Tissue-Level Integration Overrides Gradations of Differentiating Cell Identity in Beetle Extraembryonic Tissue
Abstract
:1. Introduction
2. Methods
2.1. Tribolium castaneum Stocks
2.2. RNAi and In Situ Hybridization
2.3. Live Imaging Acquisition and Analysis
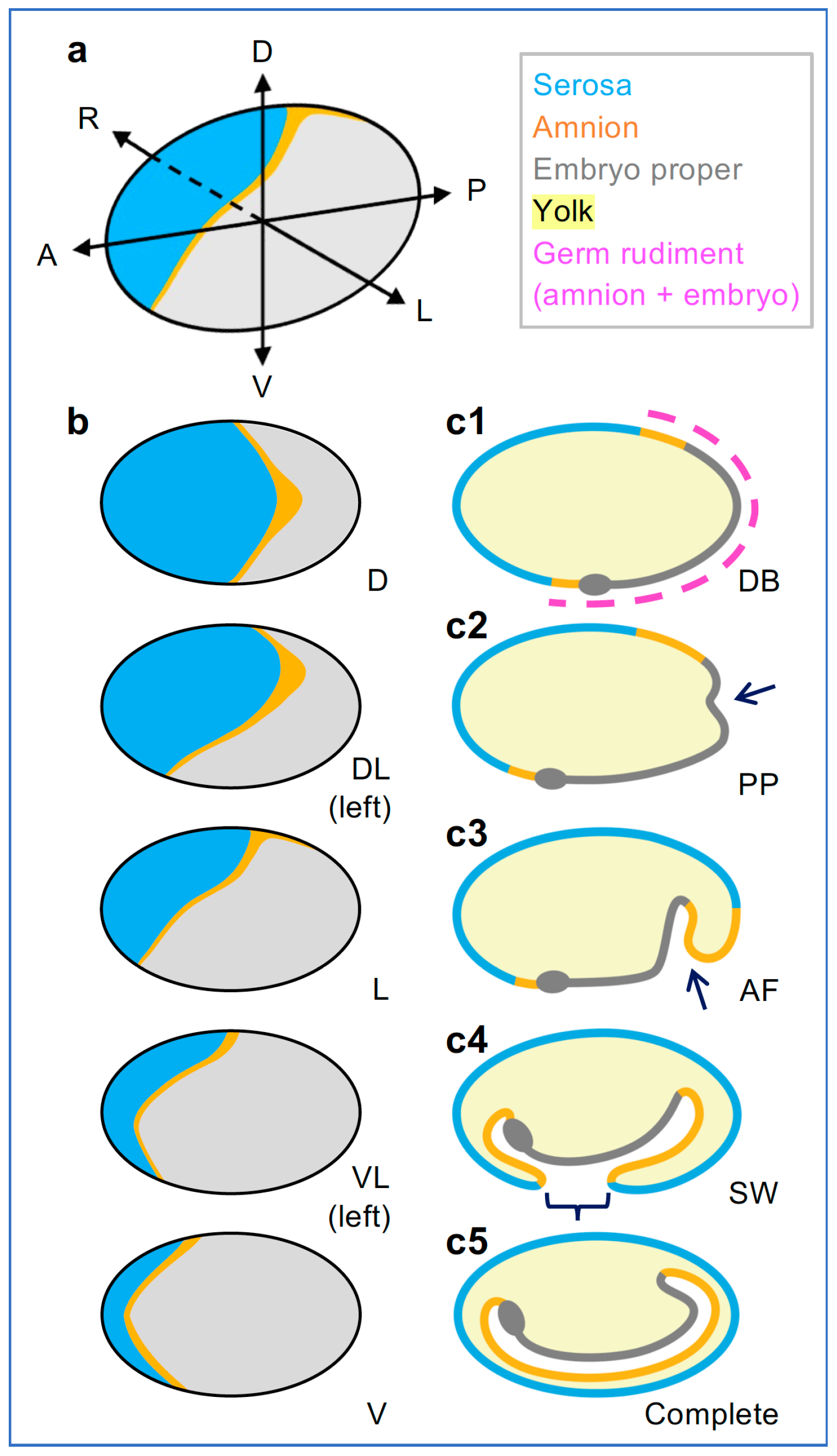
2.3.1. Spatial and Temporal Analyses
2.3.2. Nuclear Area and Cell Density
2.3.3. Head Lobe Area and Cell Density in the EE–Embryonic Tissue Border Region
2.4. Statistical Analysis
3. Results
3.1. Early Morphogenetic and Morphological Differentiation of the Serosa

3.2. Strong Tc-zen1 RNAi Leads to Loss of Serosal Tissue Identity
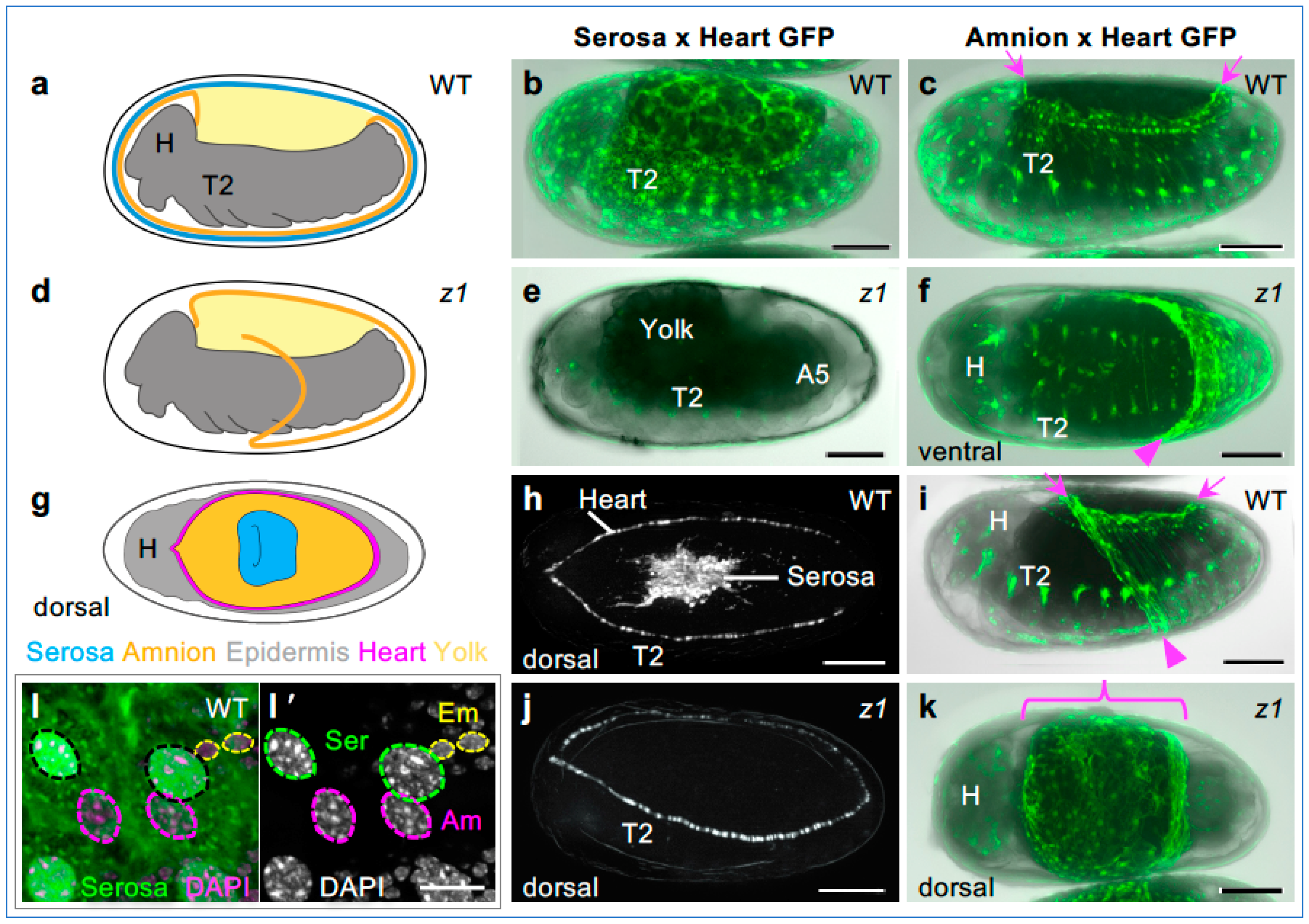
3.3. Tc-zen1 RNAi Reveals Early, Subtle Regionalization within the EE Tissue
3.4. A Phenotypic Spectrum for EE Tissue Differentiation
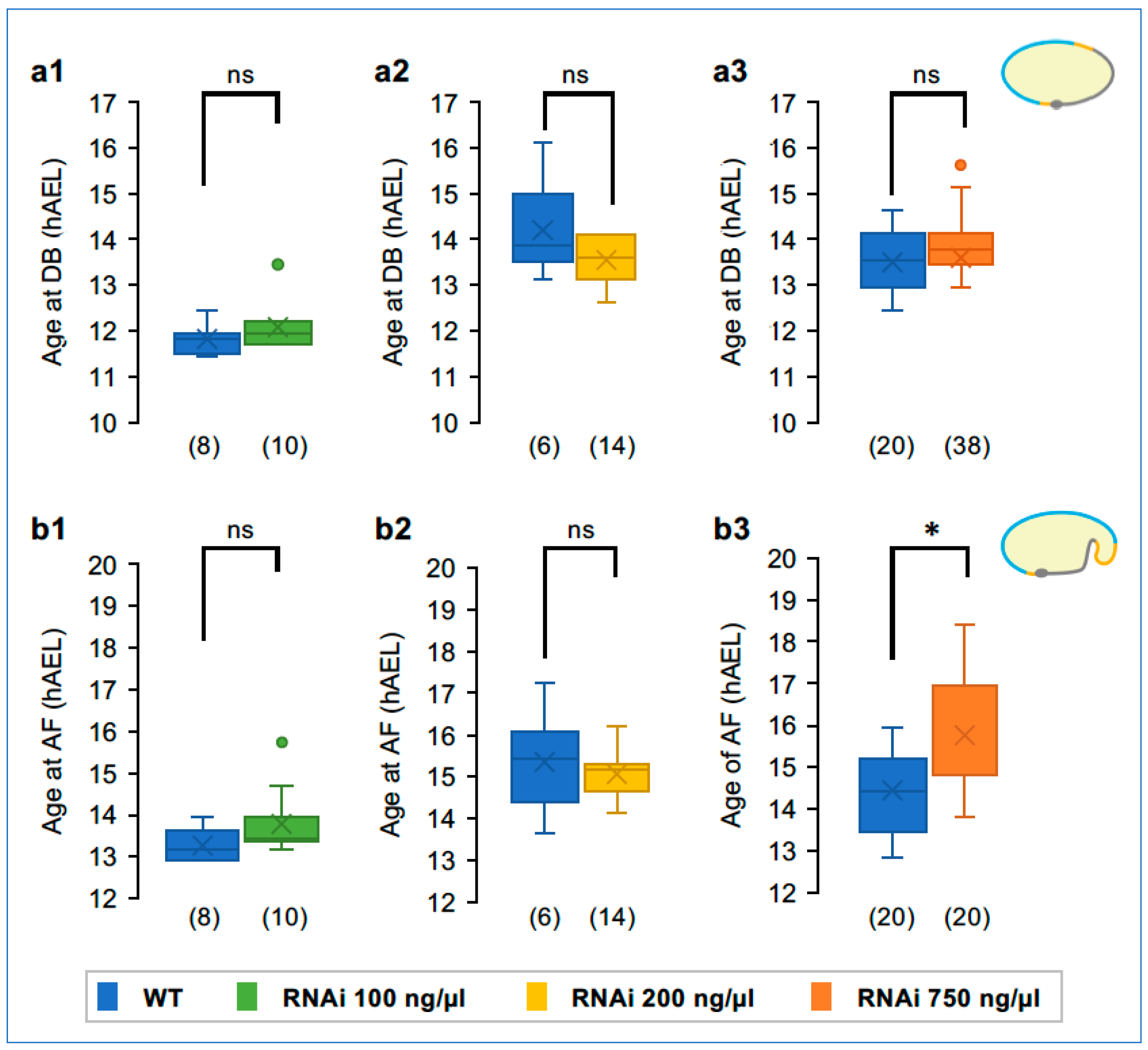
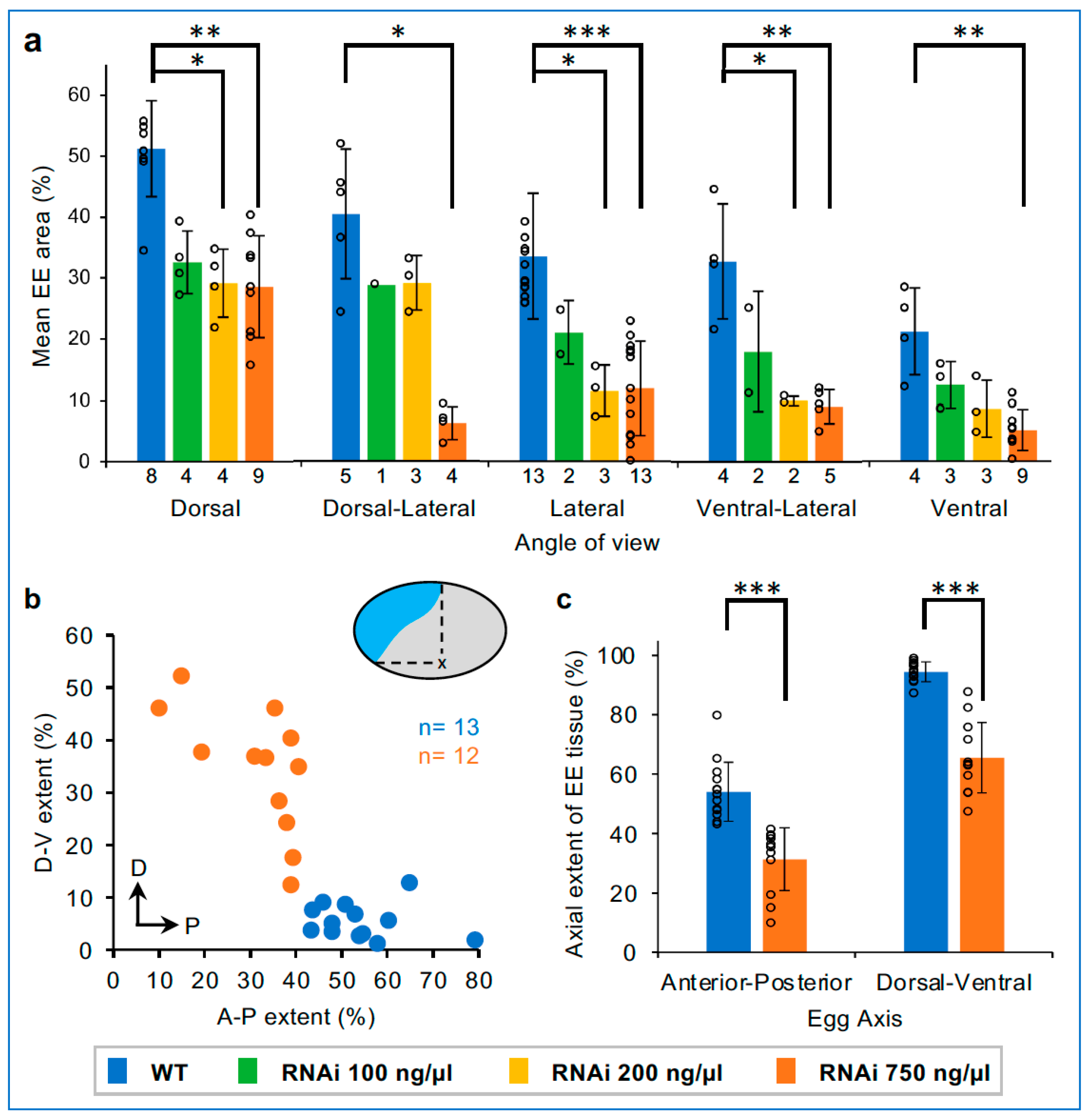
3.5. Dose-Dependent and Non-Uniform Reduction in the EE Domain after Tc-zen1 RNAi
3.6. Head Morphology Shows Conflicting Trends for Knockdown Phenotypic Response

3.7. Tc-zen1 RNAi Influences the Rate of EE Polyploidization

4. Discussion
4.1. Uncoupled and Dose-Dependent Features of Tissue Identity
4.2. Maternal Terminal Inputs Pattern Anterior Tissue Domains
4.3. A Selector Gene at the Nexus of Maternal Patterning and Zygotic EE Specification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Zee, M.; Berns, N.; Roth, S. Distinct functions of the Tribolium zerknüllt genes in serosa specification and dorsal closure. Curr. Biol. 2005, 15, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Troelenberg, N.; Anh, V.D.; Richter, T.; Bucher, G.; Klingler, M. Double abdomen in a short-germ insect: Zygotic control of axis formation revealed in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2018, 115, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Özüak, O.; Buchta, T.; Roth, S.; Lynch, J.A. Dorsoventral polarity of the Nasonia embryo primarily relies on a BMP gradient formed without input from Toll. Curr. Biol. 2014, 24, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Posnien, N.; Bolognesi, R.; Fischer, T.D.; Rayl, P.; Oberhofer, G.; Kitzmann, P.; Brown, S.J.; Bucher, G. Asymmetrically expressed axin required for anterior development in Tribolium. Proc. Natl. Acad. Sci. USA 2012, 109, 7782–7786. [Google Scholar] [CrossRef] [PubMed]
- Gurska, D.; Vargas Jentzsch, I.M.; Panfilio, K.A. Unexpected mutual regulation underlies paralogue functional diversification and promotes epithelial tissue maturation in Tribolium. Commun. Biol. 2020, 3, 552. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Fonseca, R.; von Levetzow, C.; Kalscheuer, P.; Basal, A.; van der Zee, M.; Roth, S. Self-regulatory circuits in dorsoventral axis formation of the short-germ beetle Tribolium castaneum. Dev. Cell 2008, 14, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Pers, D.; Lynch, J.A. Ankyrin domain encoding genes from an ancient horizontal transfer are functionally integrated into Nasonia developmental gene regulatory networks. Genome Biol 2018, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Ali-Murthy, Z.; Kornberg, T.B. Bicoid gradient formation and function in the Drosophila pre-syncytial blastoderm. eLife 2016, 5, e13222. [Google Scholar] [CrossRef] [PubMed]
- Gavin-Smyth, J.; Wang, Y.C.; Butler, I.; Ferguson, E.L. A genetic network conferring canalization to a bistable patterning system in Drosophila. Curr. Biol. 2013, 23, 2296–2302. [Google Scholar] [CrossRef]
- Houchmandzadeh, B.; Wieschaus, E.; Leibler, S. Establishment of developmental precision and proportions in the early Drosophila embryo. Nature 2002, 415, 798–8002. [Google Scholar] [CrossRef]
- Liu, F.; Morrison, A.H.; Gregor, T. Dynamic interpretation of maternal inputs by the Drosophila segmentation gene network. Proc. Natl. Acad. Sci. USA 2013, 110, 6724–6729. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B.; Roth, S. Dorsoventral Axis Formation in the Drosophila Embryo—Shaping and Transducing a Morphogen Gradient. Curr. Biol. 2005, 15, R887–R899. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Panfilio, K.A. Novel functions for Dorsocross in epithelial morphogenesis in the beetle Tribolium castaneum. Development 2016, 143, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- Kerridge, S.; Munjal, A.; Philippe, J.-M.; Jha, A.; Garcia de las Bayonas, A.; Saurin, A.J.; Lecuit, T. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat. Cell Biol. 2016, 18, 261–270. [Google Scholar] [CrossRef]
- Panfilio, K.A. Extraembryonic development in insects and the acrobatics of blastokinesis. Dev. Biol. 2008, 313, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Zeh, D.W.; Zeh, J.A.; Smith, R.L. Ovipositors, amnions and eggshell architecture in the diversification of terrestrial arthropods. Q. Rev. Biol. 1989, 64, 147–168. [Google Scholar] [CrossRef]
- Panfilio, K.A.; Chuva de Sousa Lopes, S.M. The extended analogy of extraembryonic development in insects and amniotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210268. [Google Scholar] [CrossRef] [PubMed]
- Bedford, G.O. The development of the egg of Didymuria violescens (Phasmatodea: Phasmatidae: Podacanthinae)—Embryology and determination of the stage at which first diapause occurs. Aust. J. Zool. 1970, 18, 155–169. [Google Scholar] [CrossRef]
- Cho, E.H.; Nijhout, H.F. Development of polyploidy of scale-building cells in the wings of Manduca sexta. Arthropod. Struct. Dev. 2013, 42, 37–46. [Google Scholar] [CrossRef]
- Darmasaputra, G.S.; van Rijnberk, L.M.; Galli, M. Functional consequences of somatic polyploidy in development. Development 2024, 151, dev202392. [Google Scholar] [CrossRef]
