Male Tract Microbiota and Male Infertility
Abstract
1. Introduction
2. Materials and Methods
3. Results
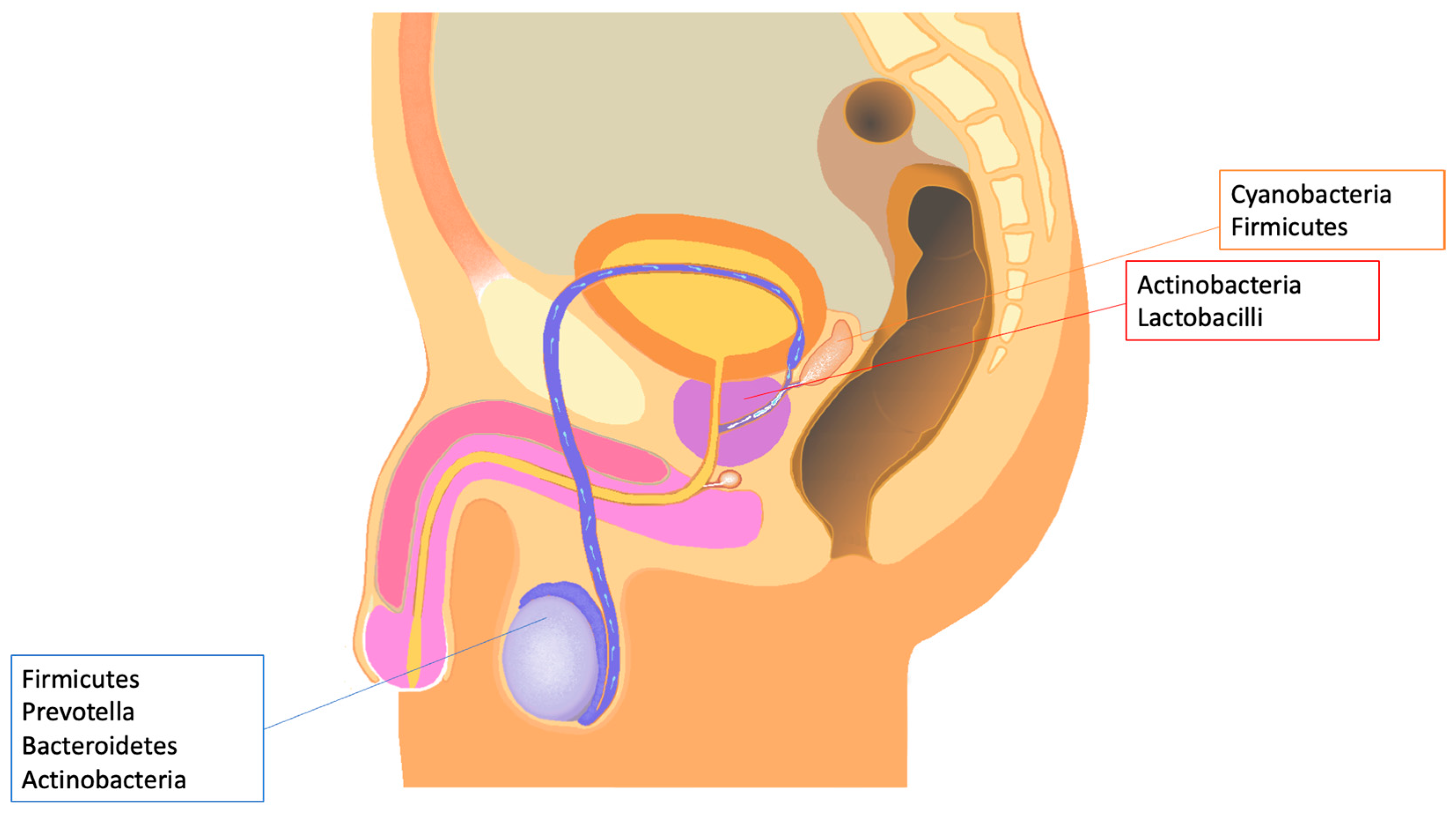
4. Semen and Male Tract Microbiota
5. Male Infertility and Microbiota
6. Leukocytospermia, Bacterial Prostatitis, and Microbiota Changes
7. Semen HPV Infection and Microbiota
8. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The Intestinal Metabolome: An Intersection between Microbiota and Host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Ahmadi Bashirzadeh, D.; Nazemalhosseini Mojarad, E.; Asadzadeh Aghdaei, H.; Norouzinia, M.; Shahrokh, S. Signature of Gut Microbiome by Conventional and Advanced Analysis Techniques: Advantages and Disadvantages. Middle East J. Dig. Dis. 2019, 12, 5–11. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Sheykhsaran, S.; Abbasi, A.; Leylabadlo, H.E.; Sadeghi, J.; Mehri, S.; Mazraeh, F.N.; Feizi, H.; Baghi, H.B. Gut microbiota and obesity: An overview of microbiota to microbial-based therapies. Postgrad. Med. J. 2023, 99, 384–402. [Google Scholar] [CrossRef]
- Tremellen, K. Gut Endotoxin Leading to a Decline IN Gonadal Function (GELDING)—A Novel Theory for the Development of Late Onset Hypogonadism in Obese Men. Basic Clin. Androl. 2016, 26, 7. [Google Scholar] [CrossRef]
- Daniel, J.A.; Abrams, M.S.; deSouza, L.; Wagner, C.G.; Whitlock, B.K.; Sartin, J.L. Endotoxin Inhibition of Luteinizing Hormone in Sheep. Domest. Anim. Endocrinol. 2003, 25, 13–19. [Google Scholar] [CrossRef]
- Shang, T.; Zhang, X.; Wang, T.; Sun, B.; Deng, T.; Han, D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology 2011, 52, 2827–2836. [Google Scholar] [CrossRef]
- Reddy, M.M.; Mahipal, S.V.K.; Subhashini, J.; Reddy, M.C.; Roy, K.R.; Reddy, G.V.; Reddy, P.R.K.; Reddanna, P. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod. Toxicol. 2006, 22, 493–500. [Google Scholar] [CrossRef]
- Hales, D.B. Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol. 2002, 57, 3–18. [Google Scholar] [CrossRef]
- Shen, P.; Ji, S.; Li, X.; Yang, Q.; Xu, B.; Wong, C.K.C.; Wang, L.; Li, L. LPS-Induced Systemic Inflammation Caused mPOA-FSH/LH Disturbance and Impaired Testicular Function. Front. Endocrinol. 2022, 13, 886085. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep. 2014, 4, 4260. [Google Scholar] [CrossRef]
- Cao, T.; Wang, S.; Pan, Y.; Guo, F.; Wu, B.; Zhang, Y.; Wang, Y.; Tian, J.; Xing, Q.; Liu, X. Characterization of the semen, gut, and urine microbiota in patients with different semen abnormalities. Front. Microbiol. 2023, 14, 1182320. [Google Scholar] [CrossRef]
- Kiessling, A.A.; Desmarais, B.M.; Yin, H.-Z.; Loverde, J.; Eyre, R.C. Detection and Identification of Bacterial DNA in Semen. Fertil. Steril. 2008, 90, 1744–1756. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sangwan, N.; Parekh, N.V.; Selvam, M.K.P.; Gupta, S.; McCaffrey, P.; Bessoff, K.; Vala, A.; Agarwal, A.; Sabanegh, E.S.; et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur. Urol. 2021, 79, 826–836. [Google Scholar] [CrossRef]
