Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease
Abstract
1. Introduction
- (1)
- A flat and elongated teardrop or cobblestone-like shape with a polygonal cell outline. The endothelial shape facilitates the blood flow dynamics (laminar blood flow due to the ECs not being studded with ciliae), and it is crucial for selective barrier formation and adaptation to environmental changes [8,9,10].
- (2)
- Possession of different junction types. The junctional complexes play a crucial role in the formation and stabilization of the endothelial barrier and in regulating permeability between the blood and surrounding tissues [11,12,13,14,15]. Barrier specialization is directly related to junction types that specifically affect the endothelial layer’s permeability, endothelial cell growth, apoptosis, and intracellular signal transmission [16,17]. Cell–cell junctional contacts impact the rearrangement of the endothelial layer through different signaling pathways (e.g., PI3Ka/MYPT1/MLCP [18], Notch, Rho GTPase, Wnt/beta-catenin, and Hippo pathways [19,20,21,22];
- (3)
- The presence of a strong extracellular matrix basal lamina, reinforcing the ECs and contributing to their trophic functions, such as survival, proliferation, and differentiation [23,24,25]. The basal lamina is associated with the vessel networks that are essential for maintaining local tissue homeostasis, post-transcriptional modifications, and possibly even the regulation of gene expression [26];
- (4)
- Polarity of the endothelial cell surface. Herein, we refer to endothelial asymmetry in the structural organization and components of the apical and basolateral surfaces of ECs. This property is one of the keys to maintaining the endothelial barrier, facilitating cell migration and vectorial transport of biomolecules and enabling proper signaling. The polarity is closely related to the positioning and distribution of the centrosomes and Golgi apparatus, centrosomal microtubule proteins (Atp6ap2, Tacc3-ch-Tog, Cep41, CG-Nap), and non-centrosomal microtubule proteins (Campsap2, Par-6, Pkc3) [27,28,29,30,31,32,33,34,35,36], as well as to the sorting of proteins and asymmetric protein surface distribution [37].
2. Endothelial Development
2.1. Endothelial Origin, Cell Specification, and Vessel Organization
| Lymphangiogenesis Growth and Development of Lymphatic Vessels in Prenatal and Postnatal Life | ||
|---|---|---|
| Mechanism and phases | Signaling and transcriptional regulators | Markers of endothelial differentiation |
| I. Prenatal lymphangiogenesis | 1. ETS domain protein [84] 2. SOXF factors: SOX7, SOX17, and SOX18 [81,83,85,86] 3. Vascular endothelial growth factor-C (VEGF-C)/vascular endothelial growth factor-F (VEGF-F)/vascular endothelial growth factor-D (VEGF-D) [59,81,82,83,84,85,86,87,88] 4. Prox1 [58,81,83,84,85,86] 5. Forkhead box C2 (FOXC2) [81,82,84,85,86] 6. Rho family GTPase (RAC-1) [85] 7. Tyrosine kinase Syk [81,85] 8. SLP 76 [81] 9. Phosphatase-Cγ2(PLCγ2) [84] 10. Semaphorin 3F (SEMA3F) [81,84] 11. Chicken ovalbumin upstream promoter transcription factor (COUP-TFII) [58,83,85] | Platelet endothelial cell adhesion (PECAM-1) [84,85] CD34 [80,81,84,87] CXCR4 (through CXCL12 stimulation) [58,85] LYVE-1 [80,82,83,84,85,86,87] Podoplanin [58,80,81,84,87] Vascular endothelial growth factor receptor 3 (VEGFR-3) [80,81,82,83,84,85,86,87] PROX-1 (Prospero homeobox protein) [58,81,83,84,85,86] CD44 [80,87] |
| (1) Classical Lymphatic vascular development: centrifugal sprouting from primary lymph sacs arises from embryonic cardinal veins (starts at E9.5-10.5 in mice and 6–7 weeks in humans) | ||
| A. Budding and sprouting lymphatic ECs from cardinal veins [82,83,84,85,86] | ||
| B. Centrifugal migration lymphatic ECs [82,83,84,85,86] | ||
| C. Proliferation lymphatic ECs and generation of a one-way network of capillaries [82,83,84,85,86] | ||
| (2) Lymphatic vascular differentiation, according to G. Oliver (2004) [87] | ||
| A. Lymphatic competence (lymphatic ECs from a vein at the E9.0-9.5 receive the ability to answer specific lymphatic-inducing signals) [87] | ||
| B. Lymphatic bias (determination of the lymphatic ECs fate, approx. E9.0-10.5) [87] | ||
| C. Lymphatic specification (lymphatic ECs differentiate into the desired phenotype independently from microenvironmental cues, approx. E10.5.-14.5) [87] | ||
| D. LEC differentiation (maturation and separation of lymphatic vessels, approx. E14.4. postnatal life) [87] | ||
| II. Postnatal lymphangiogenesis | ||
| closely related to such pathological processes as implantation and tumorogenesis (phases similar to angiogenesis) | ||
| A. Sprouting lymphangiogenesis: occurs with or without lymph flow in pre-existing vessels [58] | ||
| B. Intussusceptive lymphangiogenesis is dependent on lymph flow [58] | ||
2.2. Pathways and Factors Involved in the Regulation of Endothelial Development
3. Endothelial Ontogenesis
3.1. ECs and Stem Cells Interchange
3.2. Role of ECs in Hematopoiesis and Vasculogenesis
4. Endothelial Plasticity as the Base of Endothelial Diversity
4.1. Target Regulation of Endothelial Plasticity and Heterogeneity
4.1.1. NOTCH
4.1.2. WNT
4.1.3. BMP
4.1.4. TGFβ
4.1.5. VEGF
4.1.6. LMO2
4.1.7. ETS (ETV2)
4.1.8. TWIST-1
4.1.9. HIF1
5. Endothelial Diversity along the Vascular Bed
5.1. Diversity of Ecs in the Microcirculation
| Vasculogenesis Differentiation of Endothelial Precursor Cells (EPCs) into ECs and De Novo Formation of the Primitive Vascular Network (ECs from Mesoderm) | ||
|---|---|---|
| Phases: | Signaling and transcriptional regulators: | Markers of endothelial differentiation: |
| I. Extraembryonic vasculogenesis: starts in ~ mice E6.5 embryos, ~3 weeks in humans (yolk sac, allantois, placenta) [59,61,65] | 1. Fibroblast growth factor (FGF) family includes 18 paracrine and endocrine factors [56,62,69,73,74] 2. The hedgehog family: Shh, Ihh, Dhh [70] 3. Vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptors (VEGFRs): VEGFR-2 and VEGFR-1 [56,57,64,67,68,69,74,75,76,84] 4. Neuropilin1(NRP-1) and Neuropilin2 (NRP2) [63,65,67,68,72,76] 5. Transforming growth factor β(TGFβ) and transforming growth factor receptors (TGFRs) [56,57,63,69,70,74,75] 6. Angiopoietins 1 and 2 (ANG1 and ANG2) binds tyrosine kinase with immunoglobulin-like and EGF-like domains 1 and 2 (Tie1 and Tie2) and affect the remodeling of capillary plexuses [70] 7. Platelet-derived growth factor (PDGF) recruitment of pericytes and smooth muscle cells [57,63,65,70,72,74,76] 8. GATA proteins [61,65,67] 9 Krüppel-like factors [61,63] 10. ETS proteins regulate ECs differentiation [61] 11. Homeodomain proteins (HOXB3 and HOXD3) participate in morphogenesis of vascular tube formation [62,70,90] 12. Epidermal growth factor like domain 7(EGFL7) participation in separation and arrangement of angioblast) [59,61] 13. Fibronectin and its receptor α5β1 [65] 14. SOXF factors: SOX7, SOX17, and SOX18 [66] 15. Granulocyte colony-stimulating factor (G-CSF) attenuated delayed tPA [63,71] 16. Overexpressed hypoxia- inducible factor (HIF-1α) stimulates endothelial progenitor cells [64,76] 17. Increase in intracellular Ca2+ concentration [68] 18. Bone morphogenetic protein (BMP) signaling pathway [63] 19. retinoid acid [67] 20. Wnt β-catenin signaling [56,61,67] 21. T- box transcription factor gene 18 (TBX18) [56] 22. Wilms tumor transcription factor 1) WT1 [56] | Vascular endothelium cadherin VE-cadherin [59,61,63,65,67,71,76] von Willebrand Factor (vWF) [62] CD34 (early angioblasts and endothelial progenitors) [59,62,63,71,72,73] T-cell acute lymphocytic leukemia (TAL1) [61,65,84] platelet endothelial cell adhesion (PECAM-1)/CD31 [56,59,61,63,73] Tyrosine kinase with immunoglobulin-like and EGF-like domains (Tie-1) and tyrosine kinase with immunoglobulin-like and EGF-like domains (Tie-2) [61,63,65] Flk1 and Flt1 [61,65] Thrombospondin type1 domain-containing protein 1 (THSD1) [62] |
| A—Assemblation of blood island within the mesodermal layer (yolk sac) [61] | ||
| B—Hemangioblast formation from blood islands (hemangioblast inner part gives rise to the hematopoietic precursors, the outer part give rise to angioblast, differentiation in situ) [61] | ||
| C—Primitive extraembryonic vascular (may contain primitive erythrocytes) plexus organization and ECs differentiation happens in association with hematopoietic precursors in blood islands [61,65] | ||
| II. Intraembryonic vasculogenesis: It starts in ~ mice E7.3 embryos and gives rise to the endocardium great vessels and is not associated with blood formation. There are two types of intra-embryonic hemangioblast forming vascular plexus (usually without erythrocytes) [61,65,73] | ||
| A—Hemangioblast from splanchnopleuric mesoderm (visceral) associated with the endoderm (production of hematopoietic cells and paraaortic splanchnopleura) [63,69] | ||
| AA—Hemangioblast from somatopleuric mesoderm (parietal) associated with ectoderm (give rise to all types of cells, except hematopoietic stem cells) [63,69,73] | ||
| B—Primitive intraembryonic vascular plexuses formation: ECs differentiate from mesoderm as solitary angioblast without the concomitant differentiation of hematopoietic stem cells, except for small regions in the aorta (paraaortic clusters) [61] | ||
| III. Functional vascular network formation in vasculogenesis: primary capillary extraembryonic plexuses anastomose with intraembryonic vasculature through the vitelline arteries and veins and then connect with developing heart tube [61] | ||
| Angiogenesis Growth of primary and secondary vascular plexus from pre-existing blood vessels (vessels which lack a fully developed tunica media) in prenatal or postnatal life | ||
| Mechanism and phases: | Signaling and transcriptional regulators: | Markers of endothelial differentiation: |
| I. Sprouting mechanism—based on endothelial cell migration, proliferation, and tube formation with or without blood flow (the result is new vascular tube formation) [56,64,67,68,69,73,84] | 1. Vascular endothelial growth factor VEGF [56,57,61,62,63,65,67,69,70,71,72,74,75] 2. Transforming growth factor β,α (TGFβ,α) [64,66,70,71] 3. Angiopoietins and tyrosine kinase with immunoglobulin-like and EGF-like domain receptors (Tie receptors) [65,70,74] 4. Platelet-derived growth factor (PDGF) and Platelet-derived growth factor receptor-β (PDGFR-β) [65,73] 5. SOXF factors: SOX7 and SOX18 [66] 6. Atypical chemokine receptor CXCR7(ACKR3) [76] 7. Angiopoietins 1 and 2 (ANG1 and ANG 2) [69,70,74] 8. Granulocyte colony-stimulating factor (G-CSF) attenuated delayed tPA [63,71] 9. hypoxia-inducible factor HIF-1α [65,71] 10. Increase in intracellular Ca2+ concentration [68] 11. High mobility group box 1 (HMGB-1) [60] 12. Connective tissue growth factor (CCN2) [60] 13. Delta/jagged-NOTCH signaling [61,63,65] 14. Metalloproteinases MMP [63,69] 15. Ephrin- B (EPH-B) [63,69] 16. Semaphorins (SEMA 3 proteins) [63,65,70] 17. Rho-associated protein kinase (ROCK) [63] 18. Chicken ovalbumin upstream promoter transcription factor (Coup-TFII) [84] | Vascular endothelium cadherin (VE-cadherin) [59,61,63,65,67,71,76] von Willebrand Factor (vWF) [62] CD34 [59,62,63,71,72,73] Platelet endothelial cell adhesion (PECAM-1)/CD31 [56,59,61,63,73] Endoglin/CD105/ [65,72] THSD1 Thrombospondin type1 domain-containing protein (THSD1) 1 [62] Krüppel-like factor 4(KLF4) [63] A disintegrin-like and metalloprotease with thrombospondin type 1 repeats 13 (ADAMTS-18) [62] Aminopeptidase N APN(CD13) in tumorigenesis [72] Intercellular adhesion molecule-1(ICAM-1 or CD54) [72] |
| A. Neovessel growth—the disintegration of the basal lamina of existing vessel, migration and proliferation of ECs, lumen formation, and loops organization by sprouts and anastomoses [226,239] | ||
| B. Neovessel stabilization—delay of the endothelial proliferation, basal lamina reconstruction, coverage of the immature vessel with pericytes [63,67] | ||
| II. Intussusceptive microvascular growth (IMG) mechanism: based on the division of existing vessel lumens by formation and insertion of tissue folds and interstitial cellular columns into the lumen of pre-existing vessels (lumen expansion occurs through the organization of new units of extracellular matrix). Blood flow-dependent process [63,69] | ||
| A. Interendothelial “transluminal bridge” formation: ECs located at the opposite side of the capillary wall move near to each [63] | ||
| B. “Cylinder tissue bridge” establishment: tissue form as a cylinder bridge, which perforates endothelial bilayer and extends across the lumen. Then ECs cover the cylinder tissue bridge involving the cytoplasmic extensions of myofibroblast and their microfilaments inside the cylinder’s core [63] | ||
| C.” Pillar” formation: the framing of the pillar by pericytes close to the lateral part wall [63] | ||
| D.” Pillar” growth: pillar growth into an intercapillary mesh [63] | ||
5.2. Diversity of Ecs in the Large Blood Vessels
5.3. Lymphatic Macro/Microcirculation
6. Selected Tissue-Specific Endothelial Phenotypes
7. Alteration of Endothelial Cells and Pathologies
7.1. Cancer
| Morpho-Functional Characteristics | ECs Types | ||||
|---|---|---|---|---|---|
| Continuous | Fenestrated Endothelium | Lymphatic | |||
| Pseudo-Fenestrated Fenestrated | Glomerular Fenestrated Endothelium (True Fenestrated) | Disconntimous Fenestrated (Sinusoid) Endothelium | |||
| Localization | Brain [1,277,278] Skin [1,270] Lungs [1,295,296,302] Heart [1,270,293,295,299,302] Arteries [1,270,272,289,292,295,299] Veins [1,270,295,296,299] | Intestinal tube [270,275,278,287,293,296] Adrenal cortex [273,278,293,295] Pancreatic islets [278,293] Kidney peritubular capillaries [278,289,293,296,301] | Kidney (Glomeruli) [270,275,290,293,295,296] | Liver [270,278,293,296,299] Spleen [270,278,293] Bone-marrow [275,278,293,295,299] | Lymphatic vessels and lymph nodes [232,278,283,288,300,302] |
| Function | Highly selective barrier: transfer of water and small solutes (diameter ~6 nm), transport of big molecules occurs through channels or transcytosis [270,278] | Size and selective charge barrier: permeable for small molecules and water, but impermeable for macromolecules (e.g., albumin, peptide hormones) and blood cells [278,286,293] | Low-selective barrier: permeable for small molecules and water and macromolecules (e.g., albumin) but impermeable for cells from ultrafiltrate [290,293] | Non-selective barrier: permeable for water, macromolecules, and blood cells [291,293,297] | Non-selective barrier of lymphatic capillaries (sinusoid lymphatic ECs): permeable for macromolecules and immune cells (high permeability) Selective barrier, collecting lymphatic vessels demonstrate low permeability [288] |
| Basal lamina | Yes [270,293] | Yes [293] | Yes [293] | Absent or poorly developed [270,291,293] | Lymphatic capillaries (initial capillaries): highly incomplete perforated basal lamina and discontinuous junctions (buttons) [288,294] Collecting lymphatic vessels: continuous basal lamina, continuous junctions (zippers) [294] |
| Fenestra, nm | No [1,270,276,293] | 60–70 [273,293] | 60–100 [293,301] | “Sieve plates”: 50–100 “Gaps”: 100–200 [293,296,297] | No [288] |
| Fenestral diaphragm | No [270,276] | Yes [1,273,293] | No [296] | No [296] | No [288] |
| Glycocalyx | Yes [275] | Yes [293,296] | Yes [275,290,301] | Yes [269,377,378] | Yes [303] |