- Hannibal, R.L.; Baker, J.C. Selective amplification of the genome surrounding key placental genes in trophoblast giant cells. Curr. Biol. 2016, 26, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Orr-Weaver, T.L. When bigger is better: The role of polyploidy in organogenesis. Trends Genet. 2015, 31, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Song, J.; Ni, M.; Kang, L.; Guo, W. Regulatory mechanisms of cell polyploidy in insects. Front. Cell Dev. Biol 2020, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.O.; Davidson, J.M.; Duronio, R.J. Endoreplication: Polyploidy with purpose. Genes Dev. 2009, 23, 2461–2477. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.G.C.; Spaink, H.P.; van der Zee, M. The extraembryonic serosa is a frontier epithelium providing the insect egg with a full-range innate immune response. eLife 2014, 3, e04111. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.G.C.; van der Hulst, R.; Chen, Y.-T.; Williamson, R.P.; Roth, S.; van der Zee, M. Immune function of the serosa in hemimetabolous insect eggs. Philos. Trans. R. Soc. Lond. B 2022, 377, 20210266. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Noh, M.Y.; Moussian, B.; Specht, C.A.; Kramer, K.J.; Beeman, R.W.; Arakane, Y.; Muthukrishnan, S. Knickkopf and retroactive proteins are required for formation of laminar serosal procuticle during embryonic development of Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 60, 1–6. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Menna-Barreto, R.F.S.; Martins, A.J.; Valle, D.; Rezende, G.L. Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: Connection with distinct levels of resistance to desiccation. J. Insect Physiol. 2015, 83, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, Y.; Rezende, G.L.; Vranizan, K.; Lanzaro, G.; Valle, D.; Levine, M. Developmental and evolutionary basis for drought tolerance of the Anopheles gambiae embryo. Dev. Biol. 2009, 330, 462–470. [Google Scholar] [CrossRef]
- Jacobs, C.G.C.; Rezende, G.L.; Lamers, G.E.M.; van der Zee, M. The extraembryonic serosa protects the insect egg against desiccation. Proc. R. Soc. B 2013, 280, 20131082. [Google Scholar] [CrossRef]
- Jacobs, C.G.C.; Braak, N.; Lamers, G.E.M.; van der Zee, M. Elucidation of the serosal cuticle machinery in the beetle Tribolium by RNA sequencing and functional analysis of Knickkopf1, Retroactive and Laccase2. Insect Biochem. Mol. Biol. 2015, 60, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Panfilio, K.A.; Oberhofer, G.; Roth, S. High plasticity in epithelial morphogenesis during insect dorsal closure. Biol. Open 2013, 2, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Rezende, G.L.; Vargas, H.C.M.; Moussian, B.; Cohen, E. Composite