- Molina, N.M.; Plaza-Díaz, J.; Vilchez-Vargas, R.; Sola-Leyva, A.; Vargas, E.; Mendoza-Tesarik, R.; Galán-Lázaro, M.; Mendoza-Ladrón de Guevara, N.; Tesarik, J.; Altmäe, S. Assessing the Testicular Sperm Microbiome: A Low-Biomass Site with Abundant Contamination. Reprod. Biomed. Online 2021, 43, 523–531. [Google Scholar] [CrossRef]
- Yao, T.; Han, X.; Guan, T.; Wang, Z.; Zhang, S.; Liu, C.; Liu, C.; Chen, L. Effect of Indoor Environmental Exposure on Seminal Microbiota and Its Application in Body Fluid Identification. Forensic Sci. Int. 2020, 314, 110417. [Google Scholar] [CrossRef]
- Štšepetova, J.; Baranova, J.; Simm, J.; Parm, Ü.; Rööp, T.; Sokmann, S.; Korrovits, P.; Jaagura, M.; Rosenstein, K.; Salumets, A.; et al. The Complex Microbiome from Native Semen to Embryo Culture Environment in Human In Vitro Fertilization Procedure. Reprod. Biol. Endocrinol. 2020, 18, 3. [Google Scholar] [CrossRef]
- Campisciano, G.; Iebba, V.; Zito, G.; Luppi, S.; Martinelli, M.; Fischer, L.; De Seta, F.; Basile, G.; Ricci, G.; Comar, M. Lactobacillus Iners and Gasseri, Prevotella Bivia and HPV Belong to the Microbiological Signature Negatively Affecting Human Reproduction. Microorganisms 2020, 9, 39. [Google Scholar] [CrossRef]
- Weng, S.-L.; Chiu, C.-M.; Lin, F.-M.; Huang, W.-C.; Liang, C.; Yang, T.; Yang, T.-L.; Liu, C.-Y.; Wu, W.-Y.; Chang, Y.-A.; et al. Bacterial Communities in Semen from Men of Infertile Couples: Metagenomic Sequencing Reveals Relationships of Seminal Microbiota to Semen Quality. PLoS ONE 2014, 9, e110152. [Google Scholar] [CrossRef]
- Chen, H.; Luo, T.; Chen, T.; Wang, G. Seminal Bacterial Composition in Patients with Obstructive and Non-obstructive Azoospermia. Exp. Ther. Med. 2018, 15, 2884–2890. [Google Scholar] [CrossRef]
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The Seminal Microbiome in Health and Disease. Nat. Rev. Urol. 2019, 16, 703–721. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; del Rey, J.; Closa, L.; Garcia-Martínez, I.; Hobeich, C.; Castel, A.B.; Vidal, F.; Benet, J.; Oliver-Bonet, M. Characterization of Seminal Microbiome of Infertile Idiopathic Patients Using Third-Generation Sequencing Platform. Int. J. Mol. Sci. 2023, 24, 7867. [Google Scholar] [CrossRef]
- Opazo, M.C.; Ortega-Rocha, E.M.; Coronado-Arrázola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.; Kalergis, A.M.; Riedel, C.A. Intestinal Microbiota Influences Non-Intestinal Related Autoimmune Diseases. Front. Microbiol. 2018, 9, 432. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Kafka, J.K.; Ferreira, V.H.; Roth, K.; Kaushic, C. Innate and Adaptive Immune Responses in Male and Female Reproductive Tracts in Homeostasis and following HIV Infection. Cell. Mol. Immunol. 2014, 11, 410–427. [Google Scholar] [CrossRef]