| Non-specific Markers | CD31: Heart (low expression), skin [277,285] CD34: Heart, skin [292] von Willebrand factor (vWF): Heart (low expression), skin, lungs [277,292] CD62E or E -selectin (inducible) [270,277,292] CD62P or P selectin (inducible) [270,277,292] CD106 or VCAM-1 (inducible) [277] CD54 or ICAM (inducible) [277] Flt-1 or vascular endothelial growth factor receptor 1 (VEGFR1, inducible) [277] KDR/Flk or vascular endothelial growth factor receptor 2 (VEGFR2, inducible) [277] CD144 human [26,280] | CD31 [277,285,292] CD34 [277,285,292] von Willebrand factor (vWF) peritubular ECs (low expression) Fli-1 (nuclear) [277,285,292] CD62E or E -selectin (inducible) [277,285,292] CD62P or P selectin (inducible) [277,285,292] CD106 or VCAM-1 (inducible) [277,285,292] CD54 or ICAM (inducible) [277,285,292] Flt-1 or vascular endothelial growth factor receptor 1 (VEGFR1, inducible) [277,285,292] KDR/Flk or vascular endothelial growth factor receptor 2 (VEGFR2, inducible) [277,285,292] vWf [277,285,292] | CD31 [277,285,292] CD34 [277,285,292] Fli-1(nuclear) [277,285,292] von Willebrand factor (vWF) expression) [277,285,292] CD62E or E -selectin (inducible) [277,285,292] CD62P or P selectin (inducible) [277,285,292] CD106 or VCAM-1 (inducible) [277,285,292] CD54 or ICAM (inducible) [277,285,292] Flt-1 or vascular endothelial growth factor receptor 1 (VEGFR1, inducible) [277,285,292] KDR/Flk or vascular endothelial growth factor receptor 2 (VEGFR2, inducible) [277,285,292] | CD31: Liver, Spleen; Bone marrow [277,292] CD34: Bone marrow [277,292] von Willebrand factor (vWF): liver, spleen [61,78] Fli-1: Liver, spleen, bone marrow [277,292] CD62E or E -selectin (inducible) [277,291] CD62P or P selectin (inducible) [277] CD106 or VCAM-1 (inducible) [277] CD54 or ICAM (inducible) [277] Flt-1 or vascular endothelial growth factor receptor 1 (VEGFR1, inducible) [277] KDR/Flk or vascular endothelial growth factor receptor2 (VEGFR2, inducible) [277] | CD31 [277,285,292] CD34 [278,284,292] Fli-1 [284,292] von Willebrand factor (vWF) [284,292] CD62E or E -selectin (inducible) [277,284] CD62P or P selectin (inducible) [277] CD106 or VCAM-1 (inducible) [277] CD54 or ICAM (inducible) [277] Flt-1 or vascular endothelial growth factor receptor 1 (VEGFR1, inducible) [277] KDR/Flk or vascular endothelial growth factor receptor 2 (VEGFR2, inducible) [277] |
| Specific markers | Angiotensin-converting enzyme ACE or CD143 (human heart and lungs) [277,278] Thrombomodulin (TM): absent in brain endothelial cells [277,278] Tissue non-specific alkaline phosphatase or TNAP: brain (mouse and human and rat) [276,278] Thrombospondin type 1 domain or THSD1: vessels (mouse and human) [278,281] P-glycoprotein or MDR 1a: brain and lungs (mouse and human) [20,277] CD73/ transferrin receptor: brain (mouse and human) [277] Platelet-derived growth factor receptor: brain (human and mouse) [277,282] Sca-1 (mouse pulmonary ECs) [277] HLA-DR (human, pulmonary ECs) [280] Glut-1: brain (human and mouse) [277] | PV1 (human and mouse peritubular capillary) [293,295,296,299] MAdCAM-1 (venules intestinal) [277] Nephrin (human Pancreatic islet) [280] CD117 (mouse pancreatic islet) [278] | ADAMTS-13 (mouse and human) [298] | CD32b (human liver sinusoidal) [280,336,434] LYVE-1 (mouse liver and spleen sinusoidal) [278,304] PV-1 (mouse spleen sinusoidals) [280,296] Angiotensin-converting enzyme (ACE or CD143) Stabilin 1,2 [434] Liver-endothelial differentiationassociation protein (LEDA-1) [434] | CD90 (human and mouse) [280] Flt-4 or vascular endothelial growth factor receptor 3 (VEGFR3, human and mouse) [277] Desmoplakin [277] Podoplanin or PDPN (human) [278,280] LYVE-1 (human and mouse) [278,280] Prox-1 (human and mouse) [280,294] Clever-1 or Stabilin-1 or FEEL-1 (human and mouse) [231,280,303] |
| Junctions | Tight junctions/adherence junctions [272] | Tight junctions/gap junctions [272,279] | Tight junctions/gap junctions [272,279] | Gap junctions/tight junctions [272,279,302] | Buttons (discontinuous button-like junctions, with openings at the borders of adjacent lymphatic ECs) enriched with adherents and tight junction proteins [350,353] Zippers (continuous zipper-like junctions without openings at the borders of adjacent lymphatic ECs) enriched with adherent and tight junction proteins [350,353] |
7.2. Endothelial Turnover, Regeneration, and Repair
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AECs | arterial ECs |
| ANG1 and ANG 2 | angiopoietins 1 and 2 |
| ACE1 | Angiotensin 1-converting enzyme |
| BMP | bone morphogenetic proteins pathway |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| EMT | epithelial–mesenchymal transition |
| EndMT | endothelial-to-mesenchymal transition |
| ER71 (ETV2) | ETS variant transcription factor 2 |
| ESAM | Endothelial cell-selective adhesion molecule |
| ETS family | E twenty-six family transcription factors |
| FGF | fibroblast growth factor |
| FLI1 | friend leukemia integration-1 transcription factor |
| HES1, 2 | hairy and enhancer of split 1, 2 |
| HEY1 | hairy/enhancer of split related with YRPW motif protein 1 |
| HGF | hepatocyte growth factor |
| HIF-1α | hypoxia-inducible factor 1α |
| Hh | Hedgehog |
| ICAM-1 | intercellular adhesion molecule-1 (or CD54) |
| IHECs | intraembryonic hemogenic endothelial cells |
| LMO2 | Lim domain only 2 |
| LSECs | liver sinusoid ECs |
| LYVE 1 | lymphatic vessel endothelial hyaluronan receptor |
| MAML1,2,3 | mastermind-like protein 1,2,3 |
| MAP4K4 | mitogen-activated protein-4-kinase 4 |
| NRP 1,2 | neuropilin1, 2 |
| PI3K/Akt | phosphoinositide 3-kinase/protein kinase B |
| PDGF | Platelet-derived growth factor |
| PDGFR-β | Platelet-derived growth factor receptor-β |
| RNA | ribonucleic acid |
| RUNX1 | runt-related transcription factor 1 |
| S1PR1 | sphingosine 1-phosphate (S1P)–sphingosine 1-phosphate receptor |
| SCL/TAL1 or TAL1 or SCL | stem cell leukemia/or T-cell acute lymphocytic leukemia-1 |
| scRNA-seq analysis | single-cell RNA sequencing analysis |
| SFRP | secret frizzled-related protein |
| SMADs | homologies to SMA (“small” worm phenotype) and MAD family (“Mothers Against Decapentaplegic”) genes |
| SSECs | spleen sinusoid ECs |
| TAK-1 | transforming growth factor-beta activated kinase 1 |
| TCF7L2 | transcription factor 7-like 2 (TCF4) |
| TGFβ | α-transforming growth factor β |
| TIE | tyrosine kinase with immunoglobulin-like and EGF-like domains |
| TLR2 | toll-like receptor-2 |
| TNF-α | tumor necrosis factor-α |
| TWIST1 | twist family of basic helix-loop-helix protein 38 (bHLHa38) transcription factor 1 |
| VCAM1 | vascular cell adhesion protein1 |
| VE-cadherin | vascular endothelial cadherin (cadherin-5 or CD144) |
| VEGF | vascular endothelial growth factor |
| VEGFR-1 (Flt 1) | vascular endothelial growth factor receptor 1 |
| VEGFR2 (Flk1/KDR) | vascular endothelial growth factor receptor 2 |
| VenECs | venous ECs |
| vWf | von Willebrand factor |
| WNT | Wingless-related integration site |
References
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Raymond, T.; Frances, P. Endothelium. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; Barr Smith Press: Adelaide, Australia, 2011; pp. 1–12. ISBN 978-0-9871718-2-5. [Google Scholar]
- Michiels, C. Endothelial Cell Functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Huang, N.F.; Botham, C.M.; Sayed, N.; Cooke, J.P. Endothelial Cells Derived from Nuclear Reprogramming. Circ. Res. 2012, 111, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Al-Soudi, A.; Kaaij, M.H.; Tas, S.W. Endothelial Cells: From Innocent Bystanders to Active Participants in Immune Responses. Autoimmun. Rev. 2017, 16, 951–962. [Google Scholar] [CrossRef]
- Jeong, H.W.; Hernández-Rodríguez, B.; Kim, J.M.; Kim, K.P.; Enriquez-Gasca, R.; Yoon, J.; Adams, S.; Schöler, H.R.; Vaquerizas, J.M.; Adams, R.H. Transcriptional Regulation of Endothelial Cell Behavior during Sprouting Angiogenesis. Nat. Commun. 2017, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Singh, I. Textbook of Human Histology (with Colour Atlas and Parctical Guide), 6th ed.; Vij, J., Ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Dehli, India, 2011; ISBN 978-93-80704-34-0. [Google Scholar]
- Potter, C.M.F.; Schobesberger, S.; Lundberg, M.H.; Weinberg, P.D.; Mitchell, J.A.; Gorelik, J. Shape and Compliance of Endothelial Cells after Shear Stress In Vitro or from Different Aortic Regions: Scanning Ion Conductance Microscopy Study. PLoS ONE 2012, 7, e31228. [Google Scholar] [CrossRef]
- Malek, A.M.; Izumo, S. Mechanism of Endothelial Cell Shape Change and Cytoskeletal Remodeling in Response to Fluid Shear Stress. J. Cell Sci. 1996, 109, 713–726. [Google Scholar] [CrossRef]
- Eroschenko, V.P. Atlas of Histology, 10th ed.; Taylor, C., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; p. 19106. ISBN 13: 978-0-7817-7057-6. [Google Scholar]
- Milton, S.G.; Knutson, V.P. Comparison of the Function of the Tight Junctions of Endothelial Cells and Epithelial Cells in Regulating the Movement of Electrolytes and Macromolecules across the Cell Monolayer. J. Cell. Physiol. 1990, 144, 498–504. [Google Scholar] [CrossRef]
- Liepsch, D.W.; Levesque, M.; Nerem, R.M.; Moravec, S.T. Correlation of Laser-Doppler-Velocity Measurements and Endothelial Cell Shape in a Stenosed Dog Aorta. Adv. Exp. Med. Biol. 1988, 242, 43–50. [Google Scholar] [CrossRef]
- Palhol, J.S.C.; Balia, M.; Sánchez-Román Terán, F.; Labarchède, M.; Gontier, E.; Battefeld, A. Direct Association with the Vascular Basement Membrane Is a Frequent Feature of Myelinating Oligodendrocytes in the Neocortex. Fluids Barriers CNS 2023, 20, 24. [Google Scholar] [CrossRef]
- Stratman, A.N.; Davis, G.E. Endothelial Cell-Pericyte Interactions Stimulate Basement Membrane Matrix Assembly: Influence on Vascular Tube Remodeling, Maturation, and Stabilization. Microsc. Microanal. 2012, 18, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, M.G.; Dejana, E. Interendothelial Junctions: Structure, Signalling and Functional Roles. Curr. Opin. Cell Biol. 1997, 9, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Z.; Lei, S. Changes of Junctions of Endothelial Cells in Coronary Sclerosis: A Review. Chronic Dis. Transl. Med. 2016, 2, 22–26. [Google Scholar] [CrossRef]
- Angulo-Urarte, A.; Casado, P.; Castillo, S.D.; Kobialka, P.; Kotini, M.P.; Figueiredo, A.M.; Castel, P.; Rajeeve, V.; Milà-Guasch, M.; Millan, J.; et al. Endothelial Cell Rearrangements during Vascular Patterning Require PI3-Kinase-Mediated Inhibition of Actomyosin Contractility. Nat. Commun. 2018, 9, 4826. [Google Scholar] [CrossRef] [PubMed]
- Canse, C.; Yildirim, E.; Yaba, A. Overview of Junctional Complexes during Mammalian Early Embryonic Development. Front. Endocrinol. 2023, 14, 1150017. [Google Scholar] [CrossRef]
- Taddei, A.; Giampietro, C.; Conti, A.; Orsenigo, F.; Breviario, F.; Pirazzoli, V.; Potente, M.; Daly, C.; Dimmeler, S.; Dejana, E. Endothelial Adherens Junctions Control Tight Junctions by VE-Cadherin-Mediated Upregulation of Claudin-5. Nat. Cell Biol. 2008, 10, 923–934. [Google Scholar] [CrossRef]
- Ahmad, U.S.; Uttagomol, J.; Wan, H. The Regulation of the Hippo Pathway by Intercellular Junction Proteins. Life 2022, 12, 1792. [Google Scholar] [CrossRef]
- Nakahara, S.; Tsutsumi, K.; Zuinen, T.; Ohta, Y. FilGAP, a Rho-ROCK-Regulated GAP for Rac, Controls Adherens Junctions in MDCK Cells. J. Cell Sci. 2015, 128, 2047–2056. [Google Scholar] [CrossRef]
- Mills, S.E. Histology for Pathologists, 4th ed.; Pine, J.W., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; p. 19103. ISBN 978-1-4511-1303-7. [Google Scholar]
- Witjas, F.M.R.; van den Berg, B.M.; van den Berg, C.W.; Engelse, M.A.; Rabelink, T.J. Concise Review: The Endothelial Cell Extracellular Matrix Regulates Tissue Homeostasis and Repair. Stem Cells Transl. Med. 2019, 8, 375–382. [Google Scholar] [CrossRef]
- Rekad, Z.; Ruff, M.; Radwanska, A.; Grall, D.; Ciais, D.; Van Obberghen-Schilling, E. Coalescent RNA-Localizing and Transcriptional Activities of SAM68 Modulate Adhesion and Subendothelial Basement Membrane Assembly. Elife 2023, 12, e85165. [Google Scholar] [CrossRef] [PubMed]
- Marcu, R.; Choi, Y.J.; Xue, J.; Fortin, C.L.; Wang, Y.; Nagao, R.J.; Xu, J.; MacDonald, J.W.; Bammler, T.K.; Murry, C.E.; et al. Human Organ-Specific Endothelial Cell Heterogeneity. iScience 2018, 4, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial Cell Heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Rajan, K.C.; Blanks, A.; Li, Y.; Prieto, M.C.; Meadows, S.M. Endothelial Cell Polarity and Extracellular Matrix Composition Require Functional ATP6AP2 during Developmental and Pathological Angiogenesis. JCI Insight 2022, 7, e154379. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Veloso, A.; Wu, J.; Katrukha, E.A.; Akhmanova, A. Control of Endothelial Cell Polarity and Sprouting Angiogenesis by Noncentrosomal Microtubules. Elife 2018, 7, e33864. [Google Scholar] [CrossRef] [PubMed]
- Wolpe, A.G.; Ruddiman, C.A.; Hall, P.J.; Isakson, B.E. Polarized Proteins in Endothelium and Their Contribution to Function. J. Vasc. Res. 2021, 58, 65–91. [Google Scholar] [CrossRef]
- Castiglioni, V.G.; Pires, H.R.; Bertolini, R.R.; Riga, A.; Kerver, J.; Boxem, M. Epidermal PAR-6 and PKC-3 Are Essential for Postembryonic Development of Caenorhabditis Elegans and Control Non-Centrosomal Microtubule Organization. bioRxiv 2020, 2020.07.23.217679. [Google Scholar] [CrossRef]
- Sanchez, A.D.; Branon, T.C.; Cote, L.E.; Papagiannakis, A.; Liang, X.; Pickett, M.; Shen, K.; Jacobs-Wagner, C.; Ting, A.; Feldman, J. Proximity Labeling at Non-Centrosomal Microtubule-Organizing Centers Reveals VAB-10B and WDR-62 as Distinct Microtubule Regulators. bioRxiv 2020, 2020.08.29.272369. [Google Scholar] [CrossRef]
- Becker, R.; Vergarajauregui, S.; Billing, F.; Sharkova, M.; Lippolis, E.; Mamchaoui, K.; Ferrazzi, F.; Engel, F.B. Myogenin Controls via AKAP6 Non-Centrosomal Microtubule-Organizing Center Formation at the Nuclear Envelope. Elife 2021, 10, e65672. [Google Scholar] [CrossRef]
- Prassanawar, S.S.; Sarkar, T.; Panda, D. CEP41, a Ciliopathy-Linked Centrosomal Protein, Regulates Microtubule Assembly and Cell Division. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ong, S.T.; Chalasani, M.L.S.; Fazil, M.H.U.T.; Prasannan, P.; Kizhakeyil, A.; Wright, G.D.; Kelleher, D.; Verma, N.K. Centrosome- and Golgi-Localized Protein Kinase N-Associated Protein Serves as a Docking Platform for Protein Kinase A Signaling and Microtubule Nucleation in Migrating T-Cells. Front. Immunol. 2018, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, R.; Mukhopadhyay, S.; Bhagyanath, S.; Priya, M.R.S.D.; Manna, T.K. TACC3–Ch-TOG Interaction Regulates Spindle Microtubule Assembly by Controlling Centrosomal Recruitment of γ-TuRC. Biosci. Rep. 2023, 43, 20221882. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Schwaninger, M. Apicobasal Polarity of Brain Endothelial Cells. J. Cereb. Blood Flow Metab. 2016, 36, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Ramirez, K.; Nagayama, K.; Tomita, S.; Kubota, Y.; Yanagisawa, H. Partial Endothelial-to-Mesenchymal Transition (EndMT) Contributes to Lumen Re-Organization after Carotid Artery Ligation. bioRxiv 2021, 2021.08.13.456319. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Ledesma, S.; Chung, S.; Hoffman, J.; Schlick, T. Regulation of Chromatin Architecture by Transcription Factor Binding. Elife 2023, 12, RP91320. [Google Scholar] [CrossRef]
- Trojanowski, J.; Rippe, K. Transcription Factor Binding and Activity on Chromatin. Curr. Opin. Syst. Biol. 2022, 31, 100438. [Google Scholar] [CrossRef]
- Afshar, Y.; Ma, F.; Quach, A.; Jeong, A.; Sunshine, H.L.; Freitas, V.; Jami-Alahmadi, Y.; Helaers, R.; Li, X.; Pellegrini, M.; et al. Transcriptional Drifts Associated with Environmental Changes in Endothelial Cells. Elife 2023, 12, 81370. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.B.; Jain, M.K. Role of Krüppel-Like Transcription Factors in Endothelial Biology. Circ. Res. 2007, 100, 1686–1695. [Google Scholar] [CrossRef]
- Pasut, A.; Becker, L.M.; Cuypers, A.; Carmeliet, P. Endothelial Cell Plasticity at the Single-Cell Level. Angiogenesis 2021, 24, 311. [Google Scholar] [CrossRef]
- Rohlenova, K.; Goveia, J.; García-Caballero, M.; Subramanian, A.; Kalucka, J.; Treps, L.; Falkenberg, K.D.; de Rooij, L.P.M.H.; Zheng, Y.; Lin, L.; et al. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metab. 2020, 31, 862–877.e14. [Google Scholar] [CrossRef]
