Cytokeratin 18 as a Novel Biomarker in Patients with Hypertrophic Cardiomyopathy
Abstract
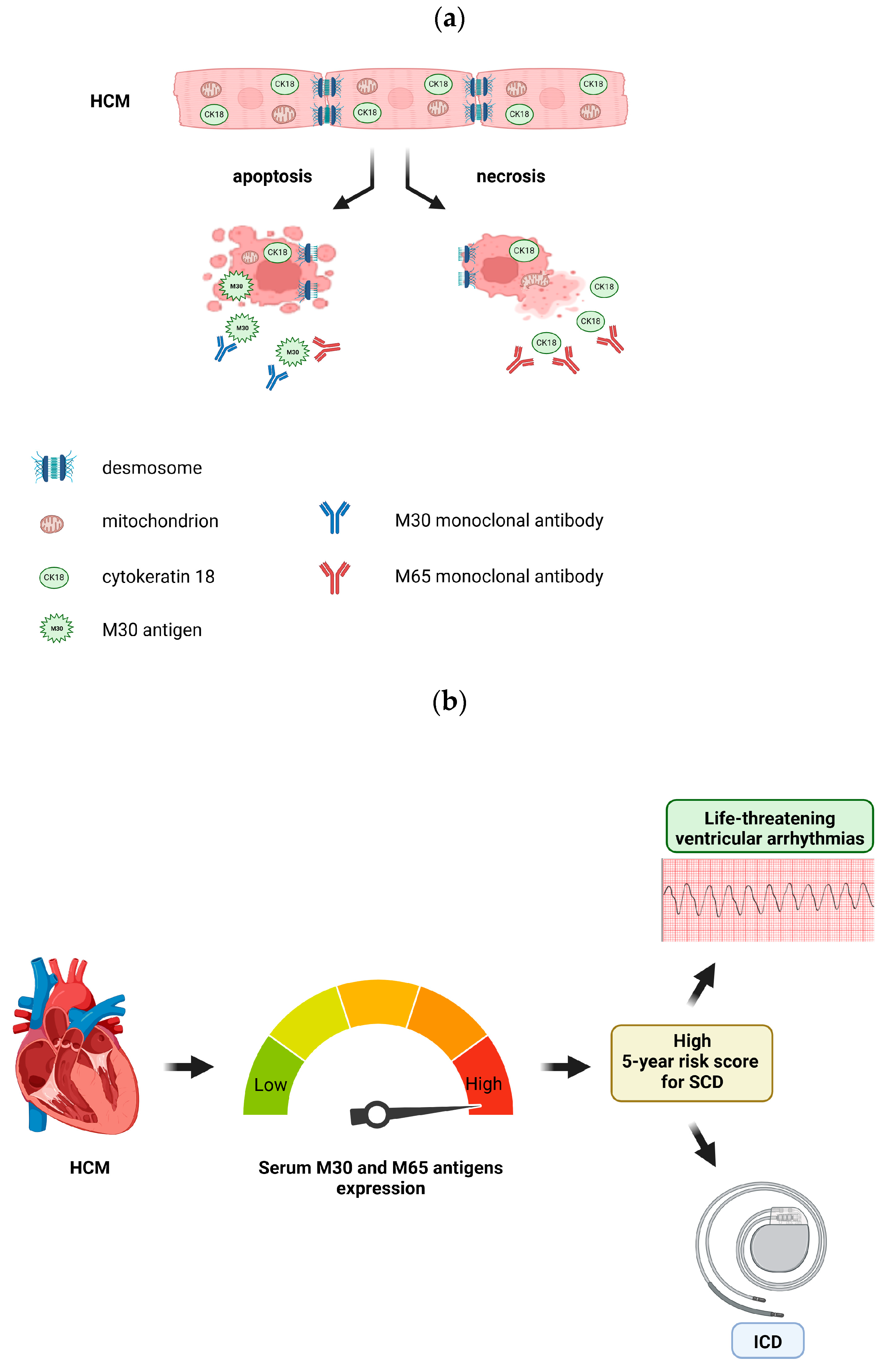
:1. Introduction
2. Materials and Methods
2.1. Study Population
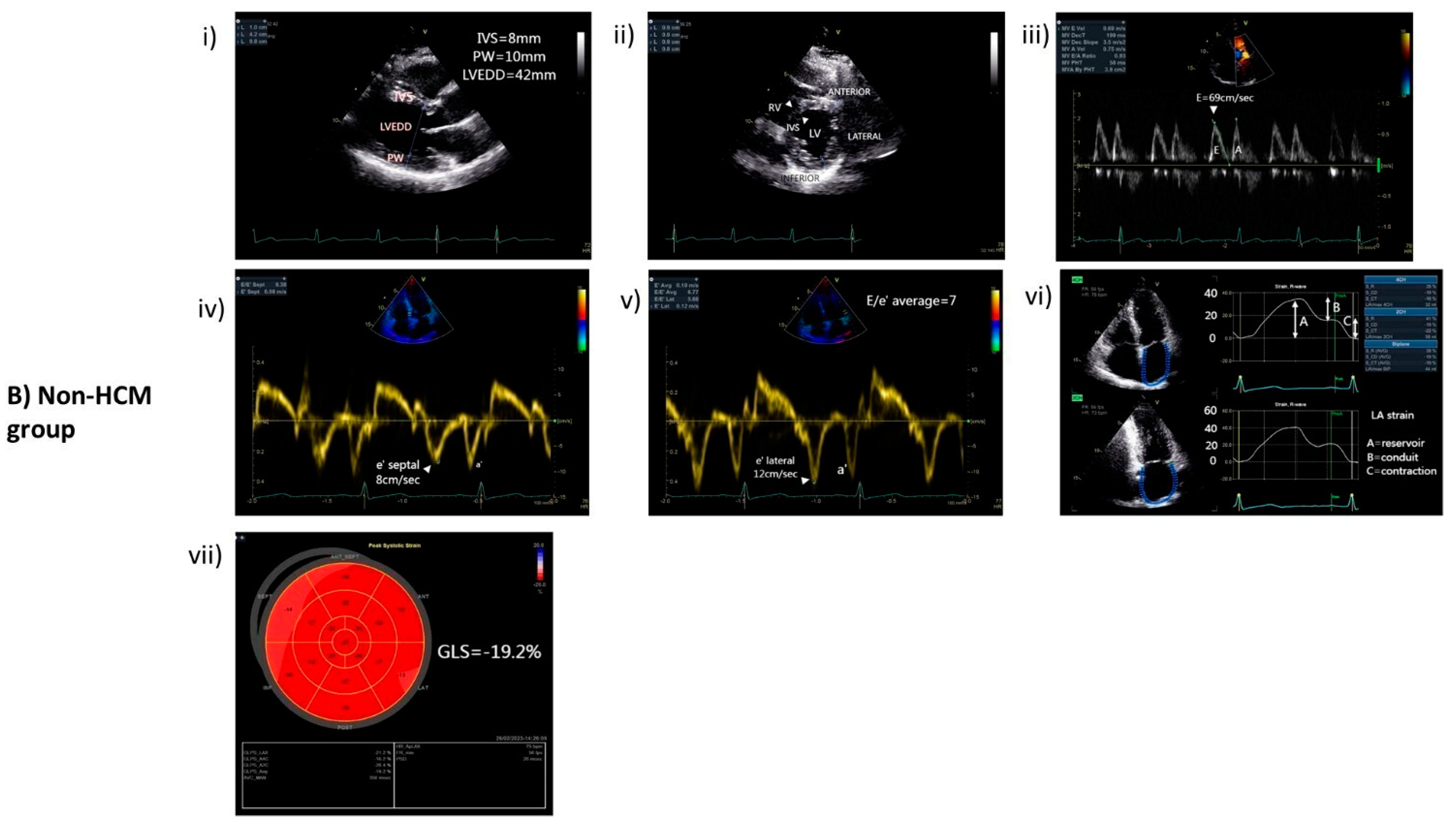
2.2. Echocardiography
2.3. Laboratory Examination
2.4. SCD Risk Assessment and Classification in HCM
2.5. Immunohistochemistry of Human Postmortem Heart Tissue
Analysis of Confocal Images
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Characteristics of HCM and Non-HCM Group
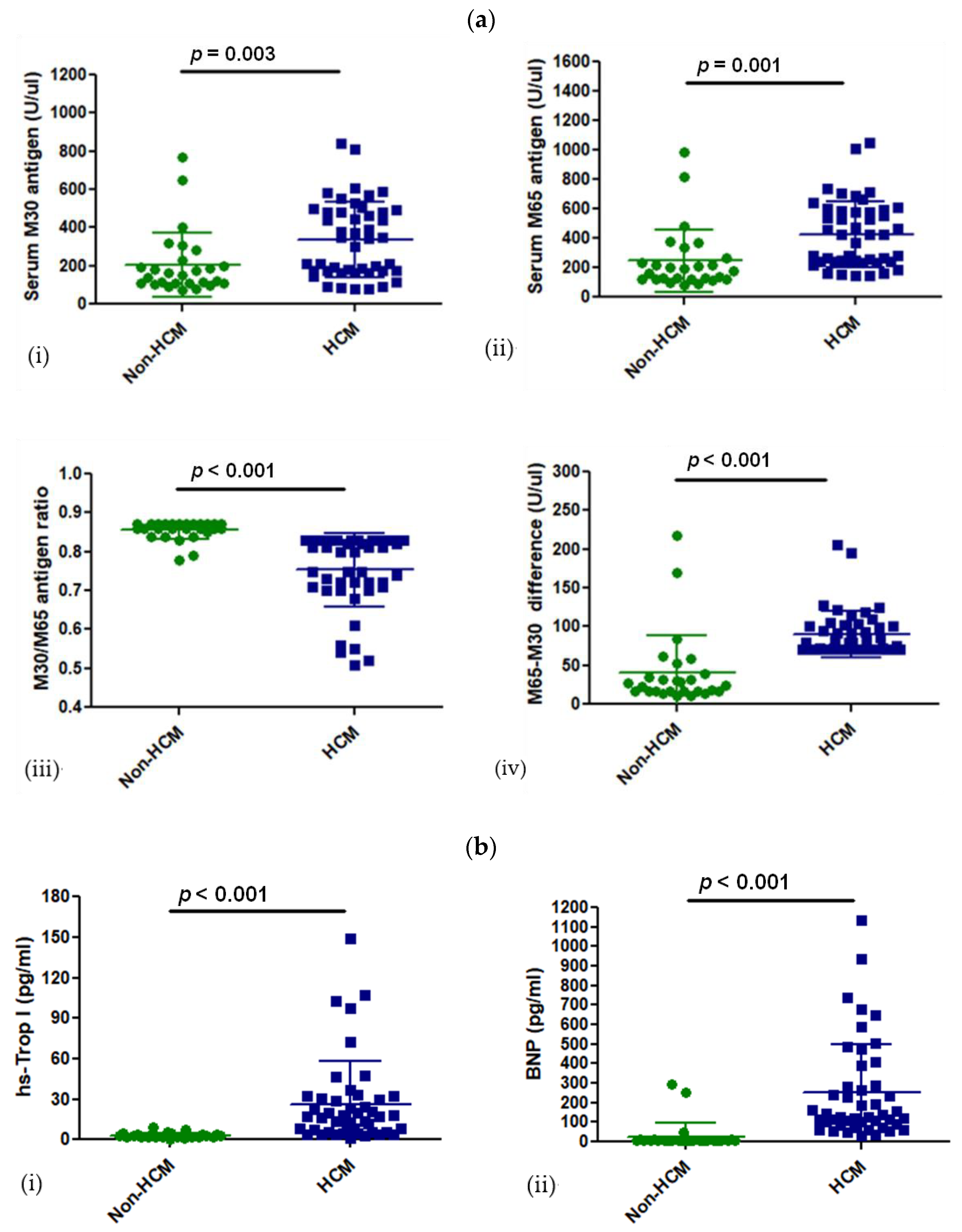
3.2. Levels of CK18 and Its Caspase-Cleaved Product in the Blood Serum of Patients with HCM
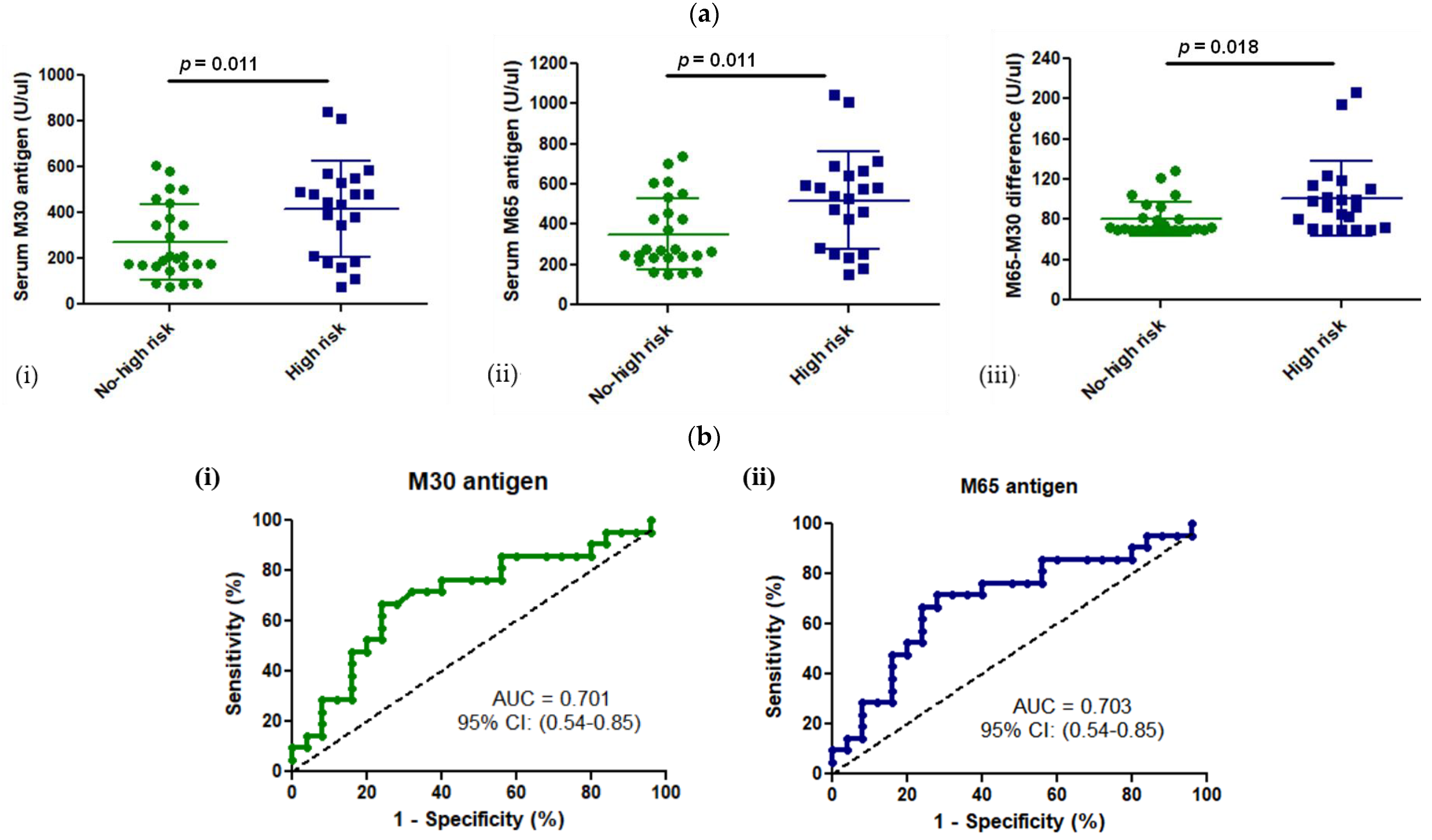
3.3. M30 and M65 Expression in HCM Group with or without High Risk for SCD
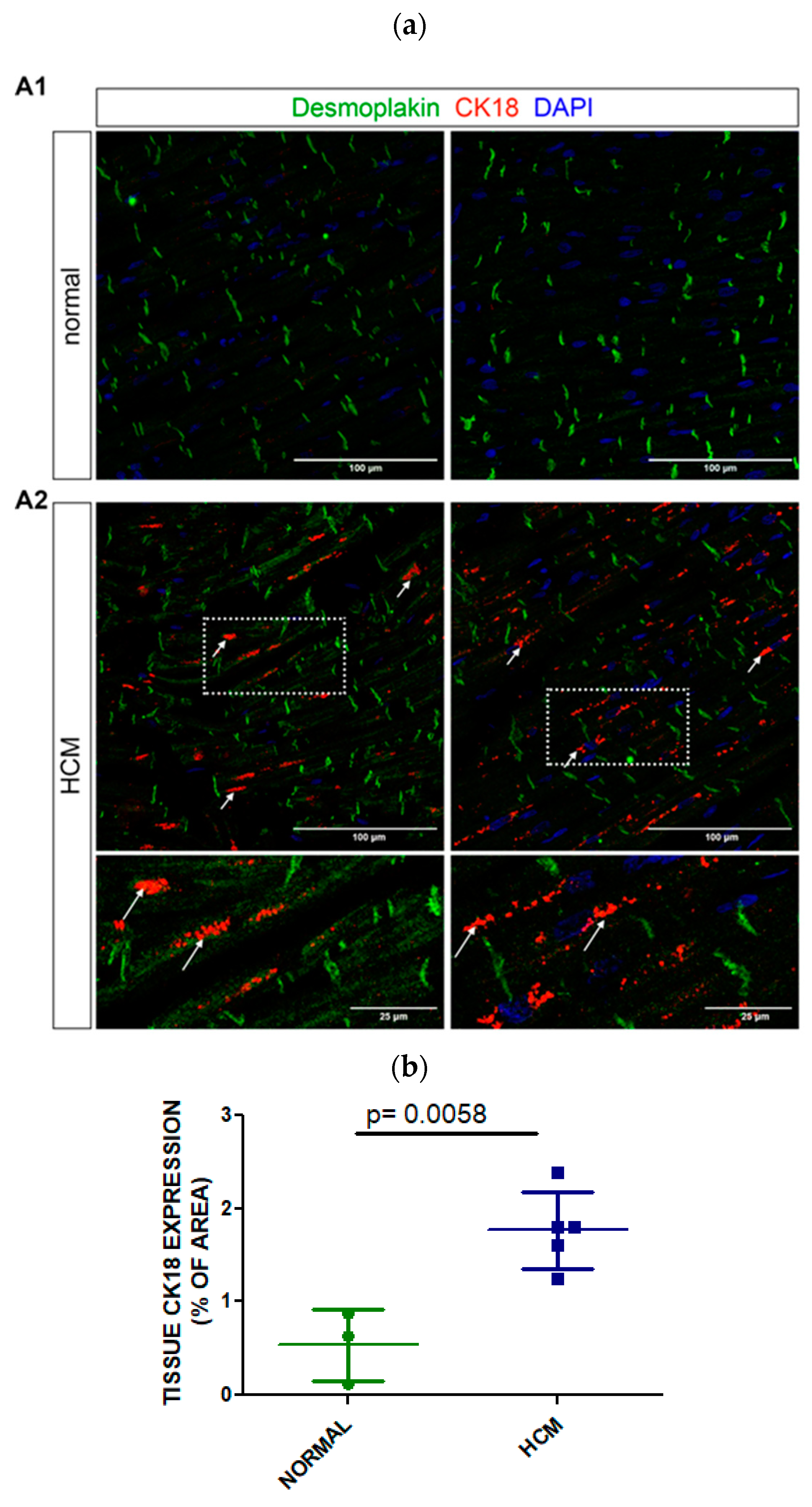
3.4. Tissue Expression of CK18 in Postmortem Myocardial Specimens from an HCM or a Normal Heart
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maron, B.J. Contemporary Insights and Strategies for Risk Stratification and Prevention of Sudden Death in Hypertrophic Cardiomyopathy. Circulation 2010, 121, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef]
- Maron, B.J. Hypertrophic Cardiomyopathy: A Systematic Review. JAMA 2002, 287, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Watkins, H.; Ashrafian, H.; Redwood, C. Inherited Cardiomyopathies. N. Engl. J. Med. 2011, 364, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Girolami, F.; Sciagrà, R.; Ackerman, M.J.; Sotgia, B.; Bos, J.M.; Nistri, S.; Sgalambro, A.; Grifoni, C.; Torricelli, F.; et al. Microvascular Function Is Selectively Impaired in Patients with Hypertrophic Cardiomyopathy and Sarcomere Myofilament Gene Mutations. J. Am. Coll. Cardiol. 2011, 58, 839–848. [Google Scholar] [CrossRef]
