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells 2024, 13, 1177. https://doi.org/10.3390/cells13141177
Bartoloni B, Mannelli M, Gamberi T, Fiaschi T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells. 2024; 13(14):1177. https://doi.org/10.3390/cells13141177
Chicago/Turabian StyleBartoloni, Bianca, Michele Mannelli, Tania Gamberi, and Tania Fiaschi. 2024. "The Multiple Roles of Lactate in the Skeletal Muscle" Cells 13, no. 14: 1177. https://doi.org/10.3390/cells13141177
APA StyleBartoloni, B., Mannelli, M., Gamberi, T., & Fiaschi, T. (2024). The Multiple Roles of Lactate in the Skeletal Muscle. Cells, 13(14), 1177. https://doi.org/10.3390/cells13141177