eggshell matrices: Chorionic layers and sub-chorionic cuticular envelopes. In Extracellular Composite Matrices in Arthropods; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Dearden, P.; Grbic, M.; Falciani, F.; Akam, M. Maternal expression and early zygotic regulation of the Hox3/zen gene in the grasshopper Schistocerca gregaria. Evol. Dev. 2000, 2, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Panfilio, K.A.; Liu, P.Z.; Akam, M.; Kaufman, T.C. Oncopeltus fasciatus zen is essential for serosal tissue function in katatrepsis. Dev. Biol. 2006, 292, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, M.; Kenny, N.J.; Pott, L.; Heger, P.; Chen, Y.T.; Buchta, T.; Özüak, O.; Lynch, J.; Roth, S. Striking parallels between dorsoventral patterning in Drosophila and Gryllus reveal a complex evolutionary history behind a model gene regulatory network. eLife 2021, 10, e68287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ott, U.; Kwan, C.W. How two extraembryonic epithelia became one: Serosa and amnion features and functions of Drosophila’s amnioserosa. Philos. Trans. R. Soc. Lond. B 2022, 377, 20210265. [Google Scholar] [CrossRef]
- Ray, R.P.; Arora, K.; Nüsslein-Volhard, C.; Gelbart, W.M. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development 1991, 113, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Rushlow, C.; Levine, M. Role of the zerknüllt gene in dorsal-ventral pattern formation in Drosophila. In Advances in Genetics: Genetic Regulatory Hierarchies in Development; Wright, T.R.F., Ed.; Academic Press: San Diego, CA, USA, 1990; Volume 27. [Google Scholar]
- Goltsev, Y.; Fuse, N.; Frasch, M.; Zinzen, R.P.; Lanzaro, G.; Levine, M. Evolution of the dorsal-ventral patterning network in the mosquito, Anopheles gambiae. Development 2007, 134, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Rafiqi, A.M.; Lemke, S.; Ferguson, S.; Stauber, M.; Schmidt-Ott, U. Evolutionary origin of the amnioserosa in cyclorrhaphan flies correlates with spatial and temporal expression changes of zen. Proc. Natl. Acad. Sci. USA 2008, 105, 234–239. [Google Scholar] [CrossRef]
- Kwan, C.W.; Gavin-Smyth, J.; Ferguson, E.L.; Schmidt-Ott, U. Functional evolution of a morphogenetic gradient. eLife 2016, 5, e20894. [Google Scholar] [CrossRef]
- Brown, S.J.; Fellers, J.P.; Shippy, T.D.; Richardson, E.A.; Maxwell, M.; Stuart, J.J.; Denell, R.E. Sequence of the Tribolium castaneum homeotic complex: The region corresponding to the Drosophila melanogaster Antennapedia Complex. Genetics 2002, 160, 1067–1074. [Google Scholar] [CrossRef]