- Capetanaki, Y.; Papathanasiou, S.; Diokmetzidou, A.; Vatsellas, G.; Tsikitis, M. Desmin Related Disease: A Matter of Cell Survival Failure. Curr. Opin. Cell Biol. 2015, 32, 113–120. [Google Scholar] [CrossRef]
- Papathanasiou, S.; Rickelt, S.; Soriano, M.E.; Schips, T.G.; Maier, H.J.; Davos, C.H.; Varela, A.; Kaklamanis, L.; Mann, D.L.; Capetanaki, Y. Tumor Necrosis Factor-α Confers Cardioprotection through Ectopic Expression of Keratins K8 and K18. Nat. Med. 2015, 21, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, M.; Galata, Z.; Mavroidis, M.; Psarras, S.; Capetanaki, Y. Intermediate Filaments in Cardiomyopathy. Biophys. Rev. 2018, 10, 1007–1031. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Omary, M.B. “Hard” and “Soft” Principles Defining the Structure, Function and Regulation of Keratin Intermediate Filaments. Curr. Opin. Cell Biol. 2002, 14, 110–122. [Google Scholar] [CrossRef]
- Kuruc, N.; Franke, W.W. Transient Coexpression of Desmin and Cytokeratins 8 and 18 in Developing Myocardial Cells of Some Vertebrate Species. Differentiation 1988, 38, 177–193. [Google Scholar] [CrossRef]
- Soleiman, A.; Lukschal, A.; Hacker, S.; Aumayr, K.; Hoetzenecker, K.; Lichtenauer, M.; Moser, B.; Untersmayr, E.; Horvat, R.; Ankersmit, H.J. Myocardial Lipofuscin-Laden Lysosomes Contain the Apoptosis Marker Caspase-Cleaved Cytokeratin-18. Eur. J. Clin. Investig. 2008, 38, 708–712. [Google Scholar] [CrossRef]
- Oshima, R.G.; Baribault, H.; Caulín, C. Oncogenic Regulation and Function of Keratins 8 and 18. Cancer Metastasis Rev. 1996, 15, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, M.; Mahmoudi, M.J.; Rose, N.R.; Rezaei, N. Proinflammatory Cytokines in Heart Failure: Double-Edged Swords. Heart Fail. Rev. 2010, 15, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Haudek, S.B.; Taffet, G.E.; Schneider, M.D.; Mann, D.L. TNF Provokes Cardiomyocyte Apoptosis and Cardiac Remodeling through Activation of Multiple Cell Death Pathways. J. Clin. Investig. 