Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. RNA Preparation and Analysis
2.3. Nuclear and Cytosolic Proteins Extraction
2.4. Western Blotting Analysis
2.5. Statistical Analysis
3. Results
3.1. KEAP1/NRF2 Expression
3.2. Antioxidant Expression Levels
3.3. Autophagic Pathway
3.4. Endoplasmic Reticulum Responses
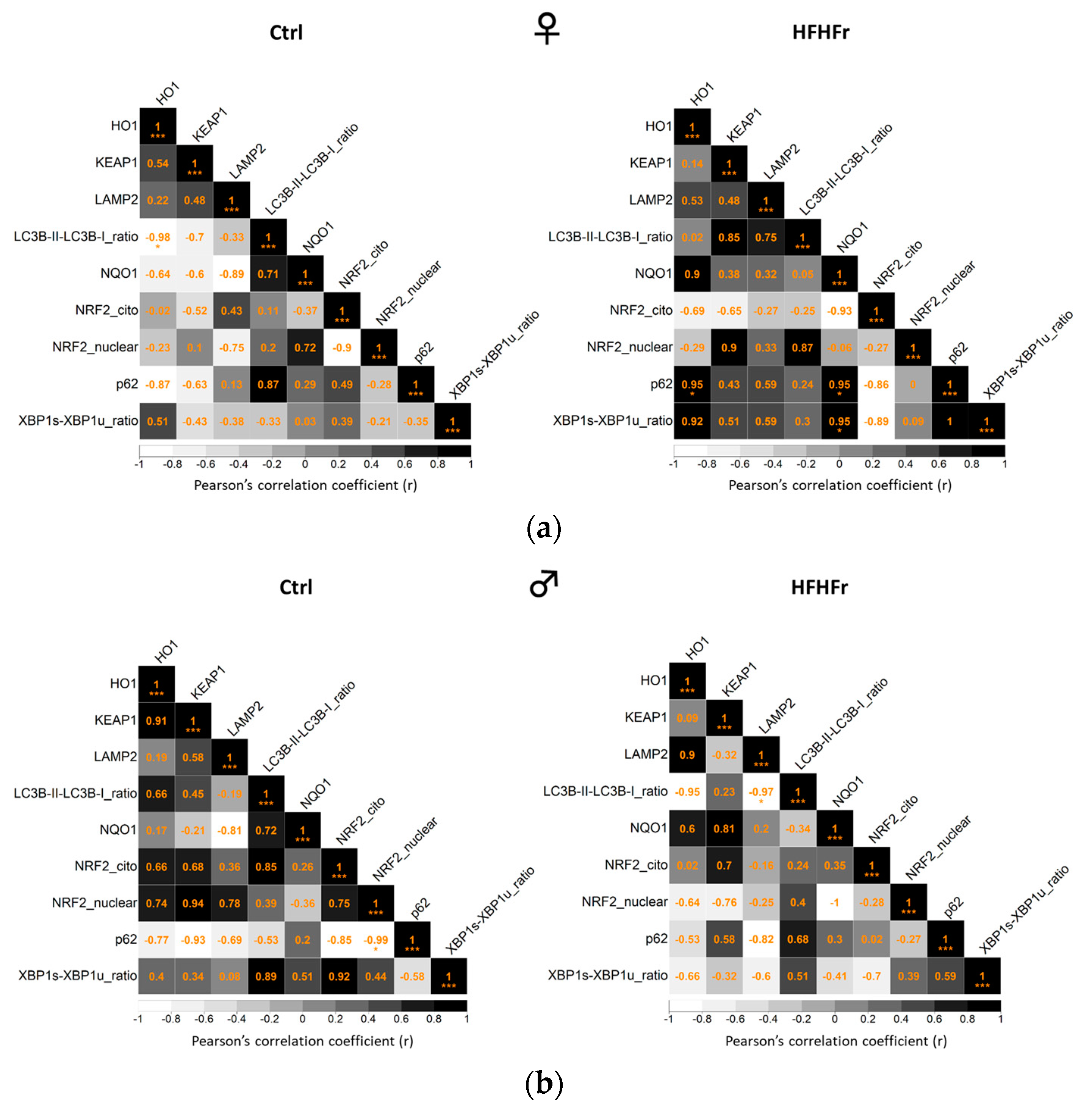
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Wilson, L.A.; Behling, C.; Guy, C.; Contos, M.; Cummings, O.; Yeh, M.; Gill, R.; Chalasani, N.; et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw. Open 2019, 2, e1912565. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Sanyal, A.J.; Neuschwander-Tetri, B.A. Improvements in Histologic Features and Diagnosis Associated with Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology 2019, 70, 522–531. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited after a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular Mechanisms of Lipotoxicity and Glucotoxicity in Nonalcoholic Fatty Liver Disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and Sugar: A Major Mediator of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Petrescu, M.; Vlaicu, S.I.; Ciumărnean, L.; Milaciu, M.V.; Mărginean, C.; Florea, M.; Vesa, Ș.C.; Popa, M. Chronic Inflammation-A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina 2022, 58, 641. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Ngo, J.; Shirihai, O.S.; Liesa, M. Mitochondrial Oxidative Function in NAFLD: Friend or Foe? Mol. Metab. 2021, 50, 101134. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, Y.F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in Chronic Liver Disease. World J. Gastroenterol. 2014, 20, 13079–13087. [Google Scholar] [CrossRef]
- Kitteringham, N.R.; Abdullah, A.; Walsh, J.; Randle, L.; Jenkins, R.E.; Sison, R.; Goldring, C.E.P.; Powell, H.; Sanderson, C.; Williams, S.; et al. Proteomic Analysis of Nrf2 Deficient Transgenic Mice Reveals Cellular Defence and Lipid Metabolism as Primary Nrf2-Dependent Pathways in the Liver. J. Proteomics 2010, 73, 1612–1631. [Google Scholar] [CrossRef]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-Related Factor 2 Inhibits Lipid Accumulation and Oxidative Stress in Mice Fed a High-Fat Diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Vomhof-DeKrey, E.E.; Picklo, M.J. The Nrf2-Antioxidant Response Element Pathway: A Target for Regulating Energy Metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Dodson, M.; Li, H.; Schmidlin, C.J.; Shakya, A.; Wei, Y.; Garcia, J.G.N.; Chapman, E.; Kiela, P.R.; Zhang, Q.Y.; et al. Non-Canonical NRF2 Activation Promotes a pro-Diabetic Shift in Hepatic Glucose Metabolism. Mol. Metab. 2021, 51, 101243. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in Prevention of High-Fat Diet-Induced Obesity by Synthetic Triterpenoid CDDO-Imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.V.; Ziros, P.G.; Psyrogiannis, A.I.; Papavassiliou, A.G.; Kyriazopoulou, V.E.; Sykiotis, G.P.; Habeos, I.G. Nrf2 Represses FGF21 during Long-Term High-Fat Diet-Induced Obesity in Mice. Diabetes 2011, 60, 2465–2473. [Google Scholar] [CrossRef]