- WHO, Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 1 May 2024).
- Ferlin, A.; Calogero, A.E.; Krausz, C.; Lombardo, F.; Paoli, D.; Rago, R.; Scarica, C.; Simoni, M.; Foresta, C.; Rochira, V.; et al. Management of Male Factor Infertility: Position Statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2022, 45, 1085–1113. [Google Scholar] [CrossRef]
- Fainberg, J.; Kashanian, J.A. Recent Advances in Understanding and Managing Male Infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef]
- Ferlin, A.; Foresta, C. Infertility: Practical Clinical Issues for Routine Investigation of the Male Partner. J. Clin. Med. 2020, 9, 1644. [Google Scholar] [CrossRef]
- Graziani, A.; Rocca, M.S.; Vinanzi, C.; Masi, G.; Grande, G.; De Toni, L.; Ferlin, A. Genetic Causes of Qualitative Sperm Defects: A Narrative Review of Clinical Evidence. Genes 2024, 15, 600. [Google Scholar] [CrossRef]
- Ferlin, A.; Dipresa, S.; Delbarba, A.; Maffezzoni, F.; Porcelli, T.; Cappelli, C.; Foresta, C. Contemporary Genetics-Based Diagnostics of Male Infertility. Expert Rev. Mol. Diagn. 2019, 19, 623–633. [Google Scholar] [CrossRef]
- Grande, G.; Graziani, A.; Ferlin, A. Guideline for Unexplained Couple Infertility: Misunderstandings on the Approach to the Male Factor. Hum. Reprod. 2024, 39, 859–860. [Google Scholar] [CrossRef]
- Jiao, J.; Xu, P.; Wang, X.; Xing, Z.; Dong, S.; Li, G.; Yao, X.; Guo, R.; Feng, T.; Yao, W.; et al. Enterotypes in Asthenospermia Patients with Obesity. Sci. Rep. 2022, 12, 16993. [Google Scholar] [CrossRef]
- Osadchiy, V.; Belarmino, A.; Kianian, R.; Sigalos, J.T.; Ancira, J.S.; Kanie, T.; Mangum, S.F.; Tipton, C.D.; Hsieh, T.-C.M.; Mills, J.N.; et al. Semen Microbiota Are Dramatically Altered in Men with Abnormal Sperm Parameters. Sci. Rep. 2024, 14, 1068. [Google Scholar] [CrossRef]
- Bottiglieri, T. S-Adenosyl-L-Methionine (SAMe): From the Bench to the Bedside--Molecular Basis of a Pleiotrophic Molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef]
- Marques, C.J.; Costa, P.; Vaz, B.; Carvalho, F.; Fernandes, S.; Barros, A.; Sousa, M. Abnormal Methylation of Imprinted Genes in Human Sperm Is Associated with Oligozoospermia. MHR Basic. Sci. Reprod. Med. 2008, 14, 67–74. [Google Scholar] [CrossRef]
- Lenzi, A.; Culasso, F.; Gandini, L.; Lombardo, F.; Dondero, F. Andrology: Placebo-Controlled, Double-Blind, Cross-over Trial of Glutathione Therapy in Male Infertility. Hum. Reprod. 1993, 8, 1657–1662. [Google Scholar] [CrossRef]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic Profiling Reveals Correlations between Spermiogram Parameters and the Metabolites Present in Human Spermatozoa and Seminal Plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Zhu, X.; Ma, M.; Chen, H.; He, J.; Liang, X.; Liu, G.; Yang, X. Interaction between Host and Microbes in the Semen of Patients with Idiopathic Nonobstructive Azoospermia. Microbiol. Spectr. 2023, 11, e0436522. [Google Scholar] [CrossRef]