2007, 117, 2692–2701. [Google Scholar] [CrossRef]
- Chen, F.; Chang, R.; Trivedi, M.; Capetanaki, Y.; Cryns, V.L. Caspase Proteolysis of Desmin Produces a Dominant-Negative Inhibitor of Intermediate Filaments and Promotes Apoptosis. J. Biol. Chem. 2003, 278, 6848–6853. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, P.; Davos, C.H.; Milner, D.J.; Varela, E.; Cameron, J.; Mann, D.L.; Capetanaki, Y. Desmin Mediates TNF-Alpha-Induced Aggregate Formation and Intercalated Disk Reorganization in Heart Failure. J. Cell Biol. 2008, 181, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Caulín, C.; Salvesen, G.S.; Oshima, R.G. Caspase Cleavage of Keratin 18 and Reorganization of Intermediate Filaments during Epithelial Cell Apoptosis. J. Cell Biol. 1997, 138, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase Cleavage of Vimentin Disrupts Intermediate Filaments and Promotes Apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kavanagh, B.D.; Thorburn, A.M.; Camidge, D.R. Preclinical and Clinical Estimates of the Basal Apoptotic Rate of a Cancer Predict the Amount of Apoptosis Induced by Subsequent Proapoptotic Stimuli. Clin. Cancer Res. 2010, 16, 4478–4489. [Google Scholar] [CrossRef]
- Linder, S.; Olofsson, M.H.; Herrmann, R.; Ulukaya, E. Utilization of Cytokeratin-Based Biomarkers for Pharmacodynamic Studies. Expert Rev. Mol. Diagn. 