- Mendes, I.K.S.; Matsuura, C.; Aguila, M.B.; Daleprane, J.B.; Martins, M.A.; Mury, W.V.; Brunini, T.M.C. Weight Loss Enhances Hepatic Antioxidant Status in a NAFLD Model Induced by High-Fat Diet. Appl. Physiol. Nutr. Metab. 2018, 43, 23–29. [Google Scholar] [CrossRef]
- Nigro, D.; Menotti, F.; Cento, A.S.; Serpe, L.; Chiazza, F.; Dal Bello, F.; Romaniello, F.; Medana, C.; Collino, M.; Aragno, M.; et al. Chronic Administration of Saturated Fats and Fructose Differently Affect SREBP Activity Resulting in Different Modulation of Nrf2 and Nlrp3 Inflammasome Pathways in Mice Liver. J. Nutr. Biochem. 2017, 42, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Bathish, B.; Robertson, H.; Dillon, J.F.; Dinkova-Kostova, A.T.; Hayes, J.D. Nonalcoholic Steatohepatitis and Mechanisms by Which It Is Ameliorated by Activation of the CNC-BZIP Transcription Factor Nrf2. Free Radic. Biol. Med. 2022, 188, 221–261. [Google Scholar] [CrossRef]
- Liu, Y.; Adachi, M.; Zhao, S.; Hareyama, M.; Koong, A.C.; Luo, D.; Rando, T.A.; Imai, K.; Shinomura, Y. Preventing Oxidative Stress: A New Role for XBP1. Cell Death Differ. 2009, 16, 847–857. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, Y.; Wang, J.J.; Yu, Q.; Plafker, K.; Plafker, S.; Zhang, S.X. Regulation of Nrf2 by X Box-Binding Protein 1 in Retinal Pigment Epithelium. Front. Genet. 2018, 9, 658. [Google Scholar] [CrossRef]
- Riz, I.; Hawley, T.S.; Marsal, J.W.; Hawley, R.G. Noncanonical SQSTM1/P62-Nrf2 Pathway Activation Mediates Proteasome Inhibitor Resistance in Multiple Myeloma Cells via Redox, Metabolic and Translational Reprogramming. Oncotarget 2016, 7, 66360–66385. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. P62/SQSTM1 Is a Target Gene for Transcription Factor NRF2 and Creates a Positive Feedback Loop by Inducing Antioxidant Response Element-Driven Gene Transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Cuadrado, A.; Rojo, A.I. Modulation of Proteostasis by Transcription Factor NRF2 and Impact in Neurodegenerative Diseases. Redox Biol. 2017, 11, 543–553. [Google Scholar] [CrossRef]
- Fernández-Ginés, R.; Encinar, J.A.; Escoll, M.; Carnicero-Senabre, D.; Jiménez-Villegas, J.; García-Yagüe, Á.J.; González-Rodríguez, Á.; Garcia-Martinez, I.; Valverde, Á.; Rojo, A.I.; et al. Specific Targeting of the NRF2/β-TrCP Axis Promotes Beneficial Effects in NASH. Redox Biol. 2024, 69, 103027. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef]
- Bentanachs, R.; Blanco, L.; Montesinos, M.; Sala-Vila, A.; Lázaro, I.; Rodríguez-Morató, J.; Sánchez, R.M.; Laguna, J.C.; Roglans, N.; Alegret, M. Adipose Tissue Protects against Hepatic Steatosis in Male Rats Fed a High-Fat Diet plus Liquid Fructose: Sex-Related Differences. Nutrients 2023, 15, 3909. [Google Scholar] [CrossRef]