- Minami, T.; Muramatsu, M.; Kume, T. Organ/Tissue-Specific Vascular Endothelial Cell Heterogeneity in Health and Disease. Biol. Pharm. Bull. 2019, 42, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M. Embryonic Origins and Assembly of Blood Vessels. Am. Rev. Respir. Dis. 1989, 140, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Hatou, S.; Shimmura, S. Review: Corneal Endothelial Cell Derivation Methods from ES/IPS Cells. Inflamm. Regen. 2019, 39, 19. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Guerrero-Zayas, M.I.; Wallingford, M.C.; Ortiz-Pineda, P.; Mager, J.; Tremblay, K.D. Visceral Endoderm Expression of Yin-Yang1 (YY1) Is Required for VEGFA Maintenance and Yolk Sac Development. PLoS ONE 2013, 8, e58828. [Google Scholar] [CrossRef]
- Havrilak, J.A.; Melton, K.R.; Shannon, J.M. Endothelial Cells Are Not Required for Specification of Respiratory Progenitors. Dev. Biol. 2017, 427, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.E. Early Embryonic Mesoderm Development. In Handbook of Stem Cells; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 273–278. ISBN 9780080533735. [Google Scholar]
- Julien, E.; El Omar, R.; Tavian, M. Origin of the Hematopoietic System in the Human Embryo. FEBS Lett. 2016, 590, 3987–4001. [Google Scholar] [CrossRef] [PubMed]
- Serrado Marques, J.; Teixeira, V.; Jacinto, A.; Tavares, A. Identification of Novel Hemangioblast Genes in the Early Chick Embryo. Cells 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Coffin, J.D.; Harrison, J.; Schwartz, S.; Heimark, R. Angioblast Differentiation and Morphogenesis of the Vascular Endothelium in the Mouse Embryo. Dev. Biol. 1991, 148, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Vokes, S.A.; Krieg, P.A. Endoderm Is Required for Vascular Endothelial Tube Formation, but Not for Angioblast Specification. Development 2002, 129, 775–785. [Google Scholar] [CrossRef]
- Borasch, K.; Richardson, K.; Plendl, J. Cardiogenesis with a Focus on Vasculogenesis and Angiogenesis. Anat. Histol. Embryol. 2020, 49, 643–655. [Google Scholar] [CrossRef]
- Darland, D.C.; D’Amore, P.A. Blood Vessel Maturation: Vascular Development Comes of Age. J. Clin. Investig. 1999, 103, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; Gayoso, S.; García, M.P.; González-Gómez, M.; Díaz-Flores, L.; Sánchez, R.; Carrasco, J.L.; Madrid, J.F. Intussusceptive Angiogenesis and Its Counterpart Intussusceptive Lymphangiogenesis. Histol. Histopathol. 2020, 35, 1083–1103. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.J. Embryonic and Adult Vasculogenesis. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fahmy-Garcia, S.; van Driel, M.; Witte-Buoma, J.; Walles, H.; van Leeuwen, J.P.T.M.; van Osch, G.J.V.M.; Farrell, E. NELL-1, HMGB1, and CCN2 Enhance Migration and Vasculogenesis, But Not Osteogenic Differentiation Compared to BMP2. Tissue Eng. Part A 2018, 24, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.E.; Kelley, R.W.; Patterson, C. Mechanisms of Endothelial Differentiation in Embryonic Vasculogenesis. Arter. Thromb. Vasc. Biol. 2005, 25, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell. Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; McClung, J.A.; Aronow, W.S. Vasculogenesis and Angiogenesis; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128023853. [Google Scholar]
- Kütscher, C.; Lampert, F.M.; Kunze, M.; Markfeld-Erol, F.; Stark, G.B.; Finkenzeller, G. Overexpression of Hypoxia-Inducible Factor-1 Alpha Improves Vasculogenesis-Related Functions of Endothelial Progenitor Cells. Microvasc. Res. 2016, 105, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P. PanVascular Medicine, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–5004. [Google Scholar] [CrossRef]
- Lilly, A.J.; Lacaud, G.; Kouskoff, V. SOXF Transcription Factors in Cardiovascular Development. Semin. Cell Dev. Biol. 2017, 63, 50–57. [Google Scholar] [CrossRef]
- Marcelo, K.L.; Goldie, L.C.; Hirschi, K.K. Regulation of Endothelial Cell Differentiation and Specification. Circ. Res. 2013, 112, 1272–1287. [Google Scholar] [CrossRef]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int. J. Mol. Sci. 2019, 20, 3962. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and Angiogenesis. Cancer Treat Res. 2004, 117, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Patel-Hett, S.; D’Amore, P.A. Signal Transduction in Vasculogenesis and Developmental Angiogenesis. Int. J. Dev. Biol. 2011, 55, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Dela Peña, I.C.; Yoo, A.; Tajiri, N.; Acosta, S.A.; Ji, X.; Kaneko, Y.; Borlongan, C.V. Granulocyte Colony-Stimulating Factor Attenuates Delayed TPA-Induced Hemorrhagic Transformation in Ischemic Stroke Rats by Enhancing Angiogenesis and Vasculogenesis. J. Cereb. Blood Flow Metab. 2015, 35, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Ruggieri, S.; Annese, T.; Crivellato, E. Surface Markers: An Identity Card of Endothelial Cells. Microcirculation 2020, 27, e12587. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell. Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kudo, M. Signaling Pathways Governing Tumor Angiogenesis. Oncology 2011, 81, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vailhé, B.; Vittet, D.; Feige, J.J. In Vitro Models of Vasculogenesis and Angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef]
- Wei, S.T.; Huang, Y.C.; Hsieh, M.L.; Lin, Y.J.; Shyu, W.C.; Chen, H.C.; Hsieh, C.H. Atypical Chemokine Receptor ACKR3/CXCR7 Controls Postnatal Vasculogenesis and Arterial Specification by Mesenchymal Stem Cells via Notch Signaling. Cell Death Dis. 2020, 11, 307. [Google Scholar] [CrossRef]
- Garcia, M.D.; Larina, I.V. Vascular Development and Hemodynamic Force in the Mouse Yolk Sac. Front. Physiol. 2014, 5, 308. [Google Scholar] [CrossRef]
- Beedie, S.L.; Diamond, A.J.; Fraga, L.R.; Figg, W.D.; Vargesson, N. Vertebrate Embryos as Tools for Anti-Angiogenic Drug Screening and Function. Reprod. Toxicol. 2017, 70, 49–59. [Google Scholar] [CrossRef]
- Mozes, G.; Gloviczki, P. Venous Embryology and Anatomy. Vein Book 2007, 15–25. [Google Scholar] [CrossRef]
- Wolf, K.; Hu, H.; Isaji, T.; Dardik, A. Molecular Identity of Arteries, Veins, and Lymphatics. J. Vasc. Surg. 2019, 69, 253–262. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in Development and Human Disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, F.; Noël, A. Lymphangiogenesis: In Vitro and in Vivo Models. FASEB J. 2010, 24, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, N.; Oliver, G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu. Rev. Cell Dev. Biol. 2016, 32, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Kaipainen, A.; Bielenberg, D.R. Hemangiogenesis versus Lymphangiogenesis. Encycl. Eye 2010, 227–232. [Google Scholar] [CrossRef]
- Norrmén, C.; Tammela, T.; Petrova, T.V.; Alitalo, K. Biological Basis of Therapeutic Lymphangiogenesis. Circulation 2011, 123, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G. Lymphatic Vasculature Development. Nat. Rev. Immunol. 2004, 4, 35–45. [Google Scholar] [CrossRef]
- Butler, M.G.; Isogai, S.; Weinstein, B.M. Lymphatic Development. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 222–231. [Google Scholar] [CrossRef]
- Matanes, E.; Gotlieb, W.H. Pathophysiological and Anatomical Basis of Lymphatic Transit of Cancer Cells and Role of the Lymphatic System: A Review of Published Literature. Chin. Clin. Oncol. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.P.; Pissas, A.; Scarato, M.; Gallon, F.; Pissas, M.H.; Amore, M.; Wu, M.; Faries, M.B.; Lund, A.W. The Lymphatic System and Sentinel Lymph Nodes: Conduit for Cancer Metastasis. Clin. Exp. Metastasis 2022, 39, 139. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, M.; Fan, J.; Odaka, Y.; Liu, N.; Hassan, A.; Duan, X.; Stump, P.; Byerly, L.; Donaldson, M.; Hao, J.; et al. YAP/TAZ-CDC42 Signaling Regulates Vascular Tip Cell Migration. Proc. Natl. Acad. Sci. USA 2017, 114, 10918–10923. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yue, R.; Wei, B.; Gao, G.; Du, J.; Pei, G. Lysophosphatidic Acid Acts as a Nutrient-derived Developmental Cue to Regulate Early Hematopoiesis. EMBO J. 2014, 33, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Mylona, A.; Andrieu-Soler, C.; Thongjuea, S.; Martella, A.; Soler, E.; Jorna, R.; Hou, J.; Kockx, C.; Van Ijcken, W.; Lenhard, B.; et al. Genome-Wide Analysis Shows That Ldb1 Controls Essential Hematopoietic Genes/Pathways in Mouse Early Development and Reveals Novel Players in Hematopoiesis. Blood 2013, 121, 2902–2913. [Google Scholar] [CrossRef] [PubMed]
- Meadows, S.M.; Myers, C.T.; Krieg, P.A. Regulation of Endothelial Cell Development by ETS Transcription Factors. Semin. Cell Dev. Biol. 2011, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Klimenko, A.; Jia, L.; Katanaev, V.L. Small Molecule Wnt Pathway Modulators from Natural Sources: History, State of the Art and Perspectives. Cells 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Vladar, E.K.; Königshoff, M. Noncanonical Wnt Planar Cell Polarity Signaling in Lung Development and Disease. Biochem. Soc. Trans. 2020, 48, 231–243. [Google Scholar] [CrossRef]
- Lories, R.J.; Monteagudo, S. Review Article: Is Wnt Signaling an Attractive Target for the Treatment of Osteoarthritis? Rheumatol. Ther. 2020, 7, 259–270. [Google Scholar] [CrossRef]
- Tocci, J.M.; Felcher, C.M.; Solá, M.E.G.; Kordon, E.C. R-Spondin-Mediated WNT Signaling Potentiation in Mammary and Breast Cancer Development. IUBMB Life 2020, 72, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Tao, F.; Zhang, X.; Zhang, Y.; Sun, X.; Wu, D. Role of Wnt/β-Catenin Signaling in the Chemoresistance Modulation of Colorectal Cancer. Biomed. Res. Int. 2020, 2020, 9390878. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, Y.; Mortier, G.; Boudin, E.; Van Hul, W. WNT Signaling and Bone: Lessons From Skeletal Dysplasias and Disorders. Front. Endocrinol. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; Rodelo-Haad, C.; Pendon-Ruiz de Mier, M.V.; Martin-Malo, A.; Santamaria, R.; Rodriguez, M. Klotho/FGF23 and Wnt Signaling as Important Players in the Comorbidities Associated with Chronic Kidney Disease. Toxins 2020, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Noelanders, R.; Vleminckx, K. How Wnt Signaling Builds the Brain: Bridging Development and Disease. Neuroscientist 2017, 23, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Bem, J.; Brożko, N.; Chakraborty, C.; Lipiec, M.A.; Koziński, K.; Nagalski, A.; Szewczyk, Ł.M.; Wiśniewska, M.B. Wnt/β-Catenin Signaling in Brain Development and Mental Disorders: Keeping TCF7L2 in Mind. FEBS Lett. 2019, 593, 1654–1674. [Google Scholar] [CrossRef] [PubMed]
- Courtwright, A.; Siamakpour-Reihani, S.; Arbiser, J.L.; Banet, N.; Hilliard, E.; Fried, L.; Livasy, C.; Ketelsen, D.; Nepal, D.B.; Perou, C.M.; et al. SFRP2 Stimulates Angiogenesis via a Calcineurin/NFAT Signaling Pathway. Cancer Res. 2009, 69, 4621. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Zheng, H.; Zhu, H.; Mai, W.; Huang, X.; Huang, Y. Multiple Roles of SFRP2 in Cardiac Development and Cardiovascular Disease. Int. J. Biol. Sci. 2020, 16, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Tsaryk, R.; Lange, M.; Wisniewski, L.; Moore, J.C.; Lawson, N.D.; Wojciechowska, K.; Schnittler, H.; Siekmann, A.F. Endothelial Notch Signalling Limits Angiogenesis via Control of Artery Formation. Nat. Cell Biol. 2017, 19, 928–940. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in Tip and Stalk Cell Selection. Cold Spring Harb. Perspect. Med. 2012, 3, a006569. [Google Scholar] [CrossRef]
- Vega, R.; Carretero, M.; Travasso, R.D.M.; Bonilla, L.L. Notch Signaling and Taxis Mechanims Regulate Early Stage Angiogenesis: A Mathematical and Computational Model. PLoS Comput. Biol. 2020, 16, e1006919. [Google Scholar] [CrossRef] [PubMed]
- MacK, J.J.; Luisa Iruela-Arispe, M. NOTCH Regulation of the Endothelial Cell Phenotype. Curr. Opin. Hematol. 2018, 25, 212–218. [Google Scholar] [CrossRef]
- Roca, C.; Adams, R.H. Regulation of Vascular Morphogenesis by Notch Signaling. Genes. Dev. 2007, 21, 2511–2524. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, X.; Zhang, H.; Wang, G.; Massey, P.A.; Barton, S.R.; Kevil, C.G.; Dong, Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am. J. Pathol. 2019, 189, 1495–1500. [Google Scholar] [CrossRef]
- Andersen, P.; Uosaki, H.; Shenje, L.T.; Kwon, C. Non-Canonical Notch Signaling: Emerging Role and Mechanism. Trends Cell Biol. 2012, 22, 257–265. [Google Scholar] [CrossRef]
- Herrick, D.B.; Guo, Z.; Jang, W.; Schnittke, N.; Schwob, J.E. Canonical Notch Signaling Directs the Fate of Differentiating Neurocompetent Progenitors in the Mammalian Olfactory Epithelium. J. Neurosci. 2018, 38, 5022–5037. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, B.; Meloty-Kapella, L.; Weinmaster, G. Canonical and Non-Canonical Notch Ligands. Curr. Top. Dev. Biol. 2010, 92, 73–129. [Google Scholar] [CrossRef]
- Beets, K.; Staring, M.W.; Criem, N.; Maas, E.; Schellinx, N.; De Sousa Lopes, S.M.C.; Umans, L.; Zwijsen, A. BMP-SMAD Signalling Output Is Highly Regionalized in Cardiovascular and Lymphatic Endothelial Networks. BMC Dev. Biol. 2016, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, L.; van Meeteren, L.A. Transforming Growth Factor β Family Members in Regulation of Vascular Function: In the Light of Vascular Conditional Knockouts. Exp. Cell Res. 2013, 319, 1264–1270. [Google Scholar] [CrossRef]
- Herrera, B.; Addante, A.; Sánchez, A. BMP Signalling at the Crossroad of Liver Fibrosis and Regeneration. Int. J. Mol. Sci. 2018, 19, 39. [Google Scholar] [CrossRef]
- Dyer, L.; Patterson, C. Development of the Endothelium: An Emphasis on Heterogeneity. Semin. Thromb. Hemost. 2010, 36, 227–235. [Google Scholar] [CrossRef][Green Version]
- Marks-Bluth, J.; Khanna, A.; Chandrakanthan, V.; Thoms, J.; Bee, T.; Eich, C.; Kang, Y.C.; Knezevic, K.; Qiao, Q.; Fitch, S.; et al. SMAD1 and SMAD5 Expression Is Coordinately Regulated by FLI1 and GATA2 during Endothelial Development. Mol. Cell. Biol. 2015, 35, 2165–2172. [Google Scholar] [CrossRef]
- Dyer, L.A.; Pi, X.; Patterson, C. The Role of BMPs in Endothelial Cell Function and Dysfunction. Trends Endocrinol. Metab. 2014, 25, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bao, L.; Zhao, M.; Cao, J.; Zheng, H. Progress in Research on the Role of FGF in the Formation and Treatment of Corneal Neovascularization. Front. Pharmacol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Oladipupo, S.S.; Smith, C.; Santeford, A.; Park, C.; Sene, A.; Wiley, L.A.; Osei-Owusu, P.; Hsu, J.; Zapata, N.; Liu, F.; et al. Endothelial Cell FGF Signaling Is Required for Injury Response but Not for Vascular Homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 13379–13384. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef]
- Garry, D.J. Etv2 Is a Master Regulator of Hematoendothelial Lineages. Trans. Am. Clin. Climatol. Assoc. 2016, 127, 212–223. [Google Scholar]
- Koyano-Nakagawa, N.; Kweon, J.; Iacovino, M.; Shi, X.; Rasmussen, T.L.; Borges, L.; Zirbes, K.M.; Li, T.; Perlingeiro, R.C.R.; Kyba, M.; et al. Etv2 Is Expressed in the Yolk Sac Hematopoietic and Endothelial Progenitors and Regulates Lmo2 Gene Expression. Stem Cells 2012, 30, 1611–1623. [Google Scholar] [CrossRef]
- Dobrzycki, T.; Lalwani, M.; Telfer, C.; Monteiro, R.; Patient, R. The Roles and Controls of GATA Factors in Blood and Cardiac Development. IUBMB Life 2020, 72, 39–44. [Google Scholar] [CrossRef]