- Krieger, J.N. NIH Consensus Definition and Classification of Prostatitis. JAMA J. Am. Med. Assoc. 1999, 282, 236–237. [Google Scholar] [CrossRef]
- Magistro, G.; Wagenlehner, F.M.E.; Grabe, M.; Weidner, W.; Stief, C.G.; Nickel, J.C. Contemporary Management of Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Eur. Urol. 2016, 69, 286–297. [Google Scholar] [CrossRef]
- Graziani, A.; Grande, G.; Martin, M.; Ferraioli, G.; Colonnello, E.; Iafrate, M.; Dal Moro, F.; Ferlin, A. Chronic Prostatitis/Chronic Pain Pelvic Syndrome and Male Infertility. Life 2023, 13, 1700. [Google Scholar] [CrossRef]
- Grande, G.; Milardi, D.; Baroni, S.; Luca, G.; Pontecorvi, A. Identification of Seminal Markers of Male Accessory Gland Inflammation: From Molecules to Proteome. Am. J. Reprod. Immunol. 2018, 80, e12992. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Korrovits, P.; Türk, S.; Ausmees, K.; Lapp, E.; Preem, J.; Oopkaup, K.; Salumets, A.; Truu, J. Seminal Microbiome in Men with and without Prostatitis. Int. J. Urol. 2017, 24, 211–216. [Google Scholar] [CrossRef]
- Yao, Y.; Qiu, X.-J.; Wang, D.-S.; Luo, J.-K.; Tang, T.; Li, Y.-H.; Zhang, C.-H.; Liu, H.; Zhou, L.; Zhao, L.-L. Semen Microbiota in Normal and Leukocytospermic Males. Asian J. Androl. 2022, 24, 398–405. [Google Scholar] [CrossRef]
- Grande, G.; Pompa, G.; Astorri, A.L.; Pontecorvi, A.; Milardi, D. Association of Probiotic Treatment with Antibiotics in Male Accessory Gland Infections. Am. J. Mens. Health 2022, 16, 155798832211190. [Google Scholar] [CrossRef]
- Bosch, F.X.; Broker, T.R.; Forman, D.; Moscicki, A.-B.; Gillison, M.L.; Doorbar, J.; Stern, P.L.; Stanley, M.; Arbyn, M.; Poljak, M.; et al. Comprehensive Control of Human Papillomavirus Infections and Related Diseases. Vaccine 2013, 31, G1–G31. [Google Scholar] [CrossRef]
- Garolla, A.; Graziani, A.; Grande, G.; Ortolani, C.; Ferlin, A. HPV-Related Diseases in Male Patients: An Underestimated Conundrum. J. Endocrinol. Investig. 2023, 47, 261–274. [Google Scholar] [CrossRef]
- Głowienka-Stodolak, M.; Bagińska-Drabiuk, K.; Szubert, S.; Hennig, E.E.; Horala, A.; Dąbrowska, M.; Micek, M.; Ciebiera, M.; Zeber-Lubecka, N. Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers 2024, 16, 399. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J.; Kero, K.; Syrjänen, S.; Collado, M.C.; Rautava, S. HPV Infection and Bacterial Microbiota in the Semen from Healthy Men. BMC Infect. Dis. 2021, 21, 373. [Google Scholar] [CrossRef]
- Allen-Vercoe, E.; Jobin, C. Fusobacterium and Enterobacteriaceae: Important Players for CRC? Immunol. Lett. 2014, 162, 54–61. [Google Scholar] [CrossRef]