- Velázquez, A.M.; Bentanachs, R.; Sala-Vila, A.; Lázaro, I.; Rodríguez-Morató, J.; Sánchez, R.M.; Alegret, M.; Roglans, N.; Laguna, J.C. ChREBP-Driven DNL and PNPLA3 Expression Induced by Liquid Fructose Are Essential in the Production of Fatty Liver and Hypertriglyceridemia in a High-Fat Diet-Fed Rat Model. Mol. Nutr. Food Res. 2022, 66, 2101115. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Navarro, M.A.; Larraga, A.; Strunk, M.; Barranquero, C.; Acín, S.; Guzman, M.A.; Iñigo, P.; Osada, J. Selection of Reference Genes for Gene Expression Studies in Rats. J. Biotechnol. 2011, 151, 325–334. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Sánchez-Martın, P.; Komatsu, M. P62/SQSTM1—Steering the Cell through Health and Disease. J. Cell Sci. 2018, 131, jcs222836. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Warabi, E.; Okada, K.; Yanagawa, T.; Ishii, T.; Kose, K.; Tokushige, K.; Ishige, K.; Mizokami, Y.; Yamagata, K.; et al. Deletion of Both P62 and Nrf2 Spontaneously Results in the Development of Nonalcoholic Steatohepatitis. Exp. Anim. 2018, 67, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kang, D.H.; Lee, D.H.; Bae, S.H. Concerted Action of P62 and Nrf2 Protects Cells from Palmitic Acid-Induced Lipotoxicity. Biochem. Biophys. Res. Commun. 2015, 466, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Yimin; Ozaki, M. Relevance of FXR-P62/SQSTM1 Pathway for Survival and Protection of Mouse Hepatocytes and Liver, Especially with Steatosis. BMC Gastroenterol. 2017, 17, 9. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, L.P.; Ye, J.Y.; Yang, L.; Huang, Y.; Jiang, X.P.; Zhang, Q.; Jia, J.Z.; Zhang, D.X.; Huang, Y. The Lysosomal Membrane Protein Lamp2 Alleviates Lysosomal Cell Death by Promoting Autophagic Flux in Ischemic Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 490155. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 MRNA Is Induced by ATF6 and Spliced by IRE1 in Response to ER Stress to Produce a Highly Active Transcription Factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Liang, Z.; Wu, Q.; Wang, M.; Yang, M.; Chen, C.; Zheng, H.; Zhu, Z.; Li, L.; Yang, G. Hepatic Lipid Accumulation Induced by a High-Fat Diet Is Regulated by Nrf2 through Multiple Pathways. FASEB J. 2022, 36, e22280. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Lanaspa, M.A.; Sanchez-Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The Fructose Survival Hypothesis for Obesity. Philos. Trans. R. Soc. B 2023, 378, 20220230. [Google Scholar] [CrossRef]
- Chan, S.M.H.; Sun, R.Q.; Zeng, X.Y.; Choong, Z.H.; Wang, H.; Watt, M.J.; Ye, J.M. Activation of PPARα Ameliorates Hepatic Insulin Resistance and Steatosis in High Fructose-Fed Mice despite Increased Endoplasmic Reticulum Stress. Diabetes 2013, 62, 2095–2105. [Google Scholar] [CrossRef]
- Huda, N.; Zou, H.; Yan, S.; Khambu, B.; Yin, X.M. Analysis of Autophagy for Liver Pathogenesis. Methods Mol. Biol. 2019, 1880, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. A Brief History of Autophagy from Cell Biology to Physiology and Disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Baena, M.; Sangüesa, G.; Hutter, N.; Sánchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose Supplementation Impairs Rat Liver Autophagy through MTORC Activation without Inducing Endoplasmic Reticulum Stress. Biochim. Biophys. Acta 2015, 1851, 107–116. [Google Scholar] [CrossRef]
- Kapuy, O. Mechanism of Decision Making between Autophagy and Apoptosis Induction upon Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2024, 25, 4368. [Google Scholar] [CrossRef]