- Whitcomb, J.; Gharibeh, L.; Nemer, M. From Embryogenesis to Adulthood: Critical Role for GATA Factors in Heart Development and Function. IUBMB Life 2020, 72, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, X.; Sun, X.; Shen, X.; Yin, H.; Wang, C.; Liu, Y.; Lu, C.; Fu, H.; Yang, S.; et al. Synergistic Effects of Dual-Presenting VEGF- A Nd BDNF-Mimetic Peptide Epitopes from Self-Assembling Peptide Hydrogels on Peripheral Nerve Regeneration. Nanoscale 2019, 11, 19943–19958. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Chauhan, S.K.; Kay, E.D.; Dana, R. Flt-1 Regulates Vascular Endothelial Cell Migration via a Protein Tyrosine Kinase-7–Dependent Pathway. Blood 2011, 117, 5762. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.C.; Ciucci, G.; Zandomenego, G.; Vuerich, R.; Ring, N.A.R.; Vodret, S.; Salton, F.; Marchesan, P.; Braga, L.; Marcuzzo, T.; et al. Flt1 Produced by Lung Endothelial Cells Impairs ATII Cell Transdifferentiation and Repair in Pulmonary Fibrosis. Cell Death Dise. 2023, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular Endothelial Growth Factor Receptor-2: Structure, Function, Intracellular Signalling and Therapeutic Inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Wei, Q.; Zhao, Z.; Chen, X.; Cui, H.; Zhang, Y. VEGF/Flk1 Mechanism Is Involved in Roxarsone Promotion of Rat Endothelial Cell Growth and B16F10 Xenograft Tumor Angiogenesis. Sci. Rep. 2019, 9, 17417. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Chen, R.; Donlon, T.A.; Kallianpur, K.J.; Masaki, K.H.; Willcox, B.J. Vascular Endothelial Growth Factor Receptor 1 Gene (FLT1) Longevity Variant Increases Lifespan by Reducing Mortality Risk Posed by Hypertension. Aging 2023, 15, 3967–3983. [Google Scholar] [CrossRef]
- Otowa, Y.; Moriwaki, K.; Sano, K.; Shirakabe, M.; Yonemura, S.; Shibuya, M.; Rossant, J.; Suda, T.; Kakeji, Y.; Hirashima, M. Flt1/VEGFR1 Heterozygosity Causes Transient Embryonic Edema. Sci. Rep. 2016, 6, 27186. [Google Scholar] [CrossRef]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Trends Endocrinol. Metab. 2016, 11, 251–281. [Google Scholar] [CrossRef]
- Harde, E.; Nicholson, L.; Furones Cuadrado, B.; Bissen, D.; Wigge, S.; Urban, S.; Segarra, M.; Ruiz de Almodóvar, C.; Acker-Palmer, A. EphrinB2 Regulates VEGFR2 during Dendritogenesis and Hippocampal Circuitry Development. Elife 2019, 8, e49819. [Google Scholar] [CrossRef]
- Large, C.L.; Vitali, H.E.; Whatley, J.D.; Red-Horse, K.; Sharma, B. In Vitro Model of Coronary Angiogenesis. J. Vis. Exp. 2020, 2020, e60558. [Google Scholar] [CrossRef]
- Real, P.J.; Ligero, G.; Ayllon, V.; Ramos-Mejia, V.; Bueno, C.; Gutierrez-Aranda, I.; Navarro-Montero, O.; Lako, M.; Menendez, P. SCL/TAL1 Regulates Hematopoietic Specification from Human Embryonic Stem Cells. Mol. Ther. 2012, 20, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Lambert, J.A.; Martin, R. SCL/TAL1 in Hematopoiesis and Cellular Reprogramming, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 118. [Google Scholar]
- Porcher, C.; Chagraoui, H.; Kristiansen, M.S. SCL/TAL1: A Multifaceted Regulator from Blood Development to Disease. Blood 2017, 129, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Matrone, G.; Meng, S.; Gu, Q.; Lv, J.; Fang, L.; Chen, K.; Cooke, J.P. Lmo2 Modulates Sphk1 and Promotes Endothelial Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Ganta, V.C.; Annex, B.H. LMO2 and Endothelial Cell Migration in Developmental and Postnatal Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Cañete, A.; Cano, E.; Muñoz-Chápuli, R.; Carmona, R. Role of Vitamin a/Retinoic Acid in Regulation of Embryonic and Adult Hematopoiesis. Nutrients 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Sugawara, A.; Uruno, A.; Kudo, M.; Kagechika, H.; Sato, Y.; Owada, Y.; Kondo, H.; Sato, M.; Kurabayashi, M.; et al. All-Trans Retinoic Acid Induces in Vitro Angiogenesis via Retinoic Acid Receptor: Possible Involvement of Paracrine Effects of Endogenous Vascular Endothelial Growth Factor Signaling. Endocrinology 2007, 148, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Bohnsack, B.L.; Niederreither, K.; Hirschi, K.K. Retinoic Acid Regulates Endothelial Cell Proliferation during Vasculogenesis. Development 2003, 130, 6465–6474. [Google Scholar] [CrossRef]
- Pawlikowski, B.; Wragge, J.; Siegenthaler, J.A. Retinoic Acid Signaling in Vascular Development. Genesis 2019, 57, e23287. [Google Scholar] [CrossRef] [PubMed]
- Binshtok, U.; Sprinzak, D. Molecular Mechanisms of Notch Signaling; Springer International Publishing: Cham, Switzerland, 2018; Volume 1066, ISBN 978-3-319-89511-6. [Google Scholar]
- Cunha, S.I.; Magnusson, P.U.; Dejana, E.; Lampugnani, M.G. Deregulated TGF-β/BMP Signaling in Vascular Malformations. Circ. Res. 2017, 121, 981–999. [Google Scholar] [CrossRef]
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-Ordinating Notch, BMP, and TGF-β Signaling during Heart Valve Development. Cell. Mol. Life Sci. 2013, 70, 2899–2917. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.F. Signaling Cross-Talk between TGF-β/BMP and Other Pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Holderfield, M.T.; Hughes, C.C.W. Cross-talk between Vascular Endothelial Growth Factor, Notch, and Transforming Growth Factor-β in Vascular Morphogenesis. Circ. Res. 2008, 102, 637–652. [Google Scholar] [CrossRef] [PubMed]
- LaFoya, B.; Munroe, J.A.; Mia, M.M.; Detweiler, M.A.; Crow, J.J.; Wood, T.; Roth, S.; Sharma, B.; Albig, A.R. Notch: A Multi-Functional Integrating System of Microenvironmental Signals. Dev. Biol. 2016, 418, 227–241. [Google Scholar] [CrossRef]
- Lyle, C.L.; Belghasem, M.; Chitalia, V.C. C-Cbl: An Important Regulator and a Target in Angiogenesis and Tumorigenesis. Cells 2019, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Pinheiro, P.; Joseph, N.; Peterkin, T.; Koth, J.; Repapi, E.; Bonkhofer, F.; Kirmizitas, A.; Patient, R. Transforming Growth Factor β Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev. Cell 2016, 38, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.J.; Pohl, S.Ö.; Deshmukh, A.; Visweswaran, M.; Ward, N.C.; Arfuso, F.; Agostino, M.; Dharmarajan, A. The Role of Wnt Signalling in Angiogenesis. Clin. Biochem. Rev. 2017, 38, 131–142. [Google Scholar] [PubMed]
- Sam, S.A.; Teel, J.; Tegge, A.N.; Bharadwaj, A.; Murali, T.M. XTALKDB: A Database of Signaling Pathway Cross-talk. Nucleic Acids Res. 2017, 45, D432–D439. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Percy Ivy, S. Controversies in Cancer Stem Cells: Targeting Embryonic Signaling Pathways. Clin. Cancer Res. 2010, 16, 3106–3112. [Google Scholar] [CrossRef]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch: Interacting Signalling Pathways in Tumour Angiogenesis. Br. J. Cancer 2008, 99, 1204–1209. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, R.-W.; Han, B.; Li, Z.; Xiong, L.; Zhang, F.-Y.; Cong, B.-B.; Zhang, B. Notch Signaling Mediated by TGF-β/Smad Pathway in Concanavalin A-Induced Liver Fibrosis in Rats. World J. Gastroenterol. 2017, 23, 2330. [Google Scholar] [CrossRef] [PubMed]
- Itoh, F.; Itoh, S.; Goumans, M.J.; Valdimarsdottir, G.; Iso, T.; Dotto, G.P.; Hamamori, Y.; Kedes, L.; Kato, M.; Ten Dijke, P. Synergy and Antagonism between Notch and BMP Receptor Signaling Pathways in Endothelial Cells. EMBO J. 2004, 23, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yao, J.; Wang, L.; Zhang, D.; Zhang, L.; Reynolds, E.X.; Yu, T.; Boström, K.I.; Yao, Y. Cross-talk between BMP and Notch Induces Sox2 in Cerebral Endothelial Cells. Cells 2019, 8, 549. [Google Scholar] [CrossRef]
- Benn, A.; Hiepen, C.; Osterland, M.; Schütte, C.; Zwijsen, A.; Knaus, P. Role of Bone Morphogenetic Proteins in Sprouting Angiogenesis: Differential BMP Receptor-Dependent Signaling Pathways Balance Stalk vs. Tip Cell Competence. FASEB J. 2017, 31, 4720–4733. [Google Scholar] [CrossRef]
- Caliceti, C.; Nigro, P.; Rizzo, P.; Ferrari, R. ROS, Notch, and Wnt Signaling Pathways: Cross-talk between Three Major Regulators of Cardiovascular Biology. Biomed Res. Int. 2014, 2014, 318714. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Zoldan, J.; Basevitch, Y.; Langer, R. Endothelial Potential of Human Embryonic Stem Cells. Blood 2007, 110, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Banno, K.; Yoder, M.C. Endothelial Stem and Progenitor Cells for Regenerative Medicine. Curr. Stem Cell Rep. 2019, 5, 101–108. [Google Scholar] [CrossRef]
- Wei, Q.; Frenette, P.S. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef]
- Gussin, H.A.E.; Bischoff, F.Z.; Hoffman, R.; Elias, S. Endothelial Precursor Cells in the Peripheral Blood of Pregnant Women. J. Soc. Gynecol. Investig. 2002, 9, 357–361. [Google Scholar] [CrossRef]
- Urbich, C.; Dimmeler, S. Endothelial Progenitor Cells: Characterization and Role in Vascular Biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S. Circulating Endothelial Precursors: Mystery, Reality, and Promise. J. Clin. Investig. 2000, 105, 17–19. [Google Scholar] [CrossRef]
- Szpera-Goździewicz, A.; Majcherek, M.; Boruczkowski, M.; Goździewicz, T.; Dworacki, G.; Wicherek, L.; Bręborowicz, G.H. Circulating Endothelial Cells, Circulating Endothelial Progenitor Cells, and von Willebrand Factor in Pregnancies Complicated by Hypertensive Disorders. Am. J. Reprod. Immunol. 2017, 77, e12625. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Mohamed, I.L.; El Asheer, O.M.; Tamer, D.M.; Abo-ELela, M.G.M.; Abdel-Rahim, M.H.; El-Badawy, O.H.B.; Elsayh, K.I. Circulating Endothelial Cells, Circulating Endothelial Progenitor Cells, and Circulating Microparticles in Type 1 Diabetes Mellitus. Clin. Appl. Thromb. 2019, 25, 1076029618825311. [Google Scholar] [CrossRef] [PubMed]
- Plein, A.; Fantin, A.; Denti, L.; Pollard, J.W.; Ruhrberg, C. Erythro-Myeloid Progenitors Contribute Endothelial Cells to Blood Vessels. Nature 2018, 562, 223–228. [Google Scholar] [CrossRef]
- Díaz del Moral, S.; Barrena, S.; Muñoz-Chápuli, R.; Carmona, R. Embryonic Circulating Endothelial Progenitor Cells. Angiogenesis 2020, 23, 531–541. [Google Scholar] [CrossRef]
- Nieda, M.; Nicol, A.; Denning-Kendall, P.; Sweetenham, J.; Bradley, B.; Hows, J. Endothelial Cell Precursors Are Normal Components of Human Umbilical Cord Blood. Br. J. Haematol. 1997, 98, 775–777. [Google Scholar] [CrossRef]
- Kirton, J.P.; Xu, Q. Endothelial Precursors in Vascular Repair. Microvasc. Res. 2010, 79, 193–199. [Google Scholar] [CrossRef]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine. Stem Cells 2011, 29, 1650–1655. [Google Scholar] [CrossRef]
- Heissig, B.; Hattori, K.; Dias, S.; Friedrich, M.; Ferris, B.; Hackett, N.R.; Crystal, R.G.; Besmer, P.; Lyden, D.; Moore, M.A.S.; et al. Recruitment of Stem and Progenitor Cells from the Bone Marrow Niche Requires MMP-9 Mediated Release of Kit-Ligand. Cell 2002, 109, 625–637. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Havens, A.M.; Pienta, K.J.; Taichman, R.S. The Bone Marrow Niche: Habitat to Hematopoietic and Mesenchymal Stem Cells, and Unwitting Host to Molecular Parasites. Leukemia 2008, 22, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.D.; Zheng, C.X.; Li, M.; Jin, Y.; Hu, C.H. Epigenetic Regulation of Mesenchymal Stem Cell Homeostasis. Trends Cell Biol. 2020, 30, 97–116. [Google Scholar] [CrossRef]
- Fielding, C.; Méndez-Ferrer, S. Neuronal Regulation of Bone Marrow Stem Cell Niches. F1000Res 2020, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.N.; Christofk, H.R. Metabolic Regulation of Tissue Stem Cells. Trends Cell Biol. 2020, 30, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan-Bogdan, M.; Zon, L.I. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, G.; Deng, L.; Kuang, B.; Li, X. The Roles of FGF10 in Vasculogenesis and Angiogenesis. Biomed. Res. 2017, 28, 1329–1332. [Google Scholar]
- Ribatti, D.; Vacca, A.; De Falco, G.; Ria, R.; Roncali, L.; Dammacco, F. Role of Hematopoietic Growth Factors in Angiogenesis. Acta Haematol. 2001, 106, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Goldie, L.C.; Nix, M.K.; Hirschi, K.K. Embryonic Vasculogenesis and Hematopoietic Specification. Organogenesis 2008, 4, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kauts, M.L.; Vink, C.S.; Dzierzak, E. Hematopoietic (Stem) Cell Development—How Divergent Are the Roads Taken? FEBS Lett. 2016, 590, 3975–3986. [Google Scholar] [CrossRef]
- Krenning, G.; Barauna, V.G.; Krieger, J.E.; Harmsen, M.C.; Moonen, J.R.A.J. Endothelial Plasticity: Shifting Phenotypes through Force Feedback. Stem Cells Int. 2016, 2016, 9762959. [Google Scholar] [CrossRef]
- Caolo, V.; Molin, D.G.M.; Post, M.J. Notch Regulation of Hematopoiesis, Endothelial Precursor Cells, and Blood Vessel Formation: Orchestrating the Vasculature. Stem Cells Int. 2012, 2012, 805602. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Role of Endothelial Progenitor Cells in Vascular Development, Homestatic Maintenance of Blood Vessels and in Injury-Mediated Reparative Response. Stem Cell Investig. 2020, 7, 7. [Google Scholar] [CrossRef]
- Kim, P.G.; Albacker, C.E.; Lu, Y.F.; Jang, I.H.; Lim, Y.; Heffner, G.C.; Arora, N.; Bowman, T.V.; Lin, M.I.; Lensch, M.W.; et al. Signaling Axis Involving Hedgehog, Notch, and Scl Promotes the Embryonic Endothelial-to-Hematopoietic Transition. Proc. Natl. Acad. Sci. USA 2013, 110, E141–E150. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. Hematopoiesis and Angiogenesis: A Link between Two Apparently Independent Processes. J. Hematother. Stem Cell Res. 2000, 19, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Dias, S.; Heissig, B.; Hackett, N.R.; Lyden, D.; Tateno, M.; Hicklin, D.J.; Zhu, Z.; Witte, L.; Crystal, R.G.; et al. Vascular Endothelial Growth Factor and Angiopoietin-1 Stimulate Postnatal Hematopoiesis by Recruitment of Vasculogenic and Hematopoietic Stem Cells. J. Exp. Med. 2001, 193, 1005–1014. [Google Scholar] [CrossRef]
- D’Alessio, A.; Moccia, F.; Li, J.H.; Micera, A.; Kyriakides, T.R. Angiogenesis and Vasculogenesis in Health and Disease. Biomed. Res. Int. 2015, 2015, 2–4. [Google Scholar] [CrossRef]
- Heissig, B.; Werb, Z.; Rafii, S.; Hattori, K. Role of C-Kit/Kit Ligand Signaling in Regulating Vasculogenesis. Thromb. Haemost. 2003, 90, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Yokota, T.; Okuzaki, D.; Uno, Y.; Mashimo, T.; Kubota, Y.; Sudo, T.; Ishibashi, T.; Shingai, Y.; Doi, Y.; et al. Endothelial Cell-Selective Adhesion Molecule Contributes to the Development of Definitive Hematopoiesis in the Fetal Liver. Stem Cell Rep. 2019, 13, 992–1005. [Google Scholar] [CrossRef]
- Cangara, H.M.; Ishida, T.; Hara, T.; Sun, L.; Toh, R.; Rikitake, Y.; Kundu, R.K.; Quertermous, T.; Hirata, K.I.; Hayashi, Y. Role of Endothelial Cell-Selective Adhesion Molecule in Hematogeneous Metastasis. Microvasc. Res. 2010, 80, 133–141. [Google Scholar] [CrossRef]
- Inoue, M.; Ishida, T.; Yasuda, T.; Toh, R.; Hara, T.; Cangara, H.M.; Rikitake, Y.; Taira, K.; Sun, L.; Kundu, R.K.; et al. Endothelial Cell-Selective Adhesion Molecule Modulates Atherosclerosis through Plaque Angiogenesis and Monocyte-Endothelial Interaction. Microvasc. Res. 2010, 80, 179–187. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular Endothelium-Gatekeeper of Vessel Health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Nova-Lampeti, E.; Aguilera, V.; Oporto, K.; Guzmán, P.; Ormazábal, V.; Zúñiga, F.; Escudero, C.; Aguayo, C. Hox Genes in Adult Tissues and Their Role in Endothelial Cell Differentiation and Angiogenesis. Endothel. Dysfunct. Old Concept. New Chall. 2018, 10. [Google Scholar] [CrossRef]
- Minami, T.; Aird, W.C. Endothelial Cell Gene Regulation. Trends Cardiovasc. Med. 2005, 15, 174.e1–174.e24. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, T.; Englund, M.C.O.; Hyllner, J. Human Embryonic Stem Cells. Regenerative Medicine-from Protocol to Patient: 2. Stem Cell Science and Technology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 27–49. [Google Scholar] [CrossRef]
- Lacorre, D.-A.; Baekkevold, E.S.; Garrido, I.; Brandtzaeg, P.; Haraldsen, G.; Amalric, F.; Girard, J.-P. Plasticity of Endothelial Cells: Rapid Dedifferentiation of Freshly Isolated High Endothelial Venule Endothelial Cells Outside the Lymphoid Tissue Microenvironment. Blood 2004, 103, 4164–4172. [Google Scholar] [CrossRef] [PubMed]
- Cleuren, A.C.A.A.; van der Ent, M.A.; Jiang, H.; Hunker, K.L.; Yee, A.; Siemieniak, D.R.; Molema, G.; Aird, W.C.; Ganesh, S.K.; Ginsburg, D. The in Vivo Endothelial Cell Translatome Is Highly Heterogeneous across Vascular Beds. Proc. Natl. Acad. Sci. USA 2019, 116, 23618–23624. [Google Scholar] [CrossRef]