- Elnaggar, J.H.; Huynh, V.O.; Lin, D.; Hillman, R.T.; Abana, C.O.; El Alam, M.B.; Tomasic, K.C.; Karpinets, T.V.; Kouzy, R.; Phan, J.L.; et al. HPV-Related Anal Cancer Is Associated with Changes in the Anorectal Microbiome during Cancer Development. Front. Immunol. 2023, 14, 1051431. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue and Patient Prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium Persistence and Antibiotic Response in Colorectal Cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Li, J.; Zhao, L.; Yan, W.; Lin, B.; Guo, X.; Wei, Y. Fusobacterium nucleatum Acts as a Pro-Carcinogenic Bacterium in Colorectal Cancer: From Association to Causality. Front. Cell Dev. Biol. 2021, 9, 710165. [Google Scholar] [CrossRef]
- Krisanaprakornkit, S.; Kimball, J.R.; Weinberg, A.; Darveau, R.P.; Bainbridge, B.W.; Dale, B.A. Inducible Expression of Human β-Defensin 2 by Fusobacterium nucleatum in Oral Epithelial Cells: Multiple Signaling Pathways and Role of Commensal Bacteria in Innate Immunity and the Epithelial Barrier. Infect. Immun. 2000, 68, 2907–2915. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the Link between Inflammation and Cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The Gut Mycobiome in Health, Disease, and Clinical Applications in Association with the Gut Bacterial Microbiome Assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef]
- Petre, G.C.; Francini-Pesenti, F.; Di Nisio, A.; De Toni, L.; Grande, G.; Mingardi, A.; Cusmano, A.; Spinella, P.; Ferlin, A.; Garolla, A. Observational Cross-Sectional Study on Mediterranean Diet and Sperm Parameters. Nutrients 2023, 15, 4989. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, Y.; Meng, R.; Tang, J.; Zhu, J.; Aldrich, M.C.; Cox, N.J.; Zhu, Y.; Li, Y.; Zhou, D. Cross-Talks between Gut Microbiota and Tobacco Smoking: A Two-Sample Mendelian Randomization Study. BMC Med. 2023, 21, 163. [Google Scholar] [CrossRef]
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Gitlin, S.; Gracia, C.; Hansen, K.; Jindal, S.; Kalra, S.; et al. Smoking and Infertility: A Committee Opinion. Fertil. Steril. 2018, 110, 611–618. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, X.; Zhong, X.; Settles, M.L.; Herring, J.; Wang, L.; Abdo, Z.; Forney, L.J.; Xu, C. Microbiota of the Seminal Fluid from Healthy and Infertile Men. Fertil. Steril. 2013, 100, 1261–1269.e3. [Google Scholar] [CrossRef]
- Mändar, R. Microbiota of Male Genital Tract: Impact on the Health of Man and His Partner. Pharmacol. Res. 2013, 69, 32–41. [Google Scholar] [CrossRef]
- Borovkova, N.; Korrovits, P.; Ausmees, K.; Türk, S.; Jõers, K.; Punab, M.; Mändar, R. Influence of Sexual Intercourse on Genital Tract Microbiota in Infertile Couples. Anaerobe 2011, 17, 414–418. [Google Scholar] [CrossRef]
- Sukarjati; Soebadi, D.M.; Hinting, A. Role of Escherichia coli Pili Adhesion Molecule to Inhibit Escherichia coli Adhesion to Human Spermatozoa In vitro. Androl. Gynecol. Curr. Res. 2014, 1, 3. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in Female Infertility Via Modulating Sperm Agglutination and Immobilization. Front. Cell Infect. Microbiol. 2021, 10, 620529. [Google Scholar] [CrossRef] [PubMed]