- Musillo, C.; Giona, L.; Ristow, M.; Zarse, K.; Siems, K.; Di Francesco, A.; Collacchi, B.; Raggi, C.; Cirulli, F.; Berry, A. Rosmarinic Acid Improves Cognitive Abilities and Glucose Metabolism in Aged C57Bl/6N Mice While Disrupting Lipid Profile in Young Adults in a Sex-Dependent Fashion. Nutrients 2023, 15, 3366. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-Fat Diet-Induced Obesity Rat Model: A Comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2015, 5, 11–21. [Google Scholar] [CrossRef]
 ) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NRF2 quantification at the gene expression level (a), at protein levels as the total NRF2 (b), in the cytosolic (c) and nuclear fractions, and (d) in the liver of female and male animals after being fed the HFHFr diet and control diet. (c,d) Western blot representative images and densitometric analysis. (e,f) KEAP1 quantification at the gene expression level and protein level. Comparison among groups was carried out via 2-way ANOVA using the HFHFr diet and sex configuration. A significant main effect of diet is indicated by §; a significant main effect of sex is indicated by #; A Tukey post hoc test or independent t-test was conducted for group comparisons and are indicated by *. Significant differences are indicated by p-values: *,§ p < 0.032; ## p < 0.0021; *** p < 0.0002; **** p < 0.0001.
) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NRF2 quantification at the gene expression level (a), at protein levels as the total NRF2 (b), in the cytosolic (c) and nuclear fractions, and (d) in the liver of female and male animals after being fed the HFHFr diet and control diet. (c,d) Western blot representative images and densitometric analysis. (e,f) KEAP1 quantification at the gene expression level and protein level. Comparison among groups was carried out via 2-way ANOVA using the HFHFr diet and sex configuration. A significant main effect of diet is indicated by §; a significant main effect of sex is indicated by #; A Tukey post hoc test or independent t-test was conducted for group comparisons and are indicated by *. Significant differences are indicated by p-values: *,§ p < 0.032; ## p < 0.0021; *** p < 0.0002; **** p < 0.0001.
 ) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NRF2 quantification at the gene expression level (a), at protein levels as the total NRF2 (b), in the cytosolic (c) and nuclear fractions, and (d) in the liver of female and male animals after being fed the HFHFr diet and control diet. (c,d) Western blot representative images and densitometric analysis. (e,f) KEAP1 quantification at the gene expression level and protein level. Comparison among groups was carried out via 2-way ANOVA using the HFHFr diet and sex configuration. A significant main effect of diet is indicated by §; a significant main effect of sex is indicated by #; A Tukey post hoc test or independent t-test was conducted for group comparisons and are indicated by *. Significant differences are indicated by p-values: *,§ p < 0.032; ## p < 0.0021; *** p < 0.0002; **** p < 0.0001.
) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NRF2 quantification at the gene expression level (a), at protein levels as the total NRF2 (b), in the cytosolic (c) and nuclear fractions, and (d) in the liver of female and male animals after being fed the HFHFr diet and control diet. (c,d) Western blot representative images and densitometric analysis. (e,f) KEAP1 quantification at the gene expression level and protein level. Comparison among groups was carried out via 2-way ANOVA using the HFHFr diet and sex configuration. A significant main effect of diet is indicated by §; a significant main effect of sex is indicated by #; A Tukey post hoc test or independent t-test was conducted for group comparisons and are indicated by *. Significant differences are indicated by p-values: *,§ p < 0.032; ## p < 0.0021; *** p < 0.0002; **** p < 0.0001.
 ) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NQO1 and HO1 quantification at the gene expression level (a,b), and at protein levels (c), in the liver of female and male animals after being fed the HFHFr diet and control diet. (c) Western blot representative images and densitometric analysis. A significant main effect of sex is indicated by # and the main effect of diet is indicated by §. Significant differences are indicated by p-values: §,# p < 0.032.
) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NQO1 and HO1 quantification at the gene expression level (a,b), and at protein levels (c), in the liver of female and male animals after being fed the HFHFr diet and control diet. (c) Western blot representative images and densitometric analysis. A significant main effect of sex is indicated by # and the main effect of diet is indicated by §. Significant differences are indicated by p-values: §,# p < 0.032.
 ) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NQO1 and HO1 quantification at the gene expression level (a,b), and at protein levels (c), in the liver of female and male animals after being fed the HFHFr diet and control diet. (c) Western blot representative images and densitometric analysis. A significant main effect of sex is indicated by # and the main effect of diet is indicated by §. Significant differences are indicated by p-values: §,# p < 0.032.
) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NQO1 and HO1 quantification at the gene expression level (a,b), and at protein levels (c), in the liver of female and male animals after being fed the HFHFr diet and control diet. (c) Western blot representative images and densitometric analysis. A significant main effect of sex is indicated by # and the main effect of diet is indicated by §. Significant differences are indicated by p-values: §,# p < 0.032.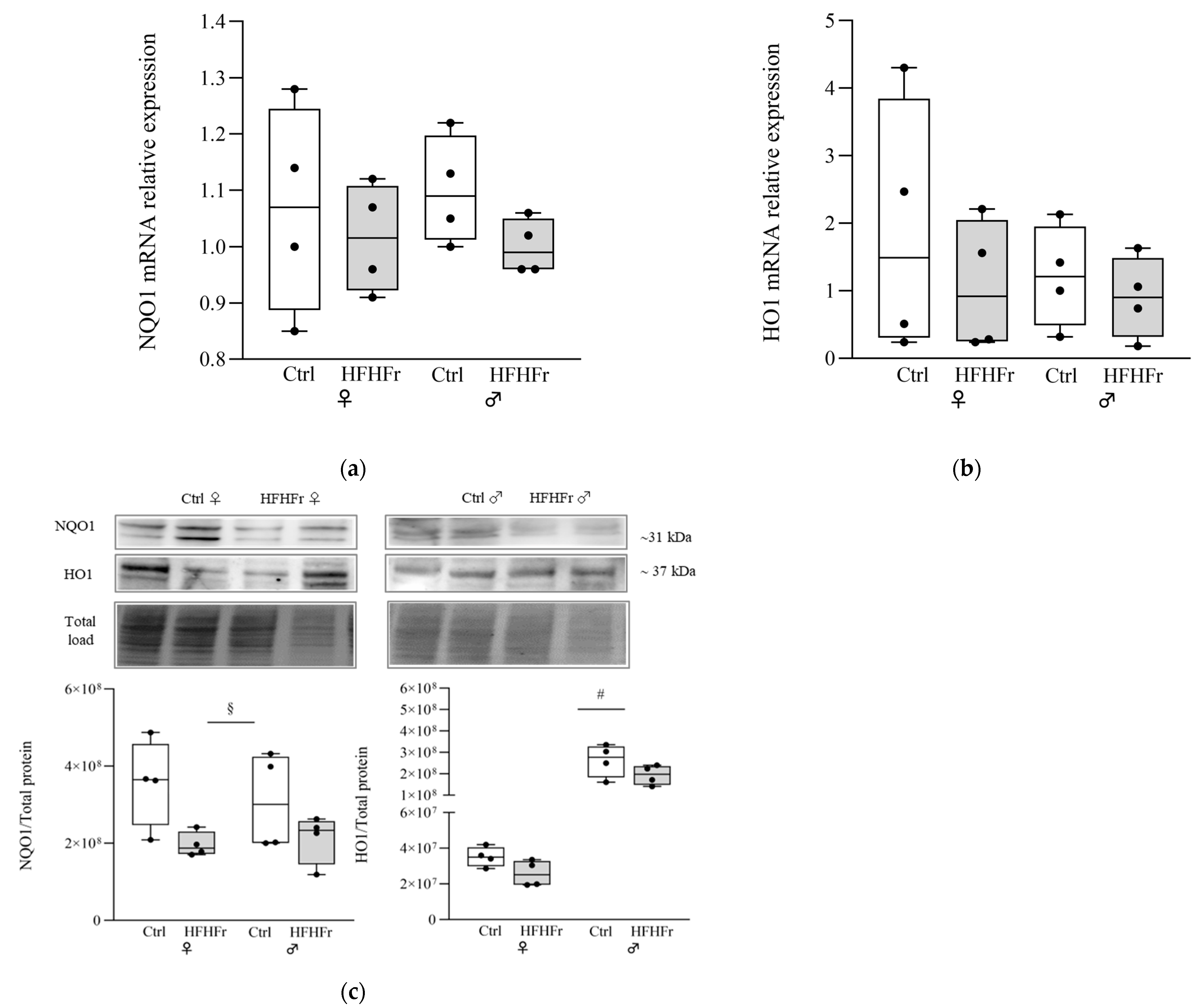
 ) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. Western blot representative images and densitometric analysis of (a) p62 and LC3B and (b) LAMP2 are shown. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). A significant main effect of diet is indicated by §. Tukey post hoc tests were conducted for group comparisons, and the results are indicated by *. Significant differences are indicated by p-values: §§,** p < 0.0021; *** p < 0.0002.
) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. Western blot representative images and densitometric analysis of (a) p62 and LC3B and (b) LAMP2 are shown. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). A significant main effect of diet is indicated by §. Tukey post hoc tests were conducted for group comparisons, and the results are indicated by *. Significant differences are indicated by p-values: §§,** p < 0.0021; *** p < 0.0002.
 ) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. Western blot representative images and densitometric analysis of (a) p62 and LC3B and (b) LAMP2 are shown. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). A significant main effect of diet is indicated by §. Tukey post hoc tests were conducted for group comparisons, and the results are indicated by *. Significant differences are indicated by p-values: §§,** p < 0.0021; *** p < 0.0002.
) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. Western blot representative images and densitometric analysis of (a) p62 and LC3B and (b) LAMP2 are shown. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). A significant main effect of diet is indicated by §. Tukey post hoc tests were conducted for group comparisons, and the results are indicated by *. Significant differences are indicated by p-values: §§,** p < 0.0021; *** p < 0.0002.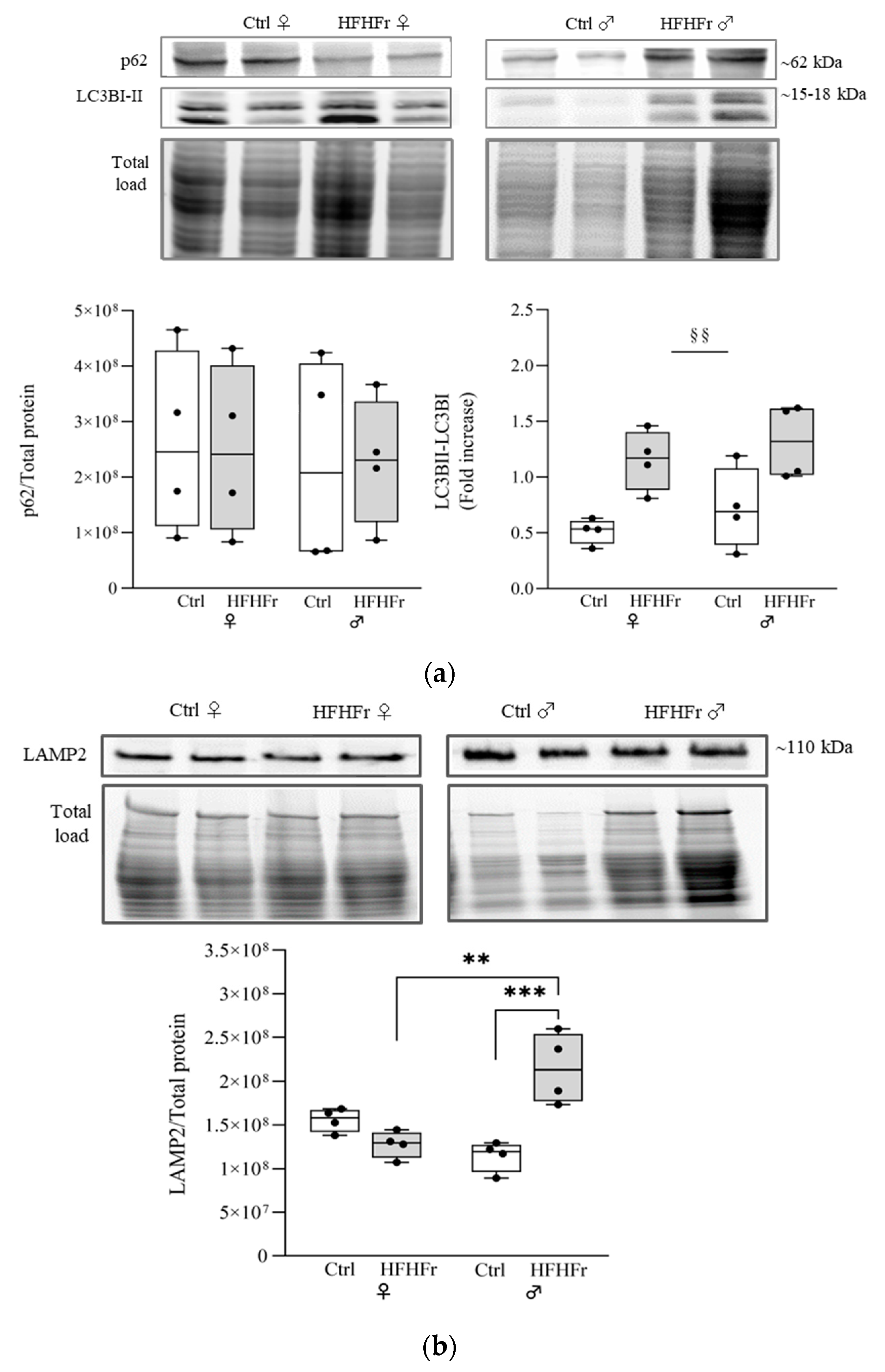
 ) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). Significant differences are indicated by p-values: * p < 0.05.
) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). Significant differences are indicated by p-values: * p < 0.05.
 ) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). Significant differences are indicated by p-values: * p < 0.05.
) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). Significant differences are indicated by p-values: * p < 0.05.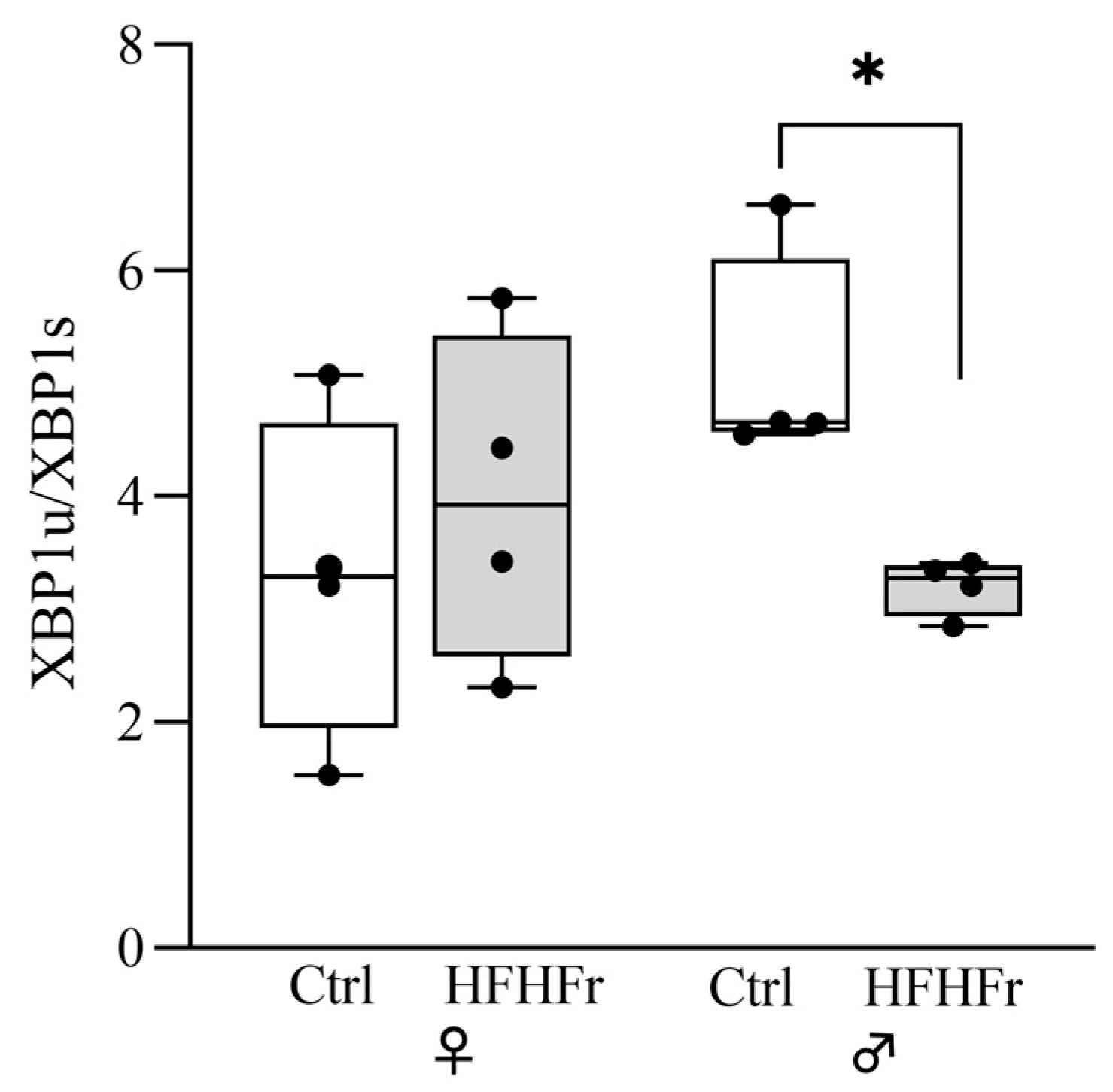
| Gene Product | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession Definition |
|---|---|---|---|