- Russell-Hallinan, A.; Watson, C.J.; O’Dwyer, D.; Grieve, D.J.; O’Neill, K.M. Epigenetic Regulation of Endothelial Cell Function by Nucleic Acid Methylation in Cardiac Homeostasis and Disease. Cardiovasc. Drugs Ther. 2021, 35, 1025. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, R.T.; Hamanaka, R.B.; Krause, M.; Oh, M.J.; Kuo, C.H.; Nigdelioglu, R.; Meliton, A.Y.; Witt, L.; Dai, G.; et al. HIF-1α Is Required for Disturbed Flow-Induced Metabolic Reprogramming in Human and Porcine Vascular Endothelium. Elife 2017, 6, e25217. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, P.; Vu, N.B.; Nguyen, H.T.; Huynh, O.T.; Truong, M.T.H. Significant Improvement of Direct Reprogramming Efficacy of Fibroblasts into Progenitor Endothelial Cells by ETV2 and Hypoxia. Stem Cell Res. Ther. 2016, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Lucero, R.; Zappulli, V.; Sammarco, A.; Murillo, O.D.; Cheah, P.S.; Srinivasan, S.; Tai, E.; Ting, D.T.; Wei, Z.; Roth, M.E.; et al. Glioma-Derived MiRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep. 2020, 30, 2065–2074.e4. [Google Scholar] [CrossRef]
- Hansen, A.; Henderson, S.; Lagos, D.; Nikitenko, L.; Coulter, E.; Roberts, S.; Gratrix, F.; Plaisance, K.; Renne, R.; Bower, M.; et al. KSHV-Encoded MiRNAs Target MAF to Induce Endothelial Cell Reprogramming. Genes. Dev. 2010, 24, 195–205. [Google Scholar] [CrossRef]
- Wang, L.; Xiang, M.; Liu, Y.; Sun, N.; Lu, M.; Shi, Y.; Wang, X.; Meng, D.; Chen, S.; Qin, J. Human Induced Pluripotent Stem Cells Derived Endothelial Cells Mimicking Vascular Inflammatory Response under Flow. Biomicrofluidics 2016, 10, 014106. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-K.; Chang, S.-H.; Cho, H.-J.; Choi, S.-B.; Ahn, H.-S.; Lee, J.; Jeong, H.; Youn, S.-W.; Lee, H.-J.; Kwon, Y.-W.; et al. Direct Conversion of Adult Skin Fibroblasts to Endothelial Cells by Defined Factors. Circulation 2014, 130, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, C.; Han, J.W.; Kim, J.Y.; Cho, K.; Kim, E.J.; Kim, S.; Lee, S.-J.; Oh, S.Y.; Tanaka, Y.; et al. Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2. Circ. Res. 2017, 120, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, K.; Das, A. TWIST1 -Reprogrammed Endothelial Cell Transplantation Potentiates Neovascularization-Mediated Diabetic Wound Tissue Regeneration. Diabetes 2020, 69, 1232–1247. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial Responses to Shear Stress in Atherosclerosis: A Novel Role for Developmental Genes. Nat. Rev. Cardiol. 2019, 17, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.; Mikhailova, N. The Role of Dll4/Notch Signaling in Normal and Pathological Ocular Angiogenesis: Dll4 Controls Blood Vessel Sprouting and Vessel Remodeling in Normal and Pathological Conditions. J. Ophthalmol. 2018, 2018, 3565292. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, F.; Clifton, N.; Sullivan, D.M.; Dalton, W.S.; Gabrilovich, D.I.; Nefedova, Y. Combined Inhibition of Notch Signaling and Bcl-2/Bcl-XL Results in Synergistic Anti-Myeloma Effect. Mol. Cancer Ther. 2010, 9, 3200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Banerjee, S.; Sarkar, F.H. Exploitation of the Notch Signaling Pathway as a Novel Target for Cancer Therapy. Anticancer Res. 2008, 28, 3621–3630. [Google Scholar] [PubMed]
- Yang, X.; Klein, R.; Tian, X.; Cheng, H.T.; Kopan, R.; Shen, J. Notch Activation Induces Apoptosis in Neural Progenitor Cells through a P53-Dependent Pathway. Dev. Biol. 2004, 269, 81–94. [Google Scholar] [CrossRef]
- Segarra, M.; Williams, C.K.; De La Luz Sierra, M.; Bernardo, M.; McCormick, P.J.; Maric, D.; Regino, C.; Choyke, P.; Tosato, G. Dll4 Activation of Notch Signaling Reduces Tumor Vascularity and Inhibits Tumor Growth. Blood 2008, 112, 1904. [Google Scholar] [CrossRef]
- Sajinovic, T.; Baier, G. New Insights into the Diverse Functions of the NR2F Nuclear Orphan Receptor Family. Front. Biosci. 2023, 28, 13. [Google Scholar] [CrossRef] [PubMed]
- Manukjan, N.; Ahmed, Z.; Fulton, D.; Blankesteijn, W.M.; Foulquier, S. A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells 2020, 9, 1545. [Google Scholar] [CrossRef]
- Laksitorini, M.D.; Yathindranath, V.; Xiong, W.; Hombach-Klonisch, S.; Miller, D.W. Modulation of Wnt/β-Catenin Signaling Promotes Blood-Brain Barrier Phenotype in Cultured Brain Endothelial Cells. Sci. Rep. 2019, 9, 19718. [Google Scholar] [CrossRef] [PubMed]
- Corada, M.; Nyqvist, D.; Orsenigo, F.; Caprini, A.; Giampietro, C.; Taketo, M.M.; Iruela-Arispe, M.L.; Adams, R.H.; Dejana, E. The Wnt/β-Catenin Pathway Modulates Vascular Remodeling and Specification by Upregulating Dll4/Notch Signaling. Dev. Cell 2010, 18, 938. [Google Scholar] [CrossRef] [PubMed]
- Korn, C.; Scholz, B.; Hu, J.; Srivastava, K.; Wojtarowicz, J.; Arnsperger, T.; Adams, R.H.; Boutros, M.; Augustin, H.G.; Augustin, I. Endothelial Cell-Derived Non-Canonical Wnt Ligands Control Vascular Pruning in Angiogenesis. Development 2014, 141, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.R.; Fortunato, I.C.; Fonseca, C.G.; Pezzarossa, A.; Barbacena, P.; Dominguez-Cejudo, M.A.; Vasconcelos, F.F.; Santos, N.C.; Carvalho, F.A.; Franco, C.A. Non-Canonical Wnt Signaling Regulates Junctional Mechanocoupling during Angiogenic Collective Cell Migration. Elife 2019, 8, e45853. [Google Scholar] [CrossRef]
- Meyer, I.S.; Jungmann, A.; Dieterich, C.; Zhang, M.; Lasitschka, F.; Werkmeister, S.; Haas, J.; Müller, O.J.; Boutros, M.; Nahrendorf, M.; et al. The Cardiac Microenvironment Uses Non-canonical WNT Signaling to Activate Monocytes after Myocardial Infarction. EMBO Mol. Med. 2017, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Zhang, Y.; Wu, J.; McVicar, A.; Chen, Y.; Zhu, S.; Zhu, G.; Lu, Y.; Zhang, J.; et al. Cbfβ Regulates Wnt/β-Catenin, Hippo/Yap, and TGFβ Signaling Pathways in Articular Cartilage Homeostasis and Protects from ACLT Surgery-Induced Osteoarthritis. Elife 2024, 13, e95640. [Google Scholar] [CrossRef]
- Gastfriend, B.D.; Nishihara, H.; Canfield, S.G.; Foreman, K.L.; Engelhardt, B.; Palecek, S.P.; Shusta, E.V. Wnt Signaling Mediates Acquisition of Blood-Brain Barrier Properties in Naive Endothelium Derived from Human Pluripotent Stem Cells. Elife 2021, 10, e70992. [Google Scholar] [CrossRef]
- Kulikauskas, M.R.; Shaka, X.; Bautch, V.L. The Versatility and Paradox of BMP Signaling in Endothelial Cell Behaviors and Blood Vessel Function. Cell. Mol. Life Sci. 2022, 79, 77. [Google Scholar] [CrossRef]
- Baeyens, N.; Larrivée, B.; Ola, R.; Hayward-Piatkowskyi, B.; Dubrac, A.; Huang, B.; Ross, T.D.; Coon, B.G.; Min, E.; Tsarfati, M.; et al. Defective Fluid Shear Stress Mechanotransduction Mediates Hereditary Hemorrhagic Telangiectasia. J. Cell Biol. 2016, 214, 807–816. [Google Scholar] [CrossRef]
- Han, O.; Pak, B.; Jin, S.W. The Role of BMP Signaling in Endothelial Heterogeneity. Front. Cell Dev. Biol. 2021, 9, 673396. [Google Scholar] [CrossRef]
- Nickel, J.; Mueller, T.D. Specification of BMP Signaling. Cells 2019, 8, 1579. [Google Scholar] [CrossRef]
- Zhu, H.; Kavsak, P.; Abdollah, S.; Wrana, J.L.; Thomsen, G.H. A SMAD Ubiquitin Ligase Targets the BMP Pathway and Affects Embryonic Pattern Formation. Nature 1999, 400, 687–693. [Google Scholar] [CrossRef]
- Masckauchán, T.N.H.; Shawber, C.J.; Funahashi, Y.; Li, C.M.; Kitajewski, J. Wnt/Beta-Catenin Signaling Induces Proliferation, Survival and Interleukin-8 in Human Endothelial Cells. Angiogenesis 2005, 8, 43–51. [Google Scholar] [CrossRef]
- Tajadura, V.; Hansen, M.H.; Smith, J.; Charles, H.; Rickman, M.; Farrell-Dillon, K.; Claro, V.; Warboys, C.; Ferro, A. β-Catenin Promotes Endothelial Survival by Regulating ENOS Activity and Flow-Dependent Anti-Apoptotic Gene Expression. Cell Death Dis. 2020, 11, 493. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2016, 9, a022145. [Google Scholar] [CrossRef]
- Lebrin, F.; Deckers, M.; Bertolino, P.; Ten Dijke, P. TGF-β Receptor Function in the Endothelium. Cardiovasc. Res. 2005, 65, 599–608. [Google Scholar] [CrossRef]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.J.; ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef]
- Li, P.; Ferrara, N. Vascular Heterogeneity: VEGF Receptors Make Blood Vessels Special. J. Exp. Med. 2022, 219, 212984. [Google Scholar] [CrossRef]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef]
- Gurevich, D.B.; David, D.T.; Sundararaman, A.; Patel, J.; Minchiotti, G.; Fico, A.; Brakebusch, C. Endothelial Heterogeneity in Development and Wound Healing. Cells 2021, 10, 2338. [Google Scholar] [CrossRef]
- Matrone, G.; Xia, B.; Chen, K.; Denvir, M.A.; Baker, A.H.; Cooke, J.P. Fli1+ Cells Transcriptional Analysis Reveals an Lmo2-Prdm16 Axis in Angiogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2008559118. [Google Scholar] [CrossRef]
- Mattonet, K.; Riemslagh, F.W.; Guenther, S.; Prummel, K.D.; Kesavan, G.; Hans, S.; Ebersberger, I.; Brand, M.; Burger, A.; Reischauer, S.; et al. Endothelial versus Pronephron Fate Decision Is Modulated by the Transcription Factors Cloche/Npas4l, Tal1, and Lmo2. Sci. Adv. 2022, 8, 31. [Google Scholar] [CrossRef]
- Kataoka, H.; Hayashi, M.; Nakagawa, R.; Tanaka, Y.; Izumi, N.; Nishikawa, S.; Jakt, M.L.; Tarui, H.; Nishikawa, S.I. Etv2/ER71 Induces Vascular Mesoderm from Flk1+PDGFRα+ Primitive Mesoderm. Blood 2011, 118, 6975–6986. [Google Scholar] [CrossRef]
- Singh, B.N.; Sierra-Pagan, J.E.; Gong, W.; Das, S.; Theisen, J.W.M.; Skie, E.; Garry, M.G.; Garry, D.J. ETV2 (Ets Variant Transcription Factor 2)- Rhoj Cascade Regulates Endothelial Progenitor Cell Migration During Embryogenesis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2875–2890. [Google Scholar] [CrossRef]
- Kim, T.M.; Lee, R.H.; Kim, M.S.; Lewis, C.A.; Park, C. ETV2/ER71, the Key Factor Leading the Paths to Vascular Regeneration and Angiogenic Reprogramming. Stem Cell Res. Ther. 2023, 14, 41. [Google Scholar] [CrossRef]
- Morita, R.; Suzuki, M.; Kasahara, H.; Shimizu, N.; Shichita, T.; Sekiya, T.; Kimura, A.; Sasaki, K.I.; Yasukawa, H.; Yoshimura, A. ETS Transcription Factor ETV2 Directly Converts Human Fibroblasts into Functional Endothelial Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 160–165. [Google Scholar] [CrossRef]
- Martin, A.; Cano, A. Tumorigenesis: Twist1 Links EMT to Self-Renewal. Nat. Cell Biol. 2010, 12, 924–925. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Zheng, X.; Zheng, Y.; Dong, X.; Zhao, N.; Liao, S.; Sun, B. The EMT Transcription Factor, Twist1, as a Novel Therapeutic Target for Pulmonary Sarcomatoid Carcinomas. Int. J. Oncol. 2020, 56, 750–760. [Google Scholar] [CrossRef]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers 2022, 14, 6054. [Google Scholar] [CrossRef] [PubMed]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and Its Potential for Therapeutic Intervention in Malignancy and Ischemia. Yale J. Biol. Med. 2007, 80, 51. [Google Scholar]
- Fernández-Sarmiento, J.; Salazar-Pelaéz, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Microcirculation, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 2, ISBN 9780123815101. [Google Scholar]
- Gilbert, S.F. Epigenetic Landscaping: Waddington’s Use of Cell Fate Bifurcation Diagrams. Biol. Philos. 1991, 6, 135–154. [Google Scholar] [CrossRef]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial Heterogeneity across Distinct Vascular Beds during Homeostasis and Inflammation. Elife 2020, 9, e51413. [Google Scholar] [CrossRef] [PubMed]
- De Seranno, S.; Estrella, C.; Loyens, A.; Cornea, A.; Ojeda, S.R.; Beauvillain, J.-C.; Prevot, V. Development/Plasticity/Repair Vascular Endothelial Cells Promote Acute Plasticity in Ependymoglial Cells of the Neuroendocrine Brain. J. Neurosci. 2004, 24, 10353–10363. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential Mechanisms Underlying Cardiovascular Protection by Polyphenols: Role of the Endothelium. Free. Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hadland, B.K.; Varnum-Finney, B.; Poulos, M.G.; Moon, R.T.; Butler, J.M.; Rafii, S.; Bernstein, I.D. Endothelium and NOTCH Specify and Amplify Aorta-Gonad-Mesonephros-Derived Hematopoietic Stem Cells. J. Clin. Investig. 2015, 125, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Slovinski, A.P.; Hajjar, L.A.; Ince, C. Microcirculation in Cardiovascular Diseases. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3458–3468. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.P.M.H.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W. Modeling Structural Adaptation of Microcirculation. Microcirculation 2008, 15, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Inoue, S. Lamina Lucida of Basement Membrane: An Artefact. Microsc. Res. Tech. 1994, 28, 59. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar]
- Jayadev, R.; Sherwood, D.R. Basement Membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef] [PubMed]
- Sieve, I.; Münster-Kühnel, A.K.; Hilfiker-Kleiner, D. Regulation and Function of Endothelial Glycocalyx Layer in Vascular Diseases. Vasc. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef]
- Delgadillo, L.F.; Marsh, G.A.; Waugh, R.E. Endothelial Glycocalyx Layer Properties and Its Ability to Limit Leukocyte Adhesion. Biophys. J. 2020, 118, 1564–1575. [Google Scholar] [CrossRef]
- Reiterer, M.; Branco, C.M. Endothelial Cells and Organ Function: Applications and Implications of Understanding Unique and Reciprocal Remodelling. FEBS J. 2020, 287, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Azcutia, V.; Stefanidakis, M.; Tsuboi, N.; Mayadas, T.; Croce, K.J.; Fukuda, D.; Aikawa, M.; Newton, G.; Luscinskas, F.W. Endothelial CD47 Promotes Vascular Endothelial-Cadherin Tyrosine Phosphorylation and Participates in T Cell Recruitment at Sites of Inflammation In Vivo. J. Immunol. 2012, 189, 2553–2562. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Bearer, E.L.; Orci, L.; Sors, P. Endothelial Fenestral Diaphragms: A Quick-Freeze, Deep-Etch Study. J. Cell Biol. 1985, 100, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Abulrob, A.N.G.; Kandalaft, L.E.; Plummer, S.; Hollins, A.J.; Gibbs, A.; Gumbleton, M. Constitutive Expression of P-Glycoprotein in Normal Lung Alveolar Epithelium and Functionality in Primary Alveolar Epithelial Cultures. J. Pharmacol. Exp. Ther. 2003, 304, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B.; Kon, V. The Glomerulus-a View from the inside-the Endothelial Cell. J. Pharmacol. Exp. Ther. 2010, 42, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Fonta, C. Neuronal Tissue-Nonspecific Alkaline Phosphatase (TNAP); Fonta, C., Négyessy, L., Eds.; Subcellular Biochemistry; Springer Netherlands: Dordrecht, The Netherland, 2015; Volume 76, ISBN 978-94-017-7196-2. [Google Scholar]
- Garlanda, C.; Dejana, E. Brief Reviews Heterogeneity of Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1193–1202. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Popova, P.I.; Avdonin, P.P.; Kudryavtsev, I.V.; Serebryakova, M.K.; Korf, E.A.; Avdonin, P.V. Markers of Endothelial Cells in Normal and Pathological Conditions. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2020, 14, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Chakraborty, S.; Johnson, K.E.; Falk, M.M.; Wheelock, M.J.; Johnson, K.R.; Mehta, P.P. Assembly of Connexin43 into Gap Junctions Is Regulated Differentially by E-Cadherin and N-Cadherin in Rat Liver Epithelial Cells. Mol. Biol. Cell 2010, 21, 4089–4107. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.; Wanner, N.; Frimel, M.; Erzurum, S.; Asosingh, K. Comprehensive Phenotyping of Endothelial Cells Using Flow Cytometry 1: Murine. Cytom. Part A 2020, 99, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Haasdijk, R.A.; Den Dekker, W.K.; Cheng, C.; Tempel, D.; Szulcek, R.; Bos, F.L.; Hermkens, D.M.A.; Chrifi, I.; Brandt, M.M.; Van Dijk, C.; et al. THSD1 Preserves Vascular Integrity and Protects against Intraplaque Haemorrhaging in ApoE-/- Mice. Cardiovasc. Res. 2016, 110, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Hamashima, T.; Yamamoto, S.; Sasahara, M. Pathogenetic Significance and Possibility as a Therapeutic Target of Platelet Derived Growth Factor. Pathol. Int. 2017, 67, 235–246. [Google Scholar] [CrossRef]