- Benton, M.A.; Akam, M.; Pavlopoulos, A. Cell and tissue dynamics during Tribolium castaneum embryogenesis revealed by versatile fluorescence labeling approaches. Development 2013, 140, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Handel, K.; Grünfelder, C.G.; Roth, S.; Sander, K. Tribolium embryogenesis: A SEM study of cell shapes and movements from blastoderm to serosal closure. Dev. Genes Evol. 2000, 210, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, S.; Kölsch, Y.; Panfilio, K.A. Visualizing late insect embryogenesis: Extraembryonic and mesodermal enhancer trap expression in the beetle Tribolium castaneum. PLoS ONE 2014, 9, e103967. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, O.A.; Butt, F.H. Embryology of Insects and Myriapods, 1st ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1941; pp. 49–64. [Google Scholar]
- Horn, T.; Hilbrant, M.; Panfilio, K.A. Evolution of epithelial morphogenesis: Phenotypic integration across multiple levels of biological organization. Front. Genet. 2015, 6, 303. [Google Scholar] [CrossRef]
- van der Zee, M.; Stockhammer, O.; Levetzow, C.V.; Fonseca, R.N.D.; Roth, S. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc. Natl. Acad. Sci. USA 2006, 103, 16307–16312. [Google Scholar]
- Benton, M.A. A revised understanding of Tribolium morphogenesis further reconciles short and long germ development. PLoS Biol. 2018, 16, e2005093. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Beermann, A.; Schröder, R. The dynamic expression of extraembryonic marker genes in the beetle Tribolium castaneum reveals the complexity of serosa and amnion formation in a short germ insect. Gene Expr. Patt. 2013, 13, 362–371. [Google Scholar] [CrossRef]
- Ferguson, L.; Marlétaz, F.; Carter, J.-M.; Taylor, W.R.; Gibbs, M.; Breuker, C.J.; Holland, P.W.H. Ancient expansion of the Hox cluster in Lepidoptera generated four homeobox genes implicated in extra-embryonic tissue formation. PLoS Genet. 2014, 10, e1004698. [Google Scholar] [CrossRef]
- Irion, U.; Johnston, D.S. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature 2007, 445, 554–558. [Google Scholar] [CrossRef]
- Stauber, M.; Prell, A.; Schmidt-Ott, U. A single Hox3 gene with composite bicoid and zerknüllt expression characteristics in non-Cyclorrhaphan flies. Proc. Natl. Acad. Sci. USA 2002, 99, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Schoppmeier, M.; Schröder, R. Maternal Torso signaling controls body axis elongation in a short germ insect. Curr. Biol. 2005, 15, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Münster, S.; Jain, A.; Mietke, A.; Pavlopoulos, A.; Grill, S.W.; Tomancak, P. Attachment of the blastoderm to the vitelline envelope affects gastrulation of insects. Nature 2019, 568, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Shippy, T.D.; Miller, S.; Bolognesi, R.; Beeman, R.W.; Lorenzen, M.D.; Bucher, G.; Wimmer, E.A.; Klingler, M. The red flour beetle, Tribolium castaneum (Coleoptera): A model for studies of development and pest biology. Cold Spring Harb. Protoc. 