2010, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the Cardiomyopathies: A Position Statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Ring, L.; Oxborough, D.; Harkness, A.; Bennett, S.; Rana, B.; Sutaria, N.; Lo Giudice, F.; Shun-Shin, M.; Paton, M.; et al. The Assessment of Left Ventricular Diastolic Function: Guidance and Recommendations from the British Society of Echocardiography. Echo Res. Pract. 2024, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 7, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Schaff, H.V.; Lentz Carvalho, J.; Nishimura, R.A.; Geske, J.B.; Dearani, J.A.; Lahr, B.D.; Lee, A.T.; Bos, J.M.; Ackerman, M.J.; et al. Myocardial Histopathology in Patients with Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 77, 2159–2170. [Google Scholar] [CrossRef]
- Polari, L.; Alam, C.M.; Nyström, J.H.; Heikkilä, T.; Tayyab, M.; Baghestani, S.; Toivola, D.M. Keratin Intermediate Filaments in the Colon: Guardians of Epithelial Homeostasis. Int. J. Biochem. Cell Biol. 2020, 129, 105878. [Google Scholar] [CrossRef]
- Jerše, M.; Zidar, N. Apoptosis in the Developing Human Heart Resembles Apoptosis in Epithelial Tissues. Cell Tissue Res. 2011, 343, 537–543. [Google Scholar] [CrossRef]
- Senturk, T.; Aydinlar, A.; Yilmaz, Y.; Oral, A.Y.; Ozdabakoglu, O.; Ulukaya, E. Serial Changes in Circulating M30 Antigen, a Biomarker of Apoptosis, in Patients with Acute Coronary Syndromes: Relationship with the Severity of Coronary Artery Disease. Coron. Artery Dis. 2009, 20, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Adlbrecht, C.; Hoetzenecker, K.; Posch, M.; Steiner, S.; Kopp, C.; Hacker, S.; Auer, J.; Horvath, R.; Moser, B.; Roth, G.; et al. Elevated Levels of Interleukin-1β-Converting Enzyme and Caspase-Cleaved Cytokeratin-18 in Acute Myocardial Infarction. Eur. J. Clin. Investig. 2007, 37, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mattey, D.L.; Dawes, P.T.; Nixon, N.B.; Goh, L.; Banks, M.J.; Kitas, G.D. Increased Levels of Antibodies to Cytokeratin 18 in Patients with Rheumatoid Arthritis and Ischaemic Heart Disease. Ann. Rheum. Dis. 2004, 63, 420–425. [Google Scholar] [CrossRef]