- Kakei, Y.; Akashi, M.; Shigeta, T.; Hasegawa, T.; Komori, T. Alteration of Cell-Cell Junctions in Cultured Human Lymphatic Endothelial Cells with Inflammatory Cytokine Stimulation. Lymphat. Res. Biol. 2014, 12, 136–143. [Google Scholar] [CrossRef]
- Keuschnigg, J.; Karinen, S.; Auvinen, K.; Irjala, H.; Mpindi, J.P.; Kallioniemi, O.; Hautaniemi, S.; Jalkanen, S.; Salmi, M. Plasticity of Blood- and Lymphatic Endothelial Cells and Marker Identification. PLoS ONE 2013, 8, e74293. [Google Scholar] [CrossRef]
- Lalor, P.F.; Lai, W.K.; Curbishley, S.M.; Shetty, S.; Adams, D.H. Human Hepatic Sinusoidal Endothelial Cells Can Be Distinguished by Expression of Phenotypic Markers Related to Their Specialised Functions in Vivo. World J. Gastroenterol. 2006, 12, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, G.; Nagao, R.J.; Xue, J.; Choi, Y.J.; Xu, J.; Ren, S.; Aburatani, T.; Anderson, S.K.; MacDonald, J.W.; Bammler, T.K.; et al. A Novel Three-Dimensional Human Peritubular Microvascular System. J. Am. Soc. Nephrol. 2016, 27, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- DiBartola, S.P. Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice; Elsevier: Amsterdam, The Netherlands, 2012; Volume 53, ISBN 9781437706543. [Google Scholar]
- McMillan, D.B.; Harris, R.J. An Atlas of Comparative Vertebrate Histology; Donald, B., McMillan, R.J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780124104242. [Google Scholar]
- Mink, D.; Schiller, A.; Kriz, W.; Taugner, R. Interendothelial Junctions in Kidney Vessels. Cell Tissue Res. 1984, 236, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Obeidat, M.; Ballermann, B.J. Glomerular Endothelium: A Porous Sieve and Formidable Barrier. Exp. Cell Res. 2012, 318, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver Sinusoidal Endothelial Cells: Physiology and Role in Liver Diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical Expression of Endothelial Markers CD31, CD34, von Willebrand Factor, and Fli-1 in Normal Human Tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef]
- Satchell, S.C.; Braet, F. Glomerular Endothelial Cell Fenestrations: An Integral Component of the Glomerular Filtration Barrier. Am. J. Physiol. Renal. Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Krishnan, J.; Kirkin, V.; Baumann, P. Markers for the Lymphatic Endothelium: In Search of the Holy Grail? Microsc. Res. Tech. 2001, 55, 61–69. [Google Scholar] [CrossRef]
- Stan, R.V. Endothelial Stomatal and Fenestral Diaphragms in Normal Vessels and Angiogenesis. J. Cell. Mol. Med. 2007, 11, 621–643. [Google Scholar] [CrossRef]
- Stan, R.V.; Tse, D.; Deharvengt, S.J.; Smits, N.C.; Xu, Y.; Luciano, M.R.; McGarry, C.L.; Buitendijk, M.; Nemani, K.V.; Elgueta, R.; et al. The Diaphragms of Fenestrated Endothelia: Gatekeepers of Vascular Permeability and Blood Composition. Dev. Cell 2012, 23, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Svistounov, D.; Warren, A.; McNerney, G.P.; Owen, D.M.; Zencak, D.; Zykova, S.N.; Crane, H.; Huser, T.; Quinn, R.J.; Smedsrød, B.; et al. The Relationship between Fenestrations, Sieve Plates and Rafts in Liver Sinusoidal Endothelial Cells. PLoS ONE 2012, 7, e46134. [Google Scholar] [CrossRef] [PubMed]
- Tati, R.; Kristoffersson, A.C.; Ståhl, A.L.; Mörgelin, M.; Motto, D.; Satchell, S.; Mathieson, P.; Manea-Hedström, M.; Karpman, D. Phenotypic Expression of ADAMTS13 in Glomerular Endothelial Cells. PLoS ONE 2011, 6, e21587. [Google Scholar] [CrossRef]
- Tse, D.; Stan, R.V. Morphological Heterogeneity of Endothelium. Semin. Thromb. Hemost. 2010, 36, 236–245. [Google Scholar] [CrossRef]
- Wallez, Y.; Huber, P. Endothelial Adherens and Tight Junctions in Vascular Homeostasis, Inflammation and Angiogenesis. Biochim. Biophys. Acta Biomembr. 2008, 1778, 794–809. [Google Scholar] [CrossRef]
- Zhang, D.; Isaka, Y.; Imamura, R.; Ichimaru, N.; Shi, Y.; Imai, E.; Tian, Y.; Ohtsuka, A.; Takahara, S. Glycocalyx Damage Estimated Using Colloidal Iron Staining. Cell Transplant. 2008, 17, 159–163. [Google Scholar] [CrossRef]
- Zhang, F.; Zarkada, G.; Yi, S.; Eichmann, A. Lymphatic Endothelial Cell Junctions: Molecular Regulation in Physiology and Diseases. Front. Physiol. 2020, 11, 509. [Google Scholar] [CrossRef]
- Shang, T.; Liang, J.; Kapron, C.M.; Liu, J. Pathophysiology of Aged Lymphatic Vessels. Aging 2019, 11, 6602–6613. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Lockwood, G.P.; Warren, A.; Mao, H.; McCourt, P.A.G.; Le Couteur, D.G.; Cogger, V.C. Manipulating Fenestrations in Young and Old Liver Sinusoidal Endothelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G144–G154. [Google Scholar] [CrossRef]
- McCarron, J.G.; Lee, M.D.; Wilson, C. The Endothelium Solves Problems That Endothelial Cells Do Not Know Exist. Trends Pharmacol. Sci. 2017, 38, 322–338. [Google Scholar] [CrossRef]
- Orsenigo, F.; Conze, L.L.; Jauhiainen, S.; Corada, M.; Lazzaroni, F.; Malinverno, M.; Sundell, V.; Cunha, S.I.; Brännström, J.; Globisch, M.A.; et al. Mapping Endothelial-Cell Diversity in Cerebral Cavernous Malformations at Single-Cell Resolution. Elife 2020, 9, e61413. [Google Scholar] [CrossRef]
- Paz, N.G.; Amore, P.A.D. Arterial versus Venous Endothelial Cells Nathaniel. Cell Tissue Res. 2014, 335, 5–16. [Google Scholar] [CrossRef]
- Risau, W. Development and Differentiation of Endothelium. Kidney Int. Suppl. 1998, 54, 3–6. [Google Scholar] [CrossRef]
- Jones, E.; Gallimore, A.; Ager, A. Defining High Endothelial Venules and Tertiary Lymphoid Structures in Cancer. Methods Mol. Biol. 2018, 1845, 99–118. [Google Scholar] [CrossRef]
- Blanchard, L.; Girard, J.P. High Endothelial Venules (HEVs) in Immunity, Inflammation and Cancer. Angiogenesis 2021, 24, 719–753. [Google Scholar] [CrossRef]
- Vella, G.; Guelfi, S.; Bergers, G. High Endothelial Venules: A Vascular Perspective on Tertiary Lymphoid Structures in Cancer. Front. Immunol. 2021, 12, 736670. [Google Scholar] [CrossRef]
- Vallance, P. Blood Vessels and the Endothelium. In Oxford Textbook of Medicine; Oxford Textbooks: Oxford, UK, 2003; Volume 2, pp. 1715–1720. [Google Scholar] [CrossRef]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The Role of Adherens Junctions and VE-Cadherin in the Control of Vascular Permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef]
- Dejana, E. Endothelial Cell-Cell Junctions: Happy Together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Freund-Michel, V.; Muller, B.; Marthan, R.; Savineau, J.P.; Guibert, C. Expression and Role of Connexin-Based Gap Junctions in Pulmonary Inflammatory Diseases. Pharmacol. Ther. 2016, 164, 105–119. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and Tight Junctions: Structure, Function and Connections to the Actin Cytoskeleton. Biochim. Biophys. Acta Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between Tight Junctions & Adherens Junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef]
- Winkler, L.; Blasig, R.; Breitkreuz-Korff, O.; Berndt, P.; Dithmer, S.; Helms, H.C.; Puchkov, D.; Devraj, K.; Kaya, M.; Qin, Z.; et al. Tight Junctions in the Blood–Brain Barrier Promote Edema Formation and Infarct Size in Stroke–Ambivalent Effects of Sealing Proteins. J. Cereb. Blood Flow Metab. 2020, 41, 132–145. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Cross-talk. Front. Physiol. 2019, 10, 1942. [Google Scholar] [CrossRef]
- Takeshita, Y.; Sato, R.; Kanda, T. Blood–Nerve Barrier (BNB) Pathology in Diabetic Peripheral Neuropathy and In Vitro Human BNB Model. Int. J. Mol. Sci. 2020, 22, 62. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional Proteins of the Blood-Brain Barrier: New Insights into Function and Dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef]
- Wittekindt, O.H. Tight Junctions in Pulmonary Epithelia during Lung Inflammation. Pflüg. Arch. Eur. J. Physiol. 2017, 469, 135–147. [Google Scholar] [CrossRef]
- Bing, S.; Chen, Z.; Gu, J.; Tse, G.; Jiang, J.; Huang, F.; Zhao, C. Tight Junction Proteins and Gap Junction Proteins Play Important Roles in High Fat Dietary Atherosclerosis Pathogenesis. Int. J. Clin. Exp. Pathol. 2016, 9, 7969–7976. [Google Scholar]
- Tym, K.; Mathieu-Costello, O. Structural and Functional Changes in the Microvasculature of Disused Skeletal Muscle. Front. Biosci. 2001, 6, d45. [Google Scholar] [CrossRef]
- Harris, T.J.C.; Tepass, U. Adherens Junctions: From Molecules to Morphogenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 502–514. [Google Scholar] [CrossRef]
- Gonschior, H.; Haucke, V.; Lehmann, M. Super-Resolution Imaging of Tight and Adherens Junctions: Challenges and Open Questions. Int. J. Mol. Sci. 2020, 21, 744. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, S.J. VE-Cadherin and Claudin-5: It Takes Two to Tango. Nat. Cell Biol. 2008, 10, 883. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef]
- Peracchia, C. Connexin/Innexin Channels in Cytoplasmic Organelles. Are There Intracellular Gap Junctions? A Hypothesis! Int. J. Mol. Sci. 2020, 21, 2163. [Google Scholar] [CrossRef]
- Socha, M.J.; Segal, S.S. Vascular Aging: Microvascular Mechanisms Limiting Skeletal Muscle Blood Flow with Advancing Age. J. Appl. Physiol. 2018, 125, 1851. [Google Scholar] [CrossRef]
- Kovacs-Oller, T.; Ivanova, E.; Bianchimano, P.; Sagdullaev, B.T. The Pericyte Connectome: Spatial Precision of Neurovascular Coupling Is Driven by Selective Connectivity Maps of Pericytes and Endothelial Cells and Is Disrupted in Diabetes. Cell Discov. 2020, 6, 39. [Google Scholar] [CrossRef]
- Maibier, M.; Bintig, W.; Goede, A.; Höpfner, M.; Kuebler, W.M.; Secomb, T.W.; Nitzsche, B.; Pries, A.R. Gap Junctions Regulate Vessel Diameter in Chick Chorioallantoic Membrane Vasculature by Both Tone-Dependent and Structural Mechanisms. Microcirculation 2020, 27, e12590. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawamoto, E.; Takagi, Y.; Akita, N.; Hayashi, T.; Park, E.J.; Suzuki, K.; Shimaoka, M. Gap Junction-Mediated Regulation of Endothelial Cellular Stiffness. Sci. Rep. 2017, 7, 6134. [Google Scholar] [CrossRef]
- Favero, G.; Paganelli, C.; Buffoli, B.; Rodella, L.F.; Rezzani, R. Endothelium and Its Alterations in Cardiovascular Diseases: Life Style Intervention. Biomed Res. Int. 2014, 2014, 801896. [Google Scholar] [CrossRef]
- Xu, B.; Broome, U.; Uzunel, M.; Nava, S.; Ge, X.; Kumagai-Braesch, M.; Hultenby, K.; Christensson, B.; Ericzon, B.G.; Holgersson, J.; et al. Capillarization of Hepatic Sinusoid by Liver Endothelial Cell-Reactive Autoantibodies in Patients with Cirrhosis and Chronic Hepatitis. Am. J. Pathol. 2003, 163, 1275–1289. [Google Scholar] [CrossRef]
- Murray, J.W.; Spray, D.C. Gap and Tight Junctions in Liver. In The Liver; Wiley: Hoboken, NJ, USA, 2020; pp. 160–173. [Google Scholar]
- Chi, J.T.J.-T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial Cell Diversity Revealed by Global Expression Profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef]
- Turunen, M.P.; Aavik, E.; Ylä-Herttuala, S. Epigenetics and Atherosclerosis. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 886–891. [Google Scholar] [CrossRef]
- Friso, S.; Carvajal, C.A.; Fardella, C.E.; Olivieri, O. Epigenetics and Arterial Hypertension: The Challenge of Emerging Evidence. Transl. Res. 2015, 165, 154–165. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Penson, P.E.; Banach, M.; Motallebnezhad, M.; Jamialahmadi, T.; Sahebkar, A. Epigenetic Control of Atherosclerosis via DNA Methylation: A New Therapeutic Target? Life Sci. 2020, 253, 117682. [Google Scholar] [CrossRef]
- Certo, M.; Elkafrawy, H.; Pucino, V.; Cucchi, D.; Cheung, K.C.P.; Mauro, C. Endothelial Cell and T-cell Cross-talk: Targeting Metabolism as a Therapeutic Approach in Chronic Inflammation. Br. J. Pharmacol. 2021, 178, 2041. [Google Scholar] [CrossRef]
- Howe, K.L.; Cybulsky, M.; Fish, J.E. The Endothelium as a Hub for Cellular Communication in Atherogenesis: Is There Directionality to the Message? Front. Cardiovasc. Med. 2022, 9, 888390. [Google Scholar] [CrossRef]
- Przysinda, A.; Feng, W.; Li, G. Diversity of Organism-Wide and Organ-Specific Endothelial Cells. Curr. Cardiol. Rep. 2020, 22, 4–9. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Huxley, V.H.; Xie, L.; Bunyak, F.; Palaniappan, K.; Glinsky, V.V. Complex Non-Sinus-Associated Pachymeningeal Lymphatic Structures: Interrelationship with Blood Microvasculature. Front. Physiol. 2019, 10, 1364. [Google Scholar] [CrossRef]
- Xiang, M.; Grosso, R.A.; Takeda, A.; Pan, J.; Bekkhus, T.; Brulois, K.; Dermadi, D.; Nordling, S.; Vanlandewijck, M.; Jalkanen, S.; et al. A Single-Cell Transcriptional Roadmap of the Mouse and Human Lymph Node Lymphatic Vasculature. Front. Cardiovasc. Med. 2020, 7, 52. [Google Scholar] [CrossRef]
- He, W.; Jing, Y.; Wan, Q.; Xiao, K.; Chen, K.; Lu, Y.; Li, L.; Tang, Y.; Deng, Y.; Yao, Z.; et al. The Anatomy and Metabolome of the Lymphatic System in the Brain in Health and Disease. Brain Pathol. 2020, 30, 392–404. [Google Scholar] [CrossRef]
- Ma, Q.; Schlegel, F.; Bachmann, S.B.; Schneider, H.; Decker, Y.; Rudin, M.; Weller, M.; Proulx, S.T.; Detmar, M. Lymphatic Outflow of Cerebrospinal Fluid Is Reduced in Glioma. Sci. Rep. 2019, 9, 14815. [Google Scholar] [CrossRef]
- Petrova, T.V.; Koh, G.Y. Biological Functions of Lymphatic Vessels. Science 2020, 369, eaax4063. [Google Scholar] [CrossRef]
- Morfoisse, F.; Noel, A. Lymphatic and Blood Systems: Identical or Fraternal Twins? International Journal of Biochemistry and Cell Biol. 2019, 114, 105562. [Google Scholar] [CrossRef]
- Liu, X.; Gu, X.; Ma, W.; Oxendine, M.; Gil, H.J.; Davis, G.E.; Cleaver, O.; Oliver, G. Rasip1 Controls Lymphatic Vessel Lumen Maintenance by Regulating Endothelial Cell Junctions. Development 2018, 145, dev.165092. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Yao, L.C.; Baluk, P.; Srinivasan, R.S.; Oliver, G.; McDonald, D.M. Plasticity of Button-like Junctions in the Endothelium of Airway Lymphatics in Development and Inflammation. Am. J. Pathol. 2012, 180, 2561–2575. [Google Scholar] [CrossRef]
- Kazenwadel, J.; Harvey, N.L. ScienceDirect Lymphatic Endothelial Progenitor Cells: Origins and Roles in Lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 81–87. [Google Scholar] [CrossRef]
- Volk-Draper, L.D.; Hall, K.L.; Wilber, A.C.; Ran, S. Lymphatic Endothelial Progenitors Originate from Plastic Myeloid Cells Activated by Toll-like Receptor-4. PLoS ONE 2017, 12, e0179257. [Google Scholar] [CrossRef]
- Shoemaker, L.D.; Fuentes, L.F.; Santiago, S.M.; Allen, B.M.; Cook, D.J.; Steinberg, G.K.; Chang, S.D. Human Brain Arteriovenous Malformations Express Lymphatic-Associated Genes. Ann. Clin. Transl. Neurol. 2014, 1, 982–995. [Google Scholar] [CrossRef]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.H.; et al. Development and Plasticity of Meningeal Lymphatic Vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef]
- Pawlak, J.B.; Caron, K.M. Lymphatic Programing and Specialization in Hybrid Vessels. Front. Physiol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Surya, V.N.; Michalaki, E.; Huang, E.Y.; Fuller, G.G.; Dunn, A.R. Sphingosine 1-Phosphate Receptor 1 Regulates the Directional Migration of Lymphatic Endothelial Cells in Response to Fluid Shear Stress. J. R. Soc. Interface 2016, 13, 20160823. [Google Scholar] [CrossRef]
- Dellinger, M.T.; Meadows, S.M.; Wynne, K.; Cleaver, O.; Brekken, R.A. Vascular Endothelial Growth Factor Receptor-2 Promotes the Development of the Lymphatic Vasculature. PLoS ONE 2013, 8, e74686. [Google Scholar] [CrossRef]
- Semo, J.; Nicenboim, J.; Yaniv, K. Development of the Lymphatic System: New Questions and Paradigms. Development 2016, 143, 924–935. [Google Scholar] [CrossRef]
- Gancz, D.; Perlmoter, G.; Yaniv, K. Formation and Growth of Cardiac Lymphatics during Embryonic Development, Heart Regeneration, and Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037176. [Google Scholar] [CrossRef]
- Shen, B.; Shang, Z.; Wang, B.; Zhang, L.; Zhou, F.; Li, T.; Chu, M.; Jiang, H.; Wang, Y.; Qiao, T.; et al. Genetic Dissection of Tie Pathway in Mouse Lymphatic Maturation and Valve Development. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1221–1230. [Google Scholar] [CrossRef]