2009, 2009, pdb.emo126. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, A.F.; Peel, A.D.; Averof, M. A segmentation clock with two-segment periodicity in insects. Science 2012, 336, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Hilbrant, M.; Horn, T.; Koelzer, S.; Panfilio, K.A. The beetle amnion and serosa functionally interact as apposed epithelia. eLife 2016, 5, e13834. [Google Scholar] [CrossRef] [PubMed]
- Trauner, J.; Schinko, J.; Lorenzen, M.D.; Shippy, T.D.; Wimmer, E.A.; Beeman, R.W.; Klingler, M.; Bucher, G.; Brown, S.J. Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol. 2009, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Herndon, N.; Shelton, J.; Gerischer, L.; Ioannidis, P.; Ninova, M.; Dönitz, J.; Waterhouse, R.M.; Liang, C.; Damm, C.; Siemanowski, J.; et al. Enhanced genome assembly and a new official gene set for Tribolium castaneum—From a draft to a reference genome. BMC Genom. 2020, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ulman, V.; Mukherjee, A.; Prakash, M.; Cuenca, M.B.; Pimpale, L.G.; Münster, S.; Haase, R.; Panfilio, K.A.; Jug, F.; et al. Regionalized tissue fluidization is required for epithelial gap closure during insect gastrulation. Nat. Commun. 2020, 11, 5604. [Google Scholar] [CrossRef]
- Mehrotra, S.; Maqbool, S.B.; Kolpakas, A.; Murnen, K.; Calvi, B.R. Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev. 2008, 22, 3158–3171. [Google Scholar] [CrossRef]
- Meijering, E.; Dzyubachyk, O.; Smal, I. Chapter 9: Methods for cell and particle tracking. Methods Enzymol. 2012, 504, 183–200. [Google Scholar]
- Dziak, J.J.; Dierker, L.C.; Abar, B. The interpretation of statistical power after the data have been gathered. Curr. Psychol. 2020, 39, 870–877. [Google Scholar] [CrossRef]
- Griffith, K.N.; Feyman, Y. Amplifying the noise: The dangers of post hoc power analyses. J. Surg. Res. 2021, 259, A9–A11. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Heckman, M.G.; Davis, J.M., 3rd; Crowson, C.S. Post hoc power calculations: An inappropriate method for interpreting the findings of a research study. J. Rheumatol. 2022, 49, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Colegrave, N.; Ruxton, G.D. Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav. Ecol. 2003, 14, 446–447. [Google Scholar] [CrossRef]
- Benton, M.A.; Frey, N.; da Fonseca, R.N.; von Levetzow, C.; Stappert, D.; Salim, M.; Conrads, H.H.; Pechmann, M.; Panfilio, K.A.; Lynch, J.A.; et al. Fog signaling has diverse roles in epithelial morphogenesis in insects. eLife 2019, 8, e47346. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, M.; Benton, M.A.; Vazquez-Faci, T.; Lamers, G.E.M.; Jacobs, C.G.C.; Rabouille, C. Innexin7a forms junctions that stabilize the basal membrane during cellularization of the blastoderm in Tribolium castaneum. Development 2015, 142, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- van Drongelen, R.; Vazquez-Faci, T.; Huijben, T.A.P.M.; van der Zee, M.; Idema, T. Mechanics of epithelial tissue formation. J. Theor. Biol. 2018, 454, 182–189. [Google Scholar] [CrossRef]