| Nrf2 | GGTTGCCCACATTCCCAAAC | CAGGGCAAGCGACTGAAATG | NM_001399173.1 |
| Keap1 | GGACGGCAACACTGATTC | TCGTCTCGATCTGGCTCATA | NM_057152.2 |
| Hmmo1/2 | ACAGGGTGACAGAAGAGGCTAA | CTGTGAGGGACTCTGGTCTTTG | NM_012580 |
| Nqo1 | AGCCCTGATTGTATTGGCCC | GATTCGACCACCTCCCATCC | NM_017000 |
| XBP1-s | GTCCGCAGCACTCAGACTAC | ATCTGAAGAGGCAACAGCGT | NM_001004210.2 |
| XBP1-u | CCATGGATTCGGCCCTCAG | CCGAAGAAGATGGGCAGCA | NM_001399536.1 |
| Hrd1 | CCTGTGAGCACTGCAGAAGA | TGCAAACAGAGAGGGAGCTG | NM_001100739.1 |
| Beta-actin | CGCGAGTACAACCTTCTTGC | ATACCCACCATCACACCCTG | NM_031144.3 |
| Gapdh | CTTCTTGTGCAGTGCCAGCC | CAAGAGAAGGCAGCCCTGGT | NM_017008.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Veroli, B.; Bentanachs, R.; Roglans, N.; Alegret, M.; Giona, L.; Profumo, E.; Berry, A.; Saso, L.; Laguna, J.C.; Buttari, B. Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose. Cells 2024, 13, 1247. https://doi.org/10.3390/cells13151247
Di Veroli B, Bentanachs R, Roglans N, Alegret M, Giona L, Profumo E, Berry A, Saso L, Laguna JC, Buttari B. Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose. Cells. 2024; 13(15):1247. https://doi.org/10.3390/cells13151247
Chicago/Turabian StyleDi Veroli, Benedetta, Roger Bentanachs, Núria Roglans, Marta Alegret, Letizia Giona, Elisabetta Profumo, Alessandra Berry, Luciano Saso, Juan Carlos Laguna, and Brigitta Buttari. 2024. "Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose" Cells 13, no. 15: 1247. https://doi.org/10.3390/cells13151247
APA StyleDi Veroli, B., Bentanachs, R., Roglans, N., Alegret, M., Giona, L., Profumo, E., Berry, A., Saso, L., Laguna, J. C., & Buttari, B. (2024). Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose. Cells, 13(15), 1247. https://doi.org/10.3390/cells13151247