- Herzer, K.; Kneiseler, G.; Bechmann, L.P.; Post, F.; Schlattjan, M.; Sowa, J.-P.; Neumann, T.; Marggraf, G.; Erbel, R.; Gerken, G.; et al. Onset of Heart Failure Determines the Hepatic Cell Death Pattern. Ann. Hepatol. 2011, 10, 174–179. [Google Scholar] [CrossRef]
- Yang, M.-C.; Liu, H.-K.; Su, Y.-T.; Tsai, C.-C.; Wu, J.-R. Serum Apoptotic Marker M30 Is Positively Correlated with Early Diastolic Dysfunction in Adolescent Obesity. PLoS ONE 2019, 14, e0217429. [Google Scholar] [CrossRef] [PubMed]
- Cambronero, F.; Marín, F.; Roldán, V.; Hernández-Romero, D.; Valdés, M.; Lip, G.Y.H. Biomarkers of Pathophysiology in Hypertrophic Cardiomyopathy: Implications for Clinical Management and Prognosis. Eur. Heart J. 2009, 30, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Zen, K.; Irie, H.; Doue, T.; Takamiya, M.; Yamano, T.; Sawada, T.; Azuma, A.; Matsubara, H. Analysis of Circulating Apoptosis Mediators and Proinflammatory Cytokines in Patients with Idiopathic Hypertrophic Cardiomyopathy: Comparison between Nonobstructive and Dilated-Phase Hypertrophic Cardiomyopathy. Int. Heart J. 2005, 46, 231–244. [Google Scholar] [CrossRef]
- Sato, Y.; Taniguchi, R.; Nagai, K.; Makiyama, T.; Okada, H.; Yamada, T.; Matsumori, A.; Takatsu, Y. Measurements of Cardiac Troponin T in Patients with Hypertrophic Cardiomyopathy. Heart 2003, 89, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yan, Y.; Chen, Q. Clinical Significance of Serum Ck18-M65 and M30 Levels in Patients with Chronic Hepatitis B Combined with Nonalcoholic Steatohepatitis and Liver Fibrosis. Medicine 2024, 103, e38342. [Google Scholar] [CrossRef]