- Engelbrecht, E.; Levesque, M.V.; He, L.; Vanlandewijck, M.; Nitzsche, A.; Niazi, H.; Kuo, A.; Singh, S.A.; Aikawa, M.; Holton, K.; et al. Sphingosine 1-Phosphate-Regulated Transcriptomes in Heterogenous Arterial and Lymphatic Endothelium of the Aorta. Elife 2020, 9, e52690. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal Lymphatics Affect Microglia Responses and Anti-Aβ Immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef]
- Norden, P.R.; Kume, T. The Role of Lymphatic Vascular Function in Metabolic Disorders. Front. Physiol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Ho, Y.C.; Srinivasan, R.S. Lymphatic Vasculature in Energy Homeostasis and Obesity. Front. Physiol. 2020, 11, 3. [Google Scholar] [CrossRef]
- Goodall, E.F.; Leach, V.; Wang, C.; Cooper-Knock, J.; Heath, P.R.; Baker, D.; Drew, D.R.; Jill Saffrey, M.; Simpson, J.E.; Romero, I.A.; et al. Age-Associated MRNA and MiRNA Expression Changes in the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 3097. [Google Scholar] [CrossRef]
- Geevarghese, A.; Herman, I.M. Pericyte-Endothelial Cross-Talk: Implications and Opportunities for Advanced Cellular Therapies. Transl. Res. 2014, 163, 296. [Google Scholar] [CrossRef] [PubMed]
- Longden, T.A.; Zhao, G.; Hariharan, A.; Lederer, W.J. Pericytes and the Control of Blood Flow in Brain and Heart. Annu. Rev. Physiol. 2023, 85, 137. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro Oncol. 2005, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fu, J.; Huang, Y.; Duan, R.; Zhang, W.; Wang, C.; Wang, S.; Hu, X.; Zhao, H.; Wang, L.; et al. Biology and Function of Pericytes in the Vascular Microcirculation. Anim. Model Exp. Med. 2023, 6, 337–345. [Google Scholar] [CrossRef]
- Van Splunder, H.; Villacampa, P.; Martínez-Romero, A.; Graupera, M. Pericytes in the Disease Spotlight Cell Biology. Trends Cell Biol. 2024, 34, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, C.J.F.; Yetkin-Arik, B.; Serrano Martinez, P.; Bakker, N.; van Breest Smallenburg, M.E.; Schlingemann, R.O.; Klaassen, I.; Majc, B.; Habic, A.; Bogataj, U.; et al. New Insights in ATP Synthesis as Therapeutic Target in Cancer and Angiogenic Ocular Diseases. J. Histochem. Cytochem. 2024, 72, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Vinet, L.; Zhedanov, A. The Blood-Brain and Other Neural Barriers; Nag, S., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 686, ISBN 978-1-60761-937-6. [Google Scholar]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional Morphology of the Blood–Brain Barrier in Health and Disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In Vitro Models of the Blood-Brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow Metab. 2015, 36, 862–890. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.M. Endothelium-Role in Regulation of Coagulation and Inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef]
- Wilhelm, I.; Nyúl-Tóth, Á.; Suciu, M.; Hermenean, A.; Krizbai, I.A. Heterogeneity of the Blood-Brain Barrier. Tissue Barriers 2016, 4, e1143544. [Google Scholar] [CrossRef]
- Mbagwu, S.I.; Filgueira, L. Differential Expression of CD31 and Von Willebrand Factor on Endothelial Cells in Different Regions of the Human Brain: Potential Implications for Cerebral Malaria Pathogenesis. Brain Sci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual Microglia Effects on Blood Brain Barrier Permeability Induced by Systemic Inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Santisteban, M.; Ahn, S.J.; Lane, D.; Faraco, G.; Garcia-Bonilla, L.; Racchumi, G.; Poon, C.; Schaeffer, S.; Segarra, S.; Körbelin, J.; et al. Endothelium-Macrophage Cross-talk Mediates Blood-Brain Barrier Dysfunction in Hypertension. Hypertension 2020, 76, 795–807. [Google Scholar] [CrossRef]
- Tunon-Ortiz, A.; Lamb, T.J. Blood Brain Barrier Disruption in Cerebral Malaria: Beyond Endothelial Cell Activation. PLoS Pathog. 2019, 15, e1007786. [Google Scholar] [CrossRef] [PubMed]
- Bonney, S.; Seitz, S.; Ryan, C.A.; Jones, K.L.; Clarke, P.; Tyler, K.L.; Siegenthaler, J.A. Gamma Interferon Alters Junctional Integrity via Rho Kinase, Resulting in Blood-Brain Barrier Leakage in Experimental Viral Encephalitis. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Ross, O.N.; Moore, C.M.; Suggett, D.J.; Macintyre, H.L.; Geider, R.J. Endothelial-Cardiomyocyte Interactions. Annu. Rev Physiol. 2008, 53, 1835–1852. [Google Scholar] [CrossRef]
- Brutsaert, D.L.; De Keulenaer, G.W.; Fransen, P.; Mohan, P.; Kaluza, G.L.; Andries, L.J.; Rouleau, J.-L.; Sys, S.U. The Cardiac Endothelium: Functional Morphology, Development, and Physiology. Prog. Cardiovasc. Dis. 1996, 39, 239–262. [Google Scholar] [CrossRef]
- Smiljic, S. The Clinical Significance of Endocardial Endothelial Dysfunction. Medicina 2017, 53, 295–302. [Google Scholar] [CrossRef]
- Piper, H.M.; Spahr, R.; Mertens, S.; Krützfeldt, A.; Watanabe, H. Microvascular Endothelial Cells from Heart. In Cell Culture Techniques in Heart and Vessel Research; Springer: Berlin\Heidelberg, Germany, 1990; pp. 158–177. [Google Scholar]
- Taylor, J.; Fischer, A. Endothelial Cells Dictate Cardiac Fuel Source. Aging 2019, 11, 1083–1084. [Google Scholar] [CrossRef]
- Yazdani, S.; Jaldin-Fincati, J.R.; Pereira, R.V.S.; Klip, A. Endothelial Cell Barriers: Transport of Molecules between Blood and Tissues. Traffic 2019, 20, 390–403. [Google Scholar] [CrossRef]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial Cell–Cardiomyocyte Cross-talk in Heart Development and Disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Brutsaert, D.L.; De Keulenaer, G.W. Cardiac Remodeling: Endothelial Cells Have More to Say than Just NO. Front. Physiol. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Pratumvinit, B.; Reesukumal, K.; Janebodin, K.; Ieronimakis, N.; Reyes, M. Isolation, Characterization, and Transplantation of Cardiac Endothelial Cells. Biomed Res. Int. 2013, 2013, 359412. [Google Scholar] [CrossRef]
- Kongpol, K.; Nernpermpisooth, N.; Prompunt, E.; Kumphune, S. Endothelial-Cell-Derived Human Secretory Leukocyte Protease Inhibitor (SLPI) Protects Cardiomyocytes against Ischemia/Reperfusion Injury. Biomolecules 2019, 9, 678. [Google Scholar] [CrossRef]
- Talman, V.; Kivelä, R. Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef]
- Mebazaa, A.; Wetzel, R.; Cherian, M.; Abraham, M. Comparison between Endocardial and Great Vessel Endothelial Cells: Morphology, Growth, and Prostaglandin Release. Am. J. Physiol. Heart Circ. Physiol. 1995, 268, H250–H259. [Google Scholar] [CrossRef]
- Yu, T.; Jing, M.; Gao, Y.; Liu, C.; Liu, L.; Jia, H.; Liu, P.; Chang, M. Study on the Relationship between Hyperthyroidism and Vascular Endothelial Cell Damage. Sci. Rep. 2020, 10, 6992. [Google Scholar] [CrossRef]
- Dal Lin, C.; Tona, F.; Osto, E. The Cross-talk between the Cardiovascular and the Immune System. Vasc. Biol. 2019, 1, H83–H88. [Google Scholar] [CrossRef]
- Mojiri, A.; Alavi, P.; Lorenzana Carrillo, M.A.; Nakhaei-Nejad, M.; Sergi, C.M.; Thebaud, B.; Aird, W.C.; Jahroudi, N. Endothelial Cells of Different Organs Exhibit Heterogeneity in von Willebrand Factor Expression in Response to Hypoxia. Atherosclerosis 2019, 282, 1–10. [Google Scholar] [CrossRef]
- Borsig, L. Selectins in Cancer Immunity. Glycobiology 2018, 28, 648. [Google Scholar] [CrossRef]
- González-Tajuelo, R.; Silván, J.; Pérez-Frías, A.; De La Fuente-Fernández, M.; Tejedor, R.; Espartero-Santos, M.; Vicente-Rabaneda, E.; Juarranz, Á.; Muñoz-Calleja, C.; Castañeda, S.; et al. P-Selectin Preserves Immune Tolerance in Mice and Is Reduced in Human Cutaneous Lupus. Sci. Rep. 2017, 7, srep41841. [Google Scholar] [CrossRef]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; et al. Pulmonary Vascular Endothelium: The Orchestra Conductor in Respiratory Diseases. Eur. Respir. J. 2018, 51, 1700745. [Google Scholar] [CrossRef] [PubMed]
- Fundamentals of Vascular Biology; Springer Nature: Dordrecht, The Netherlands, 2019. [CrossRef]
- Niethamer, T.K.; Stabler, C.T.; Leach, J.P.; Zepp, J.A.; Morley, M.P.; Babu, A.; Zhou, S.; Morrisey, E.E. Defining the Role of Pulmonary Endothelial Cell Heterogeneity in the Response to Acute Lung Injury. Elife 2020, 9, e53072. [Google Scholar] [CrossRef] [PubMed]
- Gillich, A.; Zhang, F.; Farmer, C.G.; Travaglini, K.J.; Tan, S.Y.; Gu, M.; Zhou, B.; Feinstein, J.A.; Krasnow, M.A.; Metzger, R.J. Capillary Cell-Type Specialization in the Alveolus. Nature 2020, 7, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Millar, F.R.; Summers, C.; Griffiths, M.J.; Toshner, M.R.; Proudfoot, A.G. The Pulmonary Endothelium in Acute Respiratory Distress Syndrome: Insights and Therapeutic Opportunities. Thorax 2016, 71, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R. Human Pulmonary Endothelial Cells in Culture: Activities of Cells from Arteries and Cells from Veins. J. Clin. Investig. 1980, 65, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.N.; Verin, A.D. Pulmonary Vascular Endothelial Cells. In Endothelial Dysfunction—Old Concepts and New Challenges; IntechOpen: London, UK, 2018; Chapter 12; pp. 287–305. [Google Scholar] [CrossRef]
- Stevens, T. Functional and Molecular Heterogeneity of Pulmonary Endothelial Cells. Proc. Am. Thorac. Soc. 2011, 8, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.A.; Xu, W.; Mavrakis, L.; Aldred, M.A.; Asosingh, K.; Erzurum, S.C. Human Primary Lung Endothelial Cells in Culture. Am. J. Respir. Cell Mol. Biol. 2012, 46, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.P.; Post, S.R.; Post, G.R. ABO Blood Group Is a Determinant of von Willebrand Factor Protein Levels in Human Pulmonary Endothelial Cells. J. Clin. Pathol. 2020, 73, 347–349. [Google Scholar] [CrossRef]
- Johansson, B.B. The Role of the Endothelial Cell Surface Charge for Blood-Brain Barrier Function. In New Concepts of a Blood—Brain Barrier; Springer: Boston, MA, USA, 1995; Volume 30, pp. 63–70. [Google Scholar]
- Segal, M.S.; Baylis, C.; Johnson, R.J. Endothelial Health and Diversity in the Kidney. J. Am. Soc. Nephrol. 2006, 17, 323–324. [Google Scholar] [CrossRef]
- Maestroni, S.; Zerbini, G. Glomerular Endothelial Cells versus Podocytes as the Cellular Target in Diabetic Nephropathy. Acta Diabetol. 2018, 55, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Satchell, S.C.; Neal, C.R.; McKenzie, E.A.; Tooke, J.E.; Mathieson, P.W. Glomerular Endothelial Glycocalyx Constitutes a Barrier to Protein Permeability. J. Am. Soc. Nephrol. 2007, 18, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Lee, K.; Chuang, P.Y.; Liu, Z.H.; He, J.C. Glomerular Endothelial Cell Injury and Cross Talk in Diabetic Kidney Disease. Am. J. Physiol. Renal. Physiol. 2015, 308, F287. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Molitoris, B.A. Renal Endothelial Injury and Microvascular Dysfunction in Acute Kidney Injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium Structure and Function in Kidney Health and Disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Noroozinia, F.; Mahmoudzadeh, L.; Gharalari, F.H.; Makhdoomi, K.; Abbasi, A. Relationship between Interstitial CD34 Positive Cells and Active Phase of Lupus Nephritis. Eur. J. Rheumatol. 2018, 5, 254–257. [Google Scholar] [CrossRef]
- Masum, M.A.; Ichii, O.; Elewa, Y.H.A.; Nakamura, T.; Kon, Y. Local CD34-Positive Capillaries Decrease in Mouse Models of Kidney Disease Associating with the Severity of Glomerular and Tubulointerstitial Lesions. BMC Nephrol. 2017, 18, 280. [Google Scholar] [CrossRef][Green Version]
- Ogawa, H.; Rafiee, P.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Binion, D.G. Butyrate Modulates Gene and Protein Expression in Human Intestinal Endothelial Cells. Biochem. Biophys. Res. Commun. 2003, 309, 512–519. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Thomas, H. Intestinal Tract: Gut Endothelial Cells-Another Line of Defence. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 4. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, S.; Jiang, Z.; Zheng, C.; Gou, Z. Effects of Equol on H2O2-Induced Oxidative Stress in Primary Chicken Intestinal Epithelial Cells. Poult. Sci. 2016, 95, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal Epithelial Claudins: Expression and Regulation in Homeostasis and Inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Haraldsen, G.; Rugtveit, J.; Kvale, D.; Scholz, T.; Muller, W.A.; Hovig, T.; Brandtzaeg, P. Isolation and Longterm Culture of Human Intestinal Microvascular Endothelial Cels. Gut 1995, 37, 225–234. [Google Scholar] [CrossRef]
- Haraldsen, G.; Kvale, D.; Lien, B.; Farstad, I.N.; Brandtzaeg, P. Cytokine-Regulated Expression of E-Selectin, Intercellular Adhesion Molecule-1 (ICAM-1), and Vascular Cell Adhesion Molecule-1 (VCAM-1) in Human Microvascular Endothelial Cells. J. Immunol. 1996, 156, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Cromer, W.E.; Mathis, J.M.; Granger, D.N.; Chaitanya, G.V.; Alexander, J.S. Role of the Endothelium in Inflammatory Bowel Diseases. World J. Gastroenterol. 2011, 17, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Harris, E.N.; Kidambi, S. SECs (Sinusoidal Endothelial Cells), Liver Microenvironment, and Fibrosis. Biomed. Res. Int. 2017, 2017, 4097205. [Google Scholar] [CrossRef] [PubMed]
- Cogger, V.C.; Le Couteur, D.G. Fenestrations in the Liver Sinusoidal Endothelial Cell. In The Liver; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 389–406. ISBN 9780470723135. [Google Scholar]
- Shubham, S.; Kumar, D.; Rooge, S.; Maras, J.S.; Maheshwari, D.; Nautiyal, N.; Kumari, R.; Bhat, A.; Kumar, G.; Rastogi, A.; et al. Cellular and Functional Loss of Liver Endothelial Cells Correlates with Poor Hepatocyte Regeneration in Acute-on-Chronic Liver Failure. Hepatol. Int. 2019, 13, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Baiocchini, A.; Del Nonno, F.; Taibi, C.; Visco-Comandini, U.; D’Offizi, G.; Piacentini, M.; Falasca, L. Liver Sinusoidal Endothelial Cells (LSECs) Modifications in Patients with Chronic Hepatitis C. Sci. Rep. 2019, 9, 8760. [Google Scholar] [CrossRef]
- Géraud, C.; Schledzewski, K.; Demory, A.; Klein, D.; Kaus, M.; Peyre, F.; Sticht, C.; Evdokimov, K.; Lu, S.; Schmieder, A.; et al. Liver Sinusoidal Endothelium: A Microenvironment-Dependent Differentiation Program in Rat Including the Novel Junctional Protein Liver Endothelial Differentiation-Associated Protein-1. Hepatology 2010, 52, 313–326. [Google Scholar] [CrossRef]
- Sørensen, K.K.; Simon-Santamaria, J.; McCuskey, R.S.; Smedsrød, B. Liver Sinusoidal Endothelial Cells. Compr. Physiol. 2015, 5, 1751–1774. [Google Scholar] [CrossRef] [PubMed]
- DeLeve, L.D.; Maretti-Mira, A.C. Liver Sinusoidal Endothelial Cell: An Update. Semin. Liver Dis. 2017, 37, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.K.; Smedsrød, B. The Liver Sinusoidal Endothelial Cell. In The Liver; Wiley: Hoboken, NJ, USA, 2020; pp. 422–434. ISBN 9780470723135. [Google Scholar]
- Steiniger, B.S. Human Spleen Microanatomy: Why Mice Do Not Suffice. Immunology 2015, 145, 334–346. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, P.P.H.; Cho, Y. Contractile Structures in Endothelial Cells of Splenic Sinusoids. J. Ultrastruct. Res. 1974, 49, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Weiss, L. Electron Microscopy of the Red Pulp of Human Spleen. Am. J. Anat. 1972, 134, 425–457. [Google Scholar] [CrossRef] [PubMed]
- Structure and Functions of the Human Spleen: Relationship between Microcirculation and Splenic Functions. Available online: https://pubmed.ncbi.nlm.nih.gov/2689682/ (accessed on 4 June 2024).
- Uehara, K.; Uehara, A. Differentiated Localizations of Phosphorylated Focal Adhesion Kinase in Endothelial Cells of Rat Splenic Sinus. Cell Tissue Res. 2016, 364, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Salama, M.E.; Hu, C.S.; Li, Y.; Wang, X.; Hoffman, R. The Characteristics of Vessel Lining Cells in Normal Spleens and Their Role in the Pathobiology of Myelofibrosis. Blood Adv. 2018, 2, 1130–1145. [Google Scholar] [CrossRef]