- Teveroni, E.; Di Nicuolo, F.; Vergani, E.; Bruno, C.; Maulucci, G.; Bianchetti, G.; Astorri, A.L.; Grande, G.; Gervasoni, J.; Santucci, L.; et al. Short-Chain Fatty Acids Modulate Sperm Migration through Olfactory Receptor 51E2 Activity. Int. J. Mol. Sci. 2022, 23, 12726. [Google Scholar] [CrossRef] [PubMed]
- Koort, K.; Sõsa, K.; Türk, S.; Lapp, E.; Talving, E.; Karits, P.; Rosenstein, K.; Jaagura, M.; Sekavin, A.; Sõritsa, D.; et al. Lactobacillus crispatus-dominated Vaginal Microbiome and Acinetobacter-dominated Seminal Microbiome Support Beneficial art Outcome. Acta Obstet. Gynecol. Scand. 2023, 102, 921–934. [Google Scholar] [CrossRef] [PubMed]
- De Lima Oliveira, L.C.S.; Carvalho Costa, E.; Gomes Martins, F.D.; Sales da Rocha, A.; Brasil, G.A. Probiotics supplementation in the treatment of male infertility: A Systematic Review. JBRA Assist. Reprod. 2024, 28, 341–348. [Google Scholar] [CrossRef]
- Chen, X.L.; Gong, L.Z.; Xu, J.X. Antioxidative activity and protective effect of probiotics against high-fat diet-induced sperm damage in rats. Animal 2013, 7, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Abbasi, H.; Niroumand, H. Synbiotic (FamiLact) administration in idiopathic male infertility enhances sperm quality, DNA integrity, and chromatin status: A triple-blinded randomized clinical trial. Int. J. Reprod. Biomed. 2021, 19, 235–244. [Google Scholar] [CrossRef]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef]
| Change in Seminal Microbiota | Seminal/Clinical Alterations | References |
|---|---|---|
| Increase in Prevotella spp. | Oligozoospermia | [19] |
| Obesity-induced asthenozoospermia | [37] | |
| Increase in Pseudomonas spp. | Oligozoospermia | [38] |
| Asthenozoospermia | [37] | |
| Increase in Lactobacillus iners | Oligozoospermia | [38] |
| Reduction in Lactobacilli | Reduction in all seminal parameters | [38,43] |
| Prostatitis/Leukocytospermia | [48,49] | |
| Increase in Steptococcus spp. | Leukocytospermia | [49] |
| Increase in Fusobacteria spp. | HPV infection | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, G.; Graziani, A.; De Toni, L.; Garolla, A.; Ferlin, A. Male Tract Microbiota and Male Infertility. Cells 2024, 13, 1275. https://doi.org/10.3390/cells13151275
Grande G, Graziani A, De Toni L, Garolla A, Ferlin A. Male Tract Microbiota and Male Infertility. Cells. 2024; 13(15):1275. https://doi.org/10.3390/cells13151275
Chicago/Turabian StyleGrande, Giuseppe, Andrea Graziani, Luca De Toni, Andrea Garolla, and Alberto Ferlin. 2024. "Male Tract Microbiota and Male Infertility" Cells 13, no. 15: 1275. https://doi.org/10.3390/cells13151275
APA StyleGrande, G., Graziani, A., De Toni, L., Garolla, A., & Ferlin, A. (2024). Male Tract Microbiota and Male Infertility. Cells, 13(15), 1275. https://doi.org/10.3390/cells13151275