- Turk, H.M.; Aliyev, A.; Celik, R.S.; Seker, M.; Coban, E.; Demir, T.; Baydas, T.; Kocyigit, A. Usefulness of Serum M30 and M65 Levels to Predict Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer. Curr. Probl. Cancer 2020, 44, 100497. [Google Scholar] [CrossRef]
- Koch, A.; Yagmur, E.; Linka, J.; Schumacher, F.; Bruensing, J.; Buendgens, L.; Herbers, U.; Koek, G.H.; Weiskirchen, R.; Trautwein, C.; et al. High Circulating Caspase-Cleaved Keratin 18 Fragments (M30) Indicate Short-Term Mortality in Critically Ill Patients. Dis. Markers 2018, 2018, 8583121. [Google Scholar] [CrossRef] [PubMed]
- Oweira, H.; Sadeghi, M.; Volker, D.; Mieth, M.; Zidan, A.; Khajeh, E.; Ghamarnejad, O.; Fonouni, H.; Weiss, K.H.; Schmidt, J.; et al. Serum Caspase-Cleaved Cytokeratin (M30) Indicates Severity of Liver Dysfunction and Predicts Liver Outcome. Ann. Transplant. 2018, 23, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Gao, S.; Luo, G.; Cheng, G.; Huang, W.; Jiang, R.; Wang, Y.; Cui, T. Increased Circulating Autoantibodies Levels of IgG, IgA, IgM Against Cytokeratin 18 and Cytokeratin 19 in Chronic Obstructive Pulmonary Disease. Arch. Med. Res. 2017, 48, 79–87. [Google Scholar] [CrossRef]
- Simopoulos, C.; Tsaroucha, A.K.; Asimakopoulos, B.; Giatromanolaki, A.; Gavriilidis, P.; Polychronidis, A.; Karayiannakis, A. Total and Caspase-Cleaved Cytokeratin 18 in Chronic Cholecystitis: A Prospective Study. BMC Gastroenterol. 2008, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Krenn, C.; Brunner, M.; Moser, B.; Ploder, M.; Spittler, A.; Pelinka, L.; Sautner, T.; Wolner, E.; Boltz-Nitulescu, G.; et al. Elevated Serum Levels of Epithelial Cell Apoptosis-Specific Cytokeratin 18 Neoepitope M30 in Critically Ill Patients. Shock 2004, 22, 218–220. [Google Scholar] [CrossRef]