- Handel, K.; Basal, A.; Fan, X.; Roth, S. Tribolium castaneum twist: Gastrulation and mesoderm formation in a short-germ beetle. Dev. Genes Evol. 2005, 215, 13–31. [Google Scholar] [CrossRef]
- Prinyakupt, J.; Pluempitiwiriyawej, C. Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. Biomed. Eng. Online 2015, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.A.; Kong, J.; Gutman, D.A.; Wang, F.; Gao, J.; Appin, C.; Cholleti, S.; Pan, T.; Sharma, A.; Scarpace, L.; et al. Integrated morphologic analysis for the identification and characterization of disease subtypes. J. Am. Med. Inform. Assoc. 2012, 19, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Gilkes, D.M.; Phillip, J.M.; Narkar, A.; Cheng, T.W.; Marchand, J.; Lee, M.H.; Li, R.; Wirtz, D. Single-cell morphology encodes metastatic potential. Sci. Adv. 2020, 6, eaaw6938. [Google Scholar] [CrossRef]
- Bucher, G.; Klingler, M. Divergent segmentation mechanism in the short germ insect Tribolium revealed by giant expression and function. Development 2004, 131, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Forés, M.; Ajuria, L.; Samper, N.; Astigarraga, S.; Nieva, C.; Grossman, R.; González-Crespo, S.; Paroush, Z.; Jiménez, G. Origins of context-dependent gene repression by Capicua. PLoS Genet. 2015, 11, e1004902. [Google Scholar] [CrossRef] [PubMed]
- Pridöhl, F.; Weißkopf, M.; Koniszewski, N.; Sulzmaier, A.; Uebe, S.; Ekici, A.B.; Schoppmeier, M. Transcriptome sequencing reveals maelstrom as a novel target gene of the terminal system in the red flour beetle Tribolium castaneum. Development 2017, 144, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Yip, M.L.; Lamka, M.L.; Lipshitz, H.D. Control of germ-band retraction in Drosophila by the zinc-finger protein HINDSIGHT. Development 1997, 124, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Buchta, T.; Özüak, O.; Stappert, D.; Roth, S.; Lynch, J.A. Patterning the dorsal-ventral axis of the wasp Nasonia vitripennis. Dev. Biol. 2013, 381, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Rafiqi, A.M.; Lemke, S.; Schmidt-Ott, U. Postgastrular zen expression is required to develop distinct amniotic and serosal epithelia in the scuttle fly Megaselia. Dev. Biol. 2010, 341, 282–290. [Google Scholar] [CrossRef]
- Loker, R.; Sanner, J.E.; Mann, R.S. Cell-type-specific Hox regulatory strategies orchestrate tissue identity. Curr. Biol. 2021, 31, 4246–4255.e4244. [Google Scholar] [CrossRef]