- Almenar, S.; Rios-Navarro, C.; Ortega, M.; Molina, P.; Ferrandez-Izquierdo, A.; Ruiz-Sauri, A. Anatomy, Immunohistochemistry, and Numerical Distribution of Human Splenic Microvessels. Ann. Anat. 2019, 224, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Horioka, K.; Tanaka, H.; Isozaki, S.; Konishi, H.; Addo, L.; Takauji, S.; Druid, H. Low Temperature Induces Von-Willebrand Factor Expression via Increased Early Growth Response 1 Transcriptional Activity in Splenic Sinusoidal Endothelial Cells: Activation of the Splenic Vascular Endothelium in Low Temperatures. Biochem. Biophys. Res. Commun. 2020, 526, 239–245. [Google Scholar] [CrossRef]
- Williams, I.M.; Wu, J.C. Generation of Endothelial Cells from Human Pluripotent Stem Cells Methods, Considerations, and Applications. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1317–1329. [Google Scholar] [CrossRef]
- Jayaraman, A.; Kumar, P.; Marin, S.; De Atauri, P.; Mateo, F.; Thomson, T.M.; Centelles, J.J.; Graham, S.F.; Cascante, M. Untargeted Metabolomics Reveals Distinct Metabolic Reprogramming in Endothelial Cells Co-Cultured with CSC and Non-CSC Prostate Cancer Cell Subpopulations. PLoS ONE 2018, 13, e0192175. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic Mimicry in Carcinogenesis and Clinical Applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Mentzer, S.J.; Konerding, M.A. Intussusceptive Angiogenesis: Expansion and Remodeling of Microvascular Networks. Angiogenesis 2014, 17, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, E.A.; Reynolds, A.R. Vessel Co-Option and Resistance to Anti-Angiogenic Therapy. Angiogenesis 2020, 23, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Won, Y.J.; Shim, J.H.; Kim, H.J.; Kim, J.; Hong, H.N.; Kim, B.S. Morphological Characteristics of Vasculogenic Mimicry and Its Correlation with EphA2 Expression in Gastric Adenocarcinoma. Sci. Rep. 2019, 9, 3414. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Shaked, Y.; Mancuso, P.; Kerbel, R.S. The Multifaceted Circulating Endothelial Cell in Cancer: Towards Marker and Target Identification. Nat. Rev. Cancer 2006, 6, 835–845. [Google Scholar] [CrossRef]
- Werb, Z.; Lu, P. The Role of Stroma in Tumor Development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef]
- Lin, P.P. Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis. Cells 2020, 9, 1539. [Google Scholar] [CrossRef]
- Dvorak, A.M.; Feng, D. The Vesiculo-Vacuolar Organelle (VVO). A New Endothelial Cell Permeability Organelle. J. Histochem. Cytochem. 2001, 49, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Ippolito, L.; Magnelli, L.; Giannoni, E.; Chiarugi, P. Stromal-Induced Mitochondrial Re-Education: Impact on Epithelial-to-Mesenchymal Transition and Cancer Aggressiveness. Semin. Cell Dev. Biol. 2020, 98, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hapke, R.Y.; Haake, S.M. Hypoxia-Induced Epithelial to Mesenchymal Transition in Cancer. Cancer Lett. 2020, 487, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; ten Dijke, P.; Kostidis, S.; Giera, M.; Hornsveld, M. TGFβ-Induced Metabolic Reprogramming during Epithelial-to-Mesenchymal Transition in Cancer. Cell. Mol. Life Sci. 2020, 77, 2103–2123. [Google Scholar] [CrossRef]
- Yang, F.; Ma, J.; Wan, J.; Ha, W.; Fang, C.; Lu, H.; Zhang, W. Epithelial-Mesenchymal Transition of Circulating Tumor Cells in Prostate Cancer Is Promoted by Survivin. J. Int. Med. Res. 2020, 48, 0300060519892395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Hu, Y.; Sun, Y.; Xu, D. Endosulfan Triggers Epithelial-Mesenchymal Transition via PTP4A3-Mediated TGF-β Signaling Pathway in Prostate Cancer Cells. Sci. Total Environ. 2020, 731, 139234. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.J.; Kim, N.H.; Bae, N.Y.; Jo, H.S.; Park, S.A. DHA Inhibits Gremlin-1-Induced Epithelial-to-Mesenchymal Transition via ERK Suppression in Human Breast Cancer Cells. Biosci. Rep. 2020, 40, BSR20200164. [Google Scholar] [CrossRef]
- Choi, H.; Moon, A. Cross-talk between Cancer Cells and Endothelial Cells: Implications for Tumor Progression and Intervention. Arch. Pharm. Res. 2018, 41, 711–724. [Google Scholar] [CrossRef]
- Zhao, R.; Bei, X.; Yang, B.; Wang, X.; Jiang, C.; Shi, F.; Wang, X.; Zhu, Y.; Jing, Y.; Han, B.; et al. Endothelial Cells Promote Metastasis of Prostate Cancer by Enhancing Autophagy. J. Exp. Clin. Cancer Res. 2018, 37, 221. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Woywodt, A.; Bahlmann, F.H.; De Groot, K.; Haller, H.; Haubitz, M. Circulating Endothelial Cells: Life, Death, Detachment and Repair of the Endothelial Cell Layer. Nephrol. Dial. Transplant. 2002, 17, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F.; Remuzzitt, A.; Gordon, E.J.; Dewey, C.F.; Gimbrone, M.A. Turbulent Fluid Shear Stress Induce Vascular Endothelial Cell Turnover in Vitrio. Proc. Natl. Acad. Sci. USA 1986, 83, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Caplan, B.A.; Schwartz, C.J. Increased Endothelial Cell Turnover in Areas of in Vivo Evans Blue Uptake in the Pig Aorta. Atherosclerosis 1973, 17, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Mesa-Tejada, R.; Quick, C.M.; Bollen, A.W.; Joshi, S.; Pile-Spellman, J.; Lawton, M.T.; Young, W.L. Evidence of Increased Endothelial Cell Turnover in Brain Arteriovenous Malformations. Neurosurgery 2001, 49, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ortiz, P.; Orban, T.; Martin, M.; Habets, A.; Dequiedt, F.; Schurmans, S. Rasa3 Controls Turnover of Endothelial Cell Adhesion and Vascular Lumen Integrity by a Rap1-Dependent Mechanism. PLoS Genet 2018, 14, e1007195. [Google Scholar] [CrossRef]
- Berardi, D.E.; Tarbell, J.M. Stretch and Shear Interactions Affect Intercellular Junction Protein Expression and Turnover in Endothelial Cells. Cell. Mol. Bioeng. 2009, 2, 320–331. [Google Scholar] [CrossRef]
- Kostelnik, K.B.; Barker, A.; Schultz, C.; Mitchell, T.P.; Rajeeve, V.; White, I.J.; Aurrand-Lions, M.; Nourshargh, S.; Cutillas, P.; Nightingale, T.D. Dynamic Trafficking and Turnover of JAM-C Is Essential for Endothelial Cell Migration. PLoS Biol. 2019, 17, 320–331. [Google Scholar] [CrossRef]
- Gao, F.; Artham, S.; Sabbineni, H.; Al-Azayzih, A.; Peng, X.D.; Hay, N.; Adams, R.H.; Byzova, T.V.; Somanath, P.R. Akt1 Promotes Stimuli-Induced Endothelial-Barrier Protection through FoxO-Mediated Tight-Junction Protein Turnover. Cell. Mol. Life Sci. 2016, 73, 3917–3933. [Google Scholar] [CrossRef]
- Botros, L.; Pronk, M.C.A.; Juschten, J.; Liddle, J.; Morsing, S.K.H.; Van Buul, J.D.; Bates, R.H.; Tuinman, P.R.; Van Bezu, J.S.M.; Huveneers, S.; et al. Bosutinib Prevents Vascular Leakage by Reducing Focal Adhesion Turnover and Reinforcing Junctional Integrity. J. Cell Sci. 2020, 133, jcs240077. [Google Scholar] [CrossRef] [PubMed]
- Wylezinski, L.S.; Hawiger, J. Interleukin 2 Activates Brain Microvascular Endothelial Cells Resulting in Destabilization of Adherens Junctions. J. Biol. Chem. 2016, 291, 22913–22923. [Google Scholar] [CrossRef] [PubMed]
- Foteinos, G.; Hu, Y.; Xiao, Q.; Metzler, B.; Xu, Q. Rapid Endothelial Turnover in Atherosclerosis-Prone Areas Coincides with Stem Cell Repair in Apolipoprotein E-Deficient Mice. Circulation 2008, 117, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, M.I.; DeStefano, J.G.; Linville, R.M.; Wong, A.D.; Searson, P.C. Cerebrovascular Plasticity: Processes That Lead to Changes in the Architecture of Brain Microvessels. J. Cereb. Blood Flow Metab. 2019, 39, 1413–1432. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, J.G.; Xu, Z.S.; Williams, A.J.; Yimam, N.; Searson, P.C. Effect of Shear Stress on IPSC-Derived Human Brain Microvascular Endothelial Cells (DhBMECs). Fluids Barriers CNS 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Greif, D.M.; Eichmann, A. Basic Research: Where Do New Endothelial Cells Come from in the Injured Heart? Nat. Rev. Cardiol. 2017, 14, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Bowden, D.H. Cell Turnover in the Lung. Am. Rev. Respir. Dis. 1983, 128, 46–48. [Google Scholar] [CrossRef]
- Chen, F.; Fine, A. Stem Cells in Lung Injury and Repair. Am. J. Pathol. 2016, 186, 2544–2550. [Google Scholar] [CrossRef]
- Alban, F.T.; Gyamfi, D.; van Golen, R.F.; Heger, M. Reactive Oxygen and Nitrogen Species and Liver Ischemia-Reperfusion Injury: Role of Lipoic Acid. In The Liver; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–119. ISBN 9780128039519. [Google Scholar]
- Van Golen, R.F.; Reiniers, M.J.; Vrisekoop, N.; Zuurbier, C.J.; Olthof, P.B.; van Rheenen, J.; van Gulik, T.M.; Parsons, B.J.; Heger, M. The Mechanisms and Physiological Relevance of Glycocalyx Degradation in Hepatic Ischemia/Reperfusion Injury. Antioxid Redox Signal. 2014, 21, 1098–1118. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, N.; Huang, L. Endothelial Progenitor Cells and Spleen: New Insights in Regeneration Medicine. Cytotherapy 2010, 12, 7–16. [Google Scholar] [CrossRef]
- Crane, G.M.; Liu, Y.-C.; Chadburn, A. Spleen: Development, Anatomy and Reactive Lymphoid Proliferations. Semin. Diagn. Pathol. 2020, 38, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Collett, J.A.; Yoder, M.C. Endothelial Colony-Forming Cells and pro-Angiogenic Cells: Clarifying Definitions and Their Potential Role in Mitigating Acute Kidney Injury. Acta Physiol. 2018, 222, e12914. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.M.; Lo, Y.H.; Mah, A.T.; Kuo, C.J. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.S.; Romay, M.C.; McDonald, A.I.; Su, T.; Steel, M.E.; Iruela-Arispe, M.L. A Multi-Step Transcriptional Cascade Underlies Vascular Regeneration in Vivo. Sci. Rep. 2018, 8, 5430. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, S.; He, Y.; Hu, H.; Yao, B.; Zhang, Y. Regulation of Bone Morphogenetic Protein 4 on Epithelial Tissue. J. Cell Commun. Signal. 2020, 14, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Subileau, M.; Merdzhanova, G.; Ciais, D.; Collin-Faure, V.; Feige, J.J.; Bailly, S.; Vittet, D. Bone Morphogenetic Protein 9 Regulates Early Lymphatic-Specified Endothelial Cell Expansion during Mouse Embryonic Stem Cell Differentiation. Stem Cell Rep. 2019, 12, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.B.; Chen, K.; Zhang, Y.M.; Gong, T. Common Injuries and Repair Mechanisms in the Endothelial Lining. Chin. Med. J. 2018, 131, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhou, T.; Sekhar Pilli, V.S.; Phan, N.; Wang, Q.; Gupta, K.; Liu, Z.; Sheibani, N.; Liu, B. Novel Paracrine Functions of Smooth Muscle Cells in Supporting Endothelial Regeneration Following Arterial Injury. Circ. Res. 2019, 124, 1253–1265. [Google Scholar] [CrossRef]
- O’Carroll, L.; Wardrop, B.; Murphy, R.P.; Ross, M.D.; Harrison, M. Circulating Angiogenic Cell Response to Sprint Interval and Continuous Exercise. Eur. J. Appl. Physiol. 2019, 119, 743–752. [Google Scholar] [CrossRef]
- D’Alessio, F.R.; Kurzhagen, J.T.; Rabb, H. Reparative T Lymphocytes in Organ Injury. J. Clin. Investig. 2019, 129, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, J.; Wang, Y.; Chen, Z.; Zhang, L.; Yu, J.; Zhu, D.; Zhong, M. M2 Macrophages Promote Pulmonary Endothelial Cells Regeneration in Sepsis-Induced Acute Lung Injury. Ann. Transl. Med. 2019, 7, 142. [Google Scholar] [CrossRef]
- Osorio-Valencia, S.; Zhou, B. Roles of Macrophages and Endothelial Cells and Their Cross-talk in Acute Lung Injury. Biomedicines 2024, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Zhou, X.; Zhou, W.; Lin, H. Regulatory T Cell and Macrophage Cross-talk in Acute Lung Injury: Future Perspectives. Cell Death Discov. 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F. Autophagy in Vascular Endothelial Cells. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Han, R.; Trojanowska, M. ER Stress and Endothelial Dysfunction. IUBMB Life 2014, 66, 530. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, R.E.; Chung, E.; Reese, L.; Cox-York, K.; Seals, D.R.; Gentile, C.L. Activation of the Unfolded Protein Response in Vascular Endothelial Cells of Nondiabetic Obese Adults. J. Clin. Endocrinol. Metab. 2013, 98, E1505. [Google Scholar] [CrossRef]
- Nagane, M.; Yasui, H.; Kuppusamy, P.; Yamashita, T.; Inanami, O. DNA Damage Response in Vascular Endothelial Senescence: Implication for Radiation-Induced Cardiovascular Diseases. J. Radiat. Res. 2021, 62, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Singh, K.K. DNA Damage Response Signaling: A Common Link between Cancer and Cardiovascular Diseases. Cancer Med. 2023, 12, 4380–4404. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, W.J.; Cho, K.J. P62 Links the Autophagy Pathway and the Ubiquitin–Proteasome System in Endothelial Cells during Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 7791. [Google Scholar] [CrossRef]
- Stangl, K.; Stangl, V. The Ubiquitin-Proteasome Pathway and Endothelial (Dys)Function. Cardiovasc. Res. 2010, 85, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bhat, O.M.; Li, P.L. Lysosome Function in Cardiovascular Diseases. Cell Physiol. Biochem. 2021, 55, 277. [Google Scholar] [CrossRef]
- Lu, Y.; Nanayakkara, G.; Sun, Y.; Liu, L.; Xu, K.; Drummer, C.; Shao, Y.; Saaoud, F.; Choi, E.T.; Jiang, X.; et al. Procaspase-1 Patrolled to the Nucleus of Proatherogenic Lipid LPC-Activated Human Aortic Endothelial Cells Induces ROS Promoter CYP1B1 and Strong Inflammation. Redox Biol. 2021, 47, 102142. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of New Blood-Vessel Formation and Proliferative Heterogeneity of Endothelial Cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Jerka, D.; Bonowicz, K.; Piekarska, K.; Gokyer, S.; Derici, U.S.; Hindy, O.A.; Altunay, B.B.; Yazgan, I.; Steinbrink, K.; Kleszczyński, K.; et al. Unraveling Endothelial Cell Migration: Insights into Fundamental Forces, Inflammation, Biomaterial Applications, and Tissue Regeneration Strategies. ACS Appl. Bio Mater. 2024, 7, 2054–2069. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tu, J.; Gao, Q.; Li, X.; Zhu, Z.; Jin, Y.; Zhang, Y.; Xie, J.; Zhu, P.; Zhao, B.; et al. WITHDRAWN: BBB Recovery after Stroke by Self-Replenishing E-Pericytes Transdifferentiated from Local Endothelial Cells. bioRxiv 2024, 2024.01.07.573171. [Google Scholar] [CrossRef]
- Kalies, K.; Knöpp, K.; Wurmbrand, L.; Korte, L.; Dutzmann, J.; Pilowski, C.; Koch, S.; Sedding, D. Isolation of Circulating Endothelial Cells Provides Tool to Determine Endothelial Cell Senescence in Blood Samples. Sci. Rep. 2024, 14, 4271. [Google Scholar] [CrossRef] [PubMed]
- Lallo, V.; Bracaglia, L.G. Influencing Endothelial Cells’ Roles in Inflammation and Wound Healing Through Nucleic Acid Delivery. Tissue Eng. Part A 2024, 30, 272–286. [Google Scholar] [CrossRef]
- Pinto, T.S.; Feltran, G.D.S.; Fernandes, C.J.D.C.; de Camargo Andrade, A.F.; Coque, A.D.C.; Silva, S.L.; Abuderman, A.A.; Zambuzzi, W.F.; Foganholi da Silva, R.A. Epigenetic Changes in Shear-Stressed Endothelial Cells. Cell Biol. Int. 2024, 48, 665–681. [Google Scholar] [CrossRef]
- Alvandi, Z.; Bischoff, J. Endothelial-Mesenchymal Transition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2357–2369. [Google Scholar] [CrossRef]

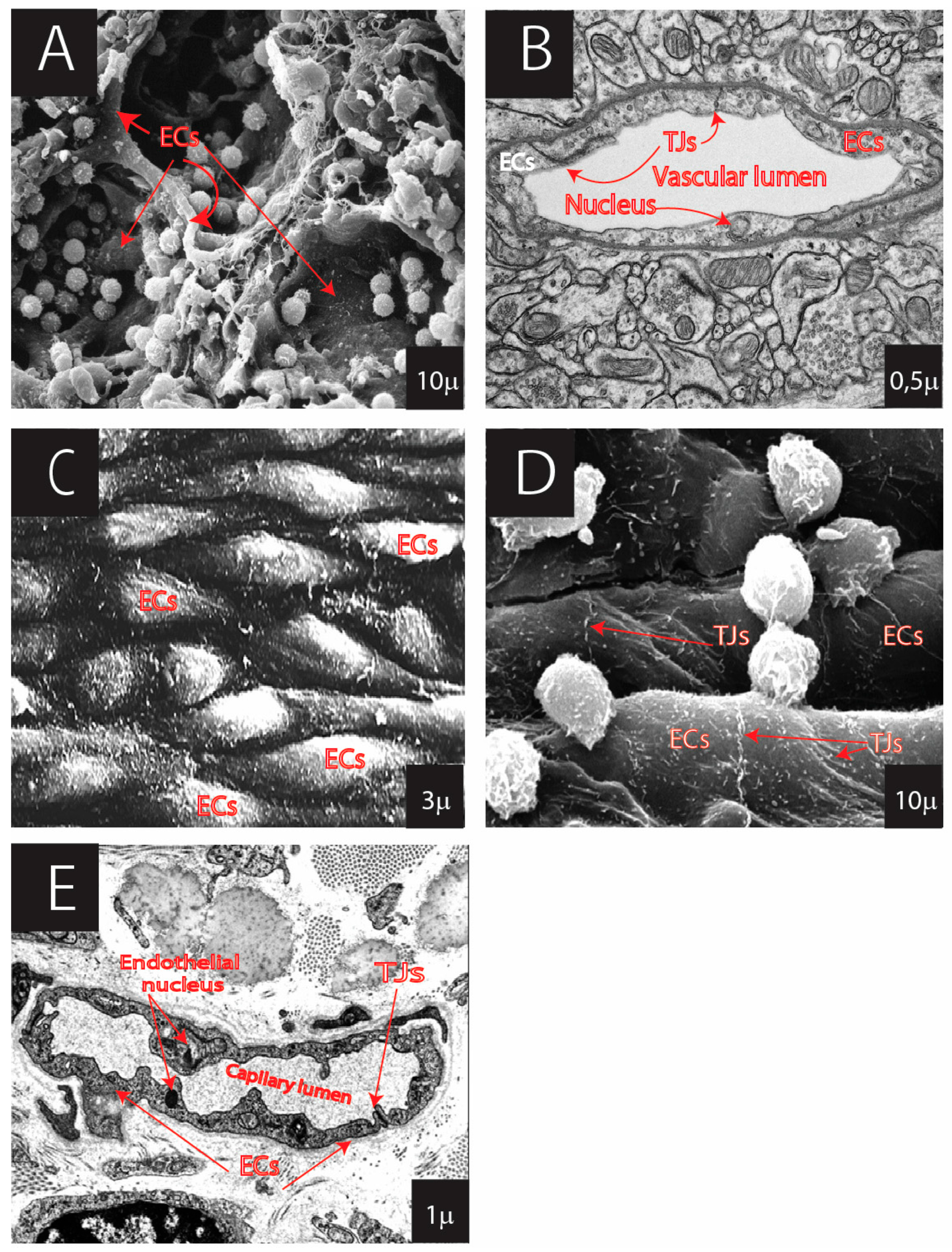

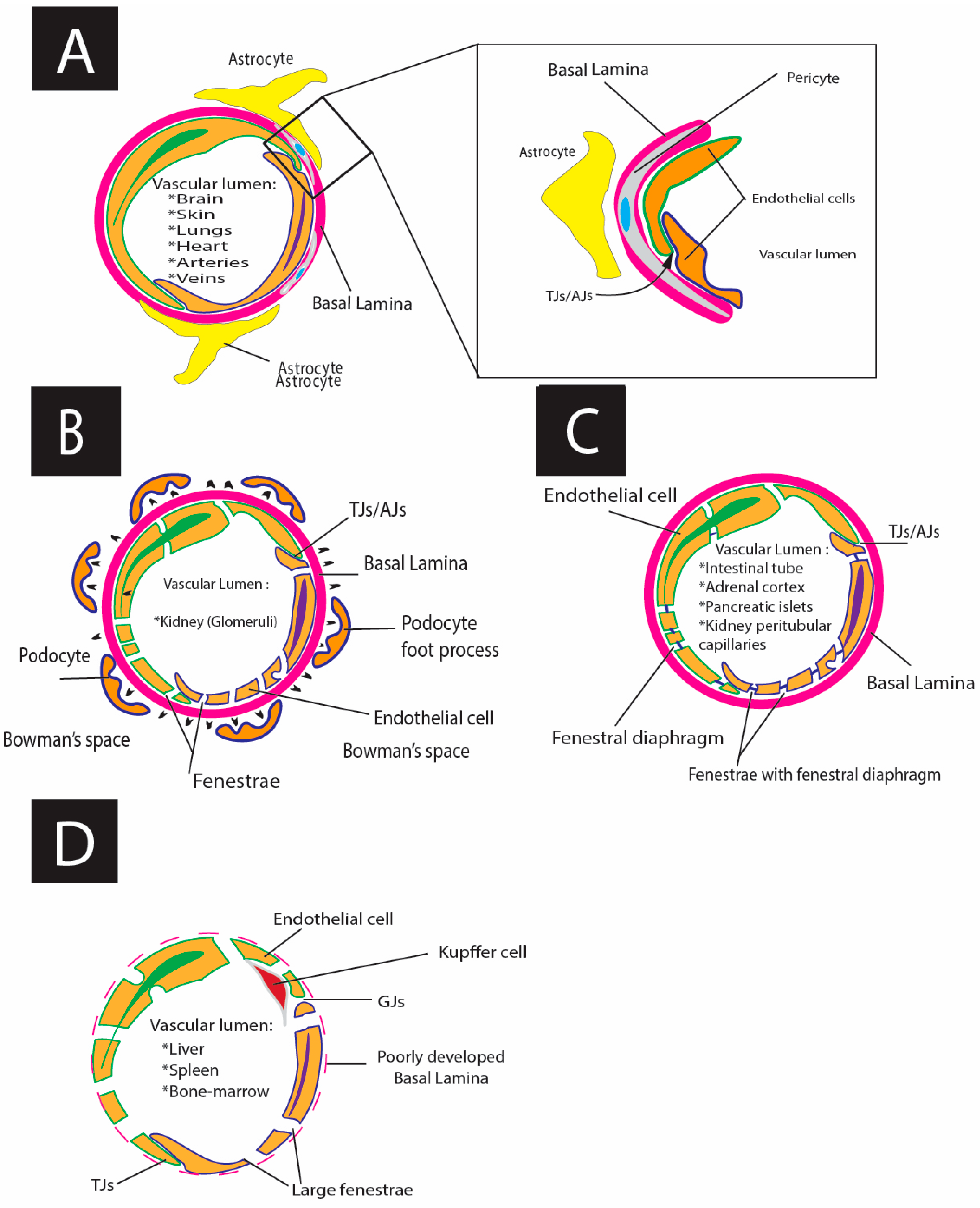
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larionov, A.; Hammer, C.M.; Fiedler, K.; Filgueira, L. Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease. Cells 2024, 13, 1276. https://doi.org/10.3390/cells13151276
Larionov A, Hammer CM, Fiedler K, Filgueira L. Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease. Cells. 2024; 13(15):1276. https://doi.org/10.3390/cells13151276
Chicago/Turabian StyleLarionov, Alexey, Christian Manfred Hammer, Klaus Fiedler, and Luis Filgueira. 2024. "Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease" Cells 13, no. 15: 1276. https://doi.org/10.3390/cells13151276
APA StyleLarionov, A., Hammer, C. M., Fiedler, K., & Filgueira, L. (2024). Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease. Cells, 13(15), 1276. https://doi.org/10.3390/cells13151276