- Högye, M.; Mándi, Y.; Csanády, M.; Sepp, R.; Buzás, K. Comparison of Circulating Levels of Interleukin-6 and Tumor Necrosis Factor-Alpha in Hypertrophic Cardiomyopathy and in Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2004, 94, 249–251. [Google Scholar] [CrossRef]
- Dirkx, E.; da Costa Martins, P.A.; De Windt, L.J. Regulation of Fetal Gene Expression in Heart Failure. Biochim. Biophys. Acta 2013, 1832, 2414–2424. [Google Scholar] [CrossRef]

| HCM (n = 46) | Non-HCM (n = 27) | Significance (p-Value) | |
|---|---|---|---|
| Age (years) | 50 (±11) | 50 (±10) | 0.731 |
| Male/Female | 36 (78.3%)/10 (21.7%) | 20 (74.1%)/7 (25.9%) | 0.777 |
| BMI (kg/m2) | 29.8 (±5) | 29.5 (±6) | 0.807 |
| Smoking | 21 (45.7%) | 12 (44.4%) | 1.000 |
| Diabetes Mellitus | 9 (19.6%) | 4 (14.8%) | 0.756 |
| Hypertension | 21 (45.7%) | 11 (40.7%) | 0.808 |
| Dyslipidemia | 25 (54.3%) | 17 (63%) | 0.624 |
| Atrial Fibrillation | 11 (23.9%) | 6 (22.2%) | 1.000 |
| WBC (K/uL) | 7.2 (±1.7) | 7.9 (±2.5) | 0.185 |
| Hgb (mg/dL) | 14 (±1) | 14 (±1) | 0.184 |
| PLTs (K/uL) | 225 (±60) | 253 (±55) | 0.054 |
| SGOT (U/L) | 28 (±11) | 25 (±8) | 0.157 |
| SGPT (U/L) | 33 (±22) | 31 (±18) | 0.748 |
| gGT (U/L) | 37 (±30) | 29 (±14) | 0.252 |
| ALP (U/L) | 73 (±22) | 72 (±18) | 0.754 |
| TB (mg/dL) | 0.8 (±0.7) | 0.7 (±0.3) | 0.460 |
| Creatinine (mg/dL) | 1.03 (±0.18) | 1.01 (±0.20) | 0.703 |
| Hs-CRP (mg/dL) | 0.53 (±0.69) | 0.25 (±0.28) | 0.050 |
| HCM (n = 46) | Non-HCM (n = 27) | Significance (p-Value) | |
|---|---|---|---|
| LVEDD (mm) | 47 (±7) | 47 (±4) | 0.893 |
| LVESD (mm) | 30 (±6) | 31 (±4) | 0.684 |
| IVS (mm) | 18 (±4) | 10 (±2) | <0.001 |