- Mann, R.S.; Carroll, S.B. Molecular mechanisms of selector gene function and evolution. Curr. Opin. Genet. Dev. 2002, 12, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Closser, M.; Guo, Y.; Wang, P.; Patel, T.; Jang, S.; Hammelman, J.; De Nooij, J.C.; Kopunova, R.; Mazzoni, E.O.; Ruan, Y.; et al. An expansion of the non-coding genome and its regulatory potential underlies vertebrate neuronal diversity. Neuron 2022, 110, 70–85.e76. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; DeCamillis, M.; Gonzalez-Charneco, K.; Denell, M.; Beeman, R.; Nie, W.; Denell, R.E. Implications of the Tribolium Deformed mutant phenotype for the evolution of Hox gene function. Proc. Natl. Acad. Sci. USA 2000, 97, 4510–4514. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulos, A.; Kontarakis, Z.; Liubicich, D.M.; Serano, J.M.; Akam, M.; Patel, N.H.; Averof, M. Probing the evolution of appendage specialization by Hox gene misexpression in an emerging model crustacean. Proc. Natl. Acad. Sci. USA 2009, 106, 13897–13902. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y.; Wheeler, S.R.; Denell, R.E. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 2005, 433, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Falciani, F.; Hausdorf, B.; Schröder, R.; Akam, M.; Tautz, D.; Denell, R.; Brown, S. Class 3 Hox genes in insects and the origin of zen. Proc. Natl. Acad. Sci. USA 1996, 93, 8479–8484. [Google Scholar] [CrossRef] [PubMed]
- Panfilio, K.A.; Akam, M. A comparison of Hox3 and Zen protein coding sequences in taxa that span the Hox3/zen divergence. Dev. Genes Evol. 2007, 217, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, P.C.; Phillips, L.A.; Deshwar, A.R.; Ryu, J.R.; Najand, N.; Brook, W.J. The selector genes midline and H15 control ventral leg pattern by both inhibiting Dpp signaling and specifying ventral fate. Dev. Biol. 2019, 455, 19–31. [Google Scholar] [CrossRef]
- Wülbeck, C.; Simpson, P. The expression of pannier and achaete-scute homologues in a mosquito suggests an ancient role of pannier as a selector gene in the regulation of the dorsal body pattern. Development 2002, 129, 3861–3871. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, K.E.; Panfilio, K.A. Tissue-Level Integration Overrides Gradations of Differentiating Cell Identity in Beetle Extraembryonic Tissue. Cells 2024, 13, 1211. https://doi.org/10.3390/cells13141211
Mann KE, Panfilio KA. Tissue-Level Integration Overrides Gradations of Differentiating Cell Identity in Beetle Extraembryonic Tissue. Cells. 2024; 13(14):1211. https://doi.org/10.3390/cells13141211
Chicago/Turabian StyleMann, Katie E., and Kristen A. Panfilio. 2024. "Tissue-Level Integration Overrides Gradations of Differentiating Cell Identity in Beetle Extraembryonic Tissue" Cells 13, no. 14: 1211. https://doi.org/10.3390/cells13141211