| PW (mm) | 11 (±2) | 9 (±1) | 0.005 |
| MWT (mm) | 19 (±3) | 10 (±2) | <0.001 |
| LV mass index (g/m2) | 137 (±30) | 77 (±23) | <0.001 |
| Location of asymmetric LV hypertrophy | |||
| IVS | 39 (84%) | - | |
| Apex | 4 (9%) | - | |
| Mixed | 3 (7%) | - | |
| LVOTO (mmHg) | 14 (30%) 61 mmHg ± 35 | - | |
| LVEF (%) | 58 (±8) | 60 (±4) | 0.435 |
| GLS (%) | −14 (±6) | −20 (±2) | <0.001 |
| LA diameter (mm) | 47 (±7) | 39 (±5) | <0.001 |
| LA volume index (mL/m2) | 51(±18) | 29 (±10) | <0.001 |
| E/e’ratio | 13 (±7) | 8 (±3) | <0.001 |
| LA strain (reservoir) | 20 (±7) | 31 (±9) | <0.001 |
| LA strain (conduit) | −10 (±5) | −15 (±5) | 0.001 |
| LA strain (contraction) | −10 (±5) | −16 (±6) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragkiadakis, K.; Ktena, N.; Kalantidou, A.; Dermitzaki, E.; Anastasiou, I.; Papathanassiou, S.; Kontaraki, J.; Kalomoirakis, P.; Kanoupakis, E.; Patrianakos, A.; et al. Cytokeratin 18 as a Novel Biomarker in Patients with Hypertrophic Cardiomyopathy. Cells 2024, 13, 1328. https://doi.org/10.3390/cells13161328
Fragkiadakis K, Ktena N, Kalantidou A, Dermitzaki E, Anastasiou I, Papathanassiou S, Kontaraki J, Kalomoirakis P, Kanoupakis E, Patrianakos A, et al. Cytokeratin 18 as a Novel Biomarker in Patients with Hypertrophic Cardiomyopathy. Cells. 2024; 13(16):1328. https://doi.org/10.3390/cells13161328
Chicago/Turabian StyleFragkiadakis, Konstantinos, Niki Ktena, Aikaterini Kalantidou, Eirini Dermitzaki, Ioannis Anastasiou, Stamatis Papathanassiou, Joanna Kontaraki, Petros Kalomoirakis, Emmanuel Kanoupakis, Alexandros Patrianakos, and et al. 2024. "Cytokeratin 18 as a Novel Biomarker in Patients with Hypertrophic Cardiomyopathy" Cells 13, no. 16: 1328. https://doi.org/10.3390/cells13